ABSTRACT
Background
The high cost and insufficient supply of HPV vaccines have substantially slowed their implementation in lower-income countries. This study aimed to assess the incremental cost-effectiveness of two doses of human papillomavirus (HPV) vaccination (bivalent 16/18 vaccine; 2vHPV) compared to a no-vaccination scenario and a three-dose scenario in one province in China.
Methods
A static Markov model was used to model a lifetime cohort of 100,000 girls aged 12 years at the start of vaccination. A two-dose vaccination schedule was assumed to be non-inferior to a three-dose schedule in terms of vaccine efficacy, and both vaccination schemes were assumed to provide lifelong protection. Incremental costs, health effects and incremental cost-effectiveness ratios were used to measure the outcomes when comparing the different strategies.
Results
Compared to no vaccination, the incremental cost-effectiveness ratio (Chinese yuan per quality-adjusted life year) of the two-dose vaccination strategy is 12,472, and the 2-dose strategy is calculated to be cost saving relative to the 3-dose vaccination strategy.
Conclusions
Introducing the 2vHPV vaccine would be highly cost effective at a per-dose vaccine price of CNY 500, which has implications for cervical cancer control in China and other resource-limited countries.
Introduction
Persistent human papillomavirus (HPV) infection is a well-established cause of cervical cancer (CC) and other types of cancer, such as anus, vulva, vagina, penis, and head and neck cancer. CC is the fourth most common cancer among women globally, with almost 85% of cases occurring in developing countries.1 China, the largest developing country, is facing an enormous disease burden, with 99,000 new CC cases in 2015, which accounted for 5.6% of all female cancer cases.Citation2 Two high-risk HPV types (type 16 and 18) were reported to be associated with 69% of CC in China on 10 December 2018 by the HPV information center.Citation3
Screening is considered an effective method of early detection, while the most promising strategy to prevent CC is vaccination against HPV. The 16/18 bivalent HPV (2vHPV) vaccine has been proven to be able to protect against persistent HPV infection and CC in clinical trialsCitation4,Citation5 and has been evaluated for cost-effectiveness in many countries.Citation6,Citation7
Some clinical trials have also suggested that a two-dose 2vHPV vaccination schedule is noninferior to a three-dose schedule.Citation8,Citation9 The World Health Organization (WHO) has recommended that girls aged 9–14 years receive a 2-dose 2vHPV vaccination schedule,Citation10 and countries such as the US,Citation11 CanadaCitation12 and SwitzerlandCitation13 have implemented two-dose vaccination schedules. The WHO has also recommended that a cost-effectiveness study be carried out before HPV vaccines are included in the expanded program on immunization (EPI) because of the high costs associated with the vaccine and its delivery.Citation14
According to the WHO database, 85 countries had introduced the HPV vaccine into the EPI by February 2019.Citation15 The current vaccination strategy for the HPV vaccine is voluntary and paid for out of pocket in China because of its high price and inadequate supply.Citation16 This situation has substantially decelerated the pace of HPV vaccine implementation in China to eliminate CC, which was called for by the WHO in May 2018.Citation17 Recently, a novel, Escherichia coli (E. coli)-produced 2vHPV 16/18 vaccine (Xiamen Innovax Biotech, Xiamen, China) was confirmed to be efficacious and safe in ChinaCitation18 and showed immunogenic noninferiority in girls aged 9–14 years who received 2 doses compared to women aged 18 to 26 years who received 3 doses.Citation19 This candidate HPV vaccine has enabled vaccination in large populations in both China and other low-income countries at lower manufacturing costs. Furthermore, vaccination strategies vary among countries, which results in confusion regarding whether the 2vHPV vaccine should be integrated into the EPI.
Despite a variety of previous cost-effectiveness studies of HPV vaccines, the generalizability of the results is not straightforward. Therefore, a cost-effectiveness analysis based on local parameters is preferred. There are several methodological innovations in this study. First, the costs of HPV-related diseases (such as cervical intraepithelial neoplasia and CC) are derived from a nationwide survey rather than experts’ opinions or estimations. Second, the cost of vaccination is based on a survey of the actual cost of the EPI in Zhejiang Province. Third, this study analyzes the candidate E. coli-produced 2vHPV vaccine with an assumed price and threshold analyses, since the vaccine will not be available on the market until 2020. Finally, this study evaluated the cost-effectiveness of a 2-dose vaccination schedule in a Chinese (Zhejiang Province) setting. Zhejiang is a relatively developed province in eastern China and plays a leading role in the EPI work in China, such as taking the lead in the EPI for 2-dose measles, mumps and rubella vaccines and the 2-dose inactivated poliomyelitis vaccine plus bivalent oral poliomyelitis vaccine. Considering the availability and large-scale production of HPV vaccines along with the current policy debate, this cost-effectiveness analysis aims to compare the two-dose and three-dose schedules for the 2vHPV vaccine.
Based on these considerations, a Markov model was used to simulate a lifetime female cohort of 100,000 people and estimate the impact and cost-effectiveness of 2-dose versus 3-dose 2vHPV vaccine schedules in Zhejiang Province. This study will provide a scientific basis for the rational and efficient use of HPV vaccines.
Materials and methods
Comparison of the strategies
To determine the best vaccination strategy, we compared 3 strategies: (1) no vaccine, which is regarded as no intervention because HPV vaccines are in short supplyCitation16,Citation17 and the vaccination rate is relatively low; (2) 2 doses of the 2vHPV vaccine; and (3) 3 doses of the 2vHPV vaccine. We assumed that both the 2-dose and 3-dose HPV vaccine schedules provide lifelong protection.
Modeling
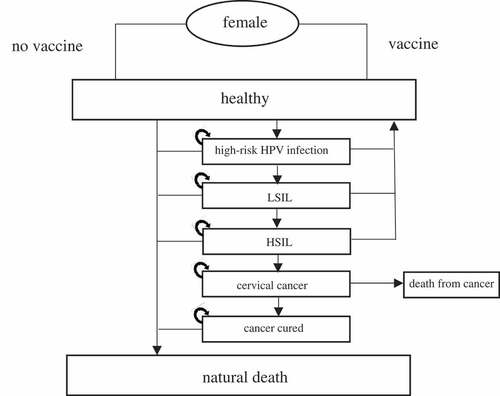
A static Markov model was used to simulate the natural history of high-risk HPV and divide the natural history into a series of health states. These states consisted of healthy, HPV infection, low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), and CC. The progression and relationship among the states are shown in . The transition probability between the states is shown in : most probabilities assumed in the model progressed annually and would be stable lifelong. The model was developed using TreeAge Pro 2016 software (TreeAge software Inc. Williamstown, United States of America) to evaluate the long-term effects and cost-effectiveness of the 2vHPV vaccine in the abovementioned 3 different strategies/scenarios. The model ran in 1-year cycle, and a total of 88 cycles were performed to model a cohort of 100,000 girls from 12 years old to 100 years old according to the defined states and transition parameters. Costs and effects were discounted at 3% annually.
Figure 2. Univariate sensitivity analysis of main parameters for 2-dose schedule versus no vaccination.
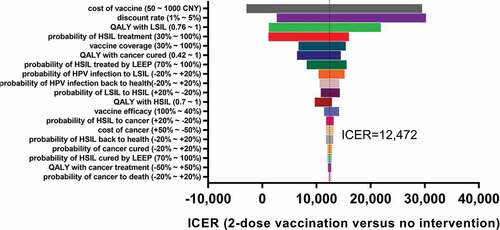
Table 1. Model parameters.
Costs
Costs related to the diagnoses and treatment of high-risk HPV-related diseases were collected by a nationwide survey in 2015 and published in 2018.Citation29 The survey was conducted through the Chinese National Health Industry Research Project and collected costs from 14 country-level hospitals and 9 provincial/municipal hospitals by stratified sampling. We assumed states of HPV infection and LSIL to have no treatment costs. We analyzed the costs from the government’s perspective; therefore, the costs include direct medical costs and direct nonmedical costs. The costs of vaccination were assumed because the candidate 2vHPV vaccine is not currently available in the market, and other costs of vaccination were obtained from an actual cost survey of the EPI carried out in Zhejiang Province,Citation30 which included human labor costs, daily maintenance costs, vaccination information system costs, and other expenses. The details are shown in .
Table 2. Costs of vaccination and treatment.
Effectiveness measurement
We measured effectiveness in terms of the numbers of CC cases and deaths, life years and quality-adjusted life years (QALYs). QALYs can assess a patient’s life years by combining morbidity and mortality in a single index, which is calculated by estimating on a cardinal scale from 0 to 1 (0 represents the worst health, while 1 represents the best health), thereby measuring effectiveness quantitatively (morbidity) and qualitatively (mortality). However, there are no published health state utilities or values of the abovementioned disease states in China. The index used in the model was from a literature review and is summarized in .
Analysis
The cost-effectiveness analysis compares the incremental costs and consequences of the 2-dose strategy compared to the no vaccination and 3-dose strategies. The incremental cost-effectiveness ratio (ICER) was used to evaluate the results. It is calculated as the incremental cost divided by the QALYs gained per woman. A strategy is considered to be “very cost-effective” if its ICER is less than the per capita gross domestic product (GDP) and “cost-effective” if its ICER is less than three times the per capita GDP according to the WHO threshold. The per capita GDP was 92,100 CNY in Zhejiang in 2017.
To address the uncertainty of parameters in the analysis, a one-way sensitivity analysis was performed with several alternative assumptions: (1) vaccine efficacy (40% to 100%); (2) vaccine price (50 to 1000 CNY); (3) discount rates for both costs and effects (1% to 5%); and (4) the remaining cost parameters and probabilities, which varied within the ranges in .
Results
Health and economic impacts
In a cohort of 100,000 females (with 70% coverage), a total of 423 cancer cases, 116 cancer deaths and 3,627 QALYs would be saved by both two-dose and three-dose strategies compared to no vaccination. In addition, both the two-dose and three-dose vaccination schemes would avert 27,500,541 CNY in medical costs compared to no vaccination. With regard to the cost, if we assume that the price of this novel vaccine equals that of a currently available 2vHPV (Cervarix®) at approximately 500 CNY per dose, then the two-dose vaccination strategy is expected to save 36,368,500 CNY in vaccination costs compared to the three-dose vaccination strategy, which shows that the 2-dose HPV vaccination scheme is cost saving compared to the three-dose vaccination scheme.
The incremental cost of the cohort is 45,236,459 CNY, and the incremental effects of that is the aforementioned 3,627 QALYs. Therefore, the two-dose vaccination versus no vaccination is clearly cost effective at an ICER of CNY 12,472 per QALY gained, and this figure is less than the per capita GDP.
Sensitivity analysis
In one-way sensitivity analysis of the two-dose vaccination strategy versus no vaccination (), we found that the results were all less than the per capita GDP despite several variations. The most sensitive factor was vaccine cost, and the 2-dose strategy remained very cost effective even when the vaccine price was as high as 1000 CNY per dose. Similarly, even if the vaccine efficacy dropped to 30% due to problems with the system or the failure of cross-protection, the ICER would still be low enough for the vaccine to be very cost effective.
Discussion
To the best of our knowledge, this is the first HPV vaccine cost-effectiveness analysis to simulate 2-dose vaccination strategies in Zhejiang Province, China. The results showed that the ICER is less than the per capita GDP for the 2-dose vaccination strategy compared to no intervention in a cohort of 12-year-old girls, and the sensitivity analysis showed that this result was robust when we varied the main parameters.
Our finding that 2-dose vaccination with this novel 2vHPV is very cost effective and has implications for CC control in both China and other resource-limited countries. Despite the heterogeneity of vaccination strategies and HPV prevalence, numerous studiesCitation31,Citation32 have shown that introducing HPV vaccines is cost effective. Especially in some resource-limited countries such as South Africa, Malaysia and India, introducing 2vHPV vaccines without screening is cost effective because of the high rates of CC in those countries. Similarly, the 2-dose schedule for the 2vHPV vaccine is cost effective in these countries when compared to a 3-dose 2vHPV or 2-dose 4vHPV vaccine regimen.Citation33,Citation34 Cost-effectiveness comparison studies of the 2-dose and 3-dose HPV vaccination strategies in the United KingdomCitation35 and CanadaCitation36 showed that the 2-dose vaccination strategy is the more cost effective of the two if protection lasts for more than 20 years or 30 years, respectively. However, the duration of the protection conferred by the 2-dose 2vHPV vaccine remains unknown.
Great importance should be attached to the influence of discounting in this study, which only focuses on vaccine efficacy and vaccine price. We used a discount rate of 3% for the base-case analysis, as most previous studies have done.Citation32,Citation33 The costs of vaccine are immediate, while the benefits are delayed. Therefore, large discounting will make the vaccination strategy less attractive.
Importantly, vaccine price is the most influential factor according to the sensitivity analysis. Because the new E. coli-produced 2vHPV vaccine can be manufactured on a large scale at low cost, the availability of an inexpensive vaccine will be favorable for resource-limited countries. If the vaccine price is dramatically reduced, then vaccination may be a very affordable policy option compared to no intervention. The new 2vHPV vaccine will soon be available on the market, and only Chinese-produced vaccines are deemed to be a reliable supply for the EPI.Citation37 The price of a vaccine is usually expected to decrease by 50–80% from the market price if the vaccine is included in the EPI. Introducing the HPV vaccine into China’s EPI would be an effective strategy for CC elimination in both urban and rural areas.Citation38 We did not take screening into consideration because of the low screening coverage and disparities in health care access in Chinese settings. A review of modeling studies in developed countriesCitation39 found that delaying the starting age of screening and extending the screening interval are important ways of increasing the cost effectiveness of screening by either cytology or HPV testing for vaccinated females.
The strength of our study was that we used the locally established costs of vaccination, epidemiological data and costs of treating HSIL and CC. Since EPI and non-EPI vaccines are regulated differently in China, it is best to use the actual EPI cost in ZhejiangCitation30 to estimate the vaccination costs other than the vaccine itself. The costs of treating HSIL and CC were underestimated by previous studies that relied on expert panels or hospital-based studies without nonmedical costs.Citation28,Citation40 With more reliable data and a robust sensitivity analysis, the result is less uncertain and sheds light on current questions pertaining to introducing HPV vaccines into the EPI. Our study evaluated only 2vHPV vaccines because of the applicability of current policies and the availability of vaccine resources. In our study, we analyzed only the cost-effectiveness of 2vHPV vaccine for girls prior to HPV exposure. Therefore, the use of a static model to stimulate costs and effects in a female cohort in a simple way can yield satisfactory results.Citation39 However, if we consider catch-up vaccination and boys’ vaccination in the future, this model is less likely to contribute reliable results. We considered the cross-protection provided by the 2vHPV vaccine in terms of vaccine efficacy and performed a sensitivity analysis in case of problems with the system or failure of cross-protection.
There are several limitations in our study. First, unlike some studies that applied dynamic transmission models to evaluate CC prevention strategies based on herd immunity among boys,Citation41–Citation44 we merely used a static Markov model for the economic evaluation, and we analyzed only the direct effects of this 2vHPV vaccine. Second, the model failed to consider other diseases caused by HPV, such as vagina, head and neck cancers, and thus may have underestimated the effects of this vaccine. Finally, because the model is based on a one-year cycle, some transition parameters are not available and were calculated roughly. For example, the case fatality rate is not used for chronic diseases such as CC. We calculated a 0.428 rate of cancer death per annum for cancer patients, which is the cumulative risk of CC incidence (0.7%) divided by the cumulative risk of CC mortality (0.3%) at 75 years old.Citation3 This method underestimated the number of CC deaths to a certain degree because some people who live beyond 75 years old are not considered.
In conclusion, introducing 2-dose 2vHPV vaccines is very cost effective at a per-dose vaccine price of CNY 500. The 2-dose vaccination schedule is a strictly dominant strategy if the per-dose vaccine price is CNY 50. The novel two-dose HPV vaccination has implications for CC control in both China and other resource-limited countries.
Disclosure of potential conflicts of interest
All authors declare that they have no conflicts of interest.
Author contributions
Zhiping Chen and Ting Wu designed the study; Yan Luo and Xuewen Tang reviewed the literature; Yan Luo and Hanqing He developed the model and performed the data analysis; Shenyu Wang and Jun Zhang estimated the validity of the model; and Yan Luo wrote the manuscript. The corresponding authors are the guarantors for the data and have full access to all data. All authors approved the final version of the manuscript.
Ethical approval
The study protocol was approved by the ethics committee of the ZJCDC.
Acknowledgments
We gratefully acknowledge Professor Ya Fang and Mr. Mingliang Luo for their technical support. Editing was performed by a specialized company.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.v68.6.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi:10.3322/caac.21338.
- Human Papillomavirus and Related Diseases Report China. http://www.hpvcentre.net/statistics.php[EB/OL].
- Zhu FC, Hu SY, Hong Y, Hu Y-M, Zhang X, Zhang Y-J, Pan Q-J, Zhang W-H, Zhao F-H, Zhang C-F. Efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine in Chinese women aged 18–25 years: event-triggered analysis of a randomized controlled trial. Cancer Med. 2017;6:12–25. doi:10.1002/cam4.2017.6.issue-1.
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow S-N, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi:10.1016/S0140-6736(09)61248-4.
- Kostaras D, Karampli E, Athanasakis K. Vaccination against HPV virus: a systematic review of economic evaluation studies for developed countries. Expert Rev Pharmacoecon Outcomes Res. 2019;19:147–58. doi:10.1080/14737167.2019.1555039.
- Setiawan D, Oktora MP, Hutubessy R, Riewpaiboon A, Postma MJ. The health-economic studies of HPV vaccination in Southeast Asian countries: a systematic review. Expert Rev Vaccines. 2017;16:933–43. doi:10.1080/14760584.2017.1357472.
- Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Behre U, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum Vaccin Immunother. 2014;10:1155–65. doi:10.4161/hv.28022.
- Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, González P, Solomon D, Jiménez S, Schiller JT, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. JNCI. 2011;103:1444–51. doi:10.1093/jnci/djr319.
- World Health Organization. Electronic address swi. Human papillomavirus vaccines: WHO position paper, May 2017-Recommendations. Vaccine. 2017;35:5753–55. doi:10.1016/j.vaccine.2017.05.069.
- Collier R. Two-dose HPV regimen recommended in US. CMAJ. 2017;189:E36. doi:10.1503/cmaj.109-5350.
- Shapiro GK, Guichon J, Kelaher M. Canadian school-based HPV vaccine programs and policy considerations. Vaccine. 2017;35:5700–07. doi:10.1016/j.vaccine.2017.07.079.
- Riesen M, Garcia V, Low N, Althaus CL. Modeling the consequences of regional heterogeneity in human papillomavirus (HPV) vaccination uptake on transmission in Switzerland. Vaccine. 2017;35:7312–21. doi:10.1016/j.vaccine.2017.10.103.
- Organization WH. Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec. 2009;84:118–31.
- https://www.who.int/immunization/monitoring_surveillance/VaccineIntroStatus.pptx?ua=1.
- The Global alliance for vaccines and immunisation, HPV supply and procurement roadmap. https://www.gavi.org/library/gavi-documents/supply-procurement/hpv-supplyand-procurement–roadmap/.
- World Health Organization. WHO director-general calls for all countries to take action to help end the suffering caused by cervical cancer. 2018; http://www.who.int/reproductivehealth/call-to-action-elimination-cervical-cancer/en/.
- Qiao YL, Wu T, Li RC, Hu Y-M, Wei L-H, Li C-G, Chen W, Huang S-J, Zhao F-H, Li M-Q, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. 2019. doi:10.1093/jnci/djz074.
- Hu Y, Guo M, Li C, Chu K, He W, Zhang J, Gu J, Li J, Zhao H, Wu X, et al. Immunogenicity noninferiority study of 2 doses and 3 doses of an Escherichia coli-produced HPV bivalent vaccine in girls vs. 3 doses in young women. Sci China Life Sci. 2019. doi:10.1007/s11427-019-9547-7.
- Zhao FH, Lewkowitz AK, Hu SY, Chen F, Li L-Y, Zhang Q-M, Wu R-F, Li C-Q, Wei L-H, Xu A-D, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. Int J Cancer. 2012;131:2929–38. doi:10.1002/ijc.27571.
- Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727–35. doi:10.1016/s0029-7844(98)00245-2.
- Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, Miller S, Clayton L, Farhat S, Broering J, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. Jama. 2001;285:2995–3002. doi:10.1001/jama.285.23.2995.
- Van de Velde N, Brisson M, Boily MC. Modeling human papillomavirus vaccine effectiveness: quantifying the impact of parameter uncertainty. Am J Epidemiol. 2007;165:762–75. doi:10.1093/aje/kwk059.
- Mo X, Gai Tobe R, Wang L, Liu X, Wu B, Luo H, Nagata C, Mori R, Nakayama T. Cost-effectiveness analysis of different types of human papillomavirus vaccination combined with a cervical cancer screening program in mainland China. BMC Infect Dis. 2017;17:502. doi:10.1186/s12879-017-2592-5.
- Sinanovic E, Moodley J, Barone MA, Mall S, Cleary S, Harries J. The potential cost-effectiveness of adding a human papillomavirus vaccine to the cervical cancer screening programme in South Africa. Vaccine. 2009;27:6196–202. doi:10.1016/j.vaccine.2009.08.004.
- Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12:186–92. doi:10.1097/00004347-199304000-00018.
- Gong W, Luo S, Hu R, Wang H, Pan J, Fei F, He Q, Yu M. Analysis of survival rate of breast, cervical, and ovarian cancer patients during 2005–2010 in Zhejiang province, China. Zhonghua Yu Fang Yi Xue Za Zhi. 2014;48:366–69.
- Zhang Q, Liu YJ, Hu SY, Zhao FH. Estimating long-term clinical effectiveness and cost-effectiveness of HPV 16/18 vaccine in China. BMC Cancer. 2016;16:848. doi:10.1186/s12885-016-2893-x.
- Tao SY, Peng JR, Wang Y, Zhang GT, Chen ZY, Zhao F, Ma JQ, Yang X, Qiao YL, Zhao FH, et al. Study on direct economic burden and influencing factors in patients with cervical cancer and precancerous lesions. Zhonghua Yu Fang Yi Xue Za Zhi. 2018;52:1281–86. doi:10.3760/cma.j.issn.0253-9624.2018.12.017.
- Zhou YHH, Deng X, Yan R, Tang XW, Xie SY. Comparative analysis on actual cost and reasonable cost of expanded program on immunization in Zhejiang. Chin Health Econ. 2018;37:86–89.
- Silas OA, Achenbach CJ, Murphy RL, Hou L, Sagay SA, Banwat E, Adoga AA, Musa J, French DD. Cost effectiveness of human papilloma virus vaccination in low and middle income countries: a systematic review of literature. Expert Rev Vaccines. 2018;17:91–98. doi:10.1080/14760584.2018.1411195.
- Fesenfeld M, Hutubessy R, Jit M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: a systematic review. Vaccine. 2013;31:3786–804. doi:10.1016/j.vaccine.2013.06.060.
- Prinja S, Bahuguna P, Faujdar DS, Jyani G, Srinivasan R, Ghoshal S, Suri V, Singh MP, Kumar R. Cost-effectiveness of human papillomavirus vaccination for adolescent girls in Punjab state: implications for India’s universal immunization program. Cancer. 2017;123:3253–60. doi:10.1002/cncr.v123.17.
- Aljunid S, Maimaiti N, Nur AM, Noor MRM, Wan Puteh SE. Cost-effectiveness of HPV vaccination regime: comparing twice versus thrice vaccinations dose regime among adolescent girls in Malaysia. BMC Public Health. 2016;16:71. doi:10.1186/s12889-016-2754-1.
- Jit M, Brisson M, Laprise JF, Choi YH. Comparison of two dose and three dose human papillomavirus vaccine schedules: cost effectiveness analysis based on transmission model. BMJ (Clinical Research Ed). 2015;350:g7584.
- Jit M, Choi YH, Laprise JF, Boily MC, Drolet M, Brisson M. Two-dose strategies for human papillomavirus vaccination: how well do they need to protect? Vaccine. 2014;32:3237–42. doi:10.1016/j.vaccine.2014.03.098.
- Pan XF, Griffiths UK, Pennington M, Yu H, Jit M. Systematic review of economic evaluations of vaccination programs in mainland China: are they sufficient to inform decision making? Vaccine. 2015;33:6164–72. doi:10.1016/j.vaccine.2015.09.081.
- Li J, Li LK, Ma JF, Wei L-H, Niyazi M, Li C-Q, Xu A-D, Wang J-B, Liang H, Belinson J. Knowledge and attitudes about human papillomavirus (HPV) and HPV vaccines among women living in metropolitan and rural regions of China. Vaccine. 2009;27:1210–15. doi:10.1016/j.vaccine.2008.12.020.
- Canfell K, Chesson H, Kulasingam SL, Berkhof J, Diaz M, Kim JJ. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine. 2012;30(Suppl 5):F157–67. doi:10.1016/j.vaccine.2012.06.091.
- Liu YJ, Zhang Q, Hu SY, Zhao FH. Effect of vaccination age on cost-effectiveness of human papillomavirus vaccination against cervical cancer in China. BMC Cancer. 2016;16:164. doi:10.1186/s12885-016-2207-3.
- Drolet M, Laprise JF, Boily MC, Franco EL, Brisson M. Potential cost-effectiveness of the nonavalent human papillomavirus (HPV) vaccine. Int J Cancer. 2014;134:2264–68. doi:10.1002/ijc.28541.
- Jit M, Chapman R, Hughes O, Choi YH. Comparing bivalent and quadrivalent human papillomavirus vaccines: economic evaluation based on transmission model. BMJ (Clinical Research Ed). 2011;343:d5775. doi:10.1136/bmj.d5775.
- Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28:6858–67. doi:10.1016/j.vaccine.2010.08.030.
- Choi YH, Jit M, Gay N, Cox A, Garnett GP, Edmunds WJ. Transmission dynamic modelling of the impact of human papillomavirus vaccination in the United Kingdom. Vaccine. 2010;28:4091–102. doi:10.1016/j.vaccine.2009.09.125.