ABSTRACT
Two vaccines, 23-valent pneumococcal polysaccharide vaccine (PPSV23) and 13-valent pneumococcal conjugate vaccine (PCV13), are widely available for the prevention of pneumococcal disease in adults. However, it is unclear how cost-effective these pneumococcal vaccine choices are in the Hong Kong healthcare environment. We aimed to assess the cost-effectiveness of a sequential administration of PCV13 followed by PPSV23 compared to a single dose of PPSV23 vaccination for pneumococcal disease control in Hong Kong adults aged ≥65 years and individuals aged 20–64 years with immunocompromising and chronic conditions. A previously developed deterministic cohort sequential model was applied to compare the outcomes of two vaccination strategies from a societal perspective. Population-specific model input, including incidence, mortality, case-fatality, risk group distribution, vaccination costs, disease management, and productivity loss, was estimated from a Hong Kong-wide electronic medical database. Costs were valued in US$ in 2017. Vaccination strategies with an incremental cost-effectiveness ratio (ICER, defined as incremental cost per QALY saved) less than one local GDP per capita ($46,193 in 2017) were defined as highly cost-effective. Deterministic sensitivity analyses (SA) were conducted. Compared with single-dose PPSV23, sequential vaccination of PCV13 followed by PPSV23 was cost-saving for adults aged ≥20 years. In the deterministic SA, the base-case results were robust for tested parameter uncertainties. Future vaccination policies should consider the cost-effectiveness of a sequential vaccination strategy as a measure to reduce the vaccine-preventable pneumococcal disease burden in Hong Kong.
Introduction
Streptococcus pneumoniae infections (pneumococcal disease) constitute a major global and regional public health problem.Citation1 In Hong Kong, annual incidence of invasive pneumococcal disease (IPD) was 2.9 per 100,000 population in 2015.Citation2 Since then, the Center for Health Protection (CHP) of the Department of Health now includes IPD (bacteremia and meningitis) as a notifiable infectious disease, requiring active case reporting. The rising resistance of pneumococcus to conventional antimicrobials is a major concern that highlights the importance of taking all preventive measures against pneumococcal disease.Citation3
Two widely available vaccines, 23-valent pneumococcal polysaccharide vaccine (PPSV23) and 13-valent pneumococcal conjugate vaccine (PCV13) are approved for the prevention of pneumococcal diseases in adults. While PPSV23 has been widely used in adults for over 30 years and has proven effectiveness against IPD, its effectiveness against noninvasive pneumococcal diseases is uncertain.Citation4-Citation7 There are also variations in the vaccine efficacy (VE) of PPSV23 depending on the age of the recipient, time since vaccination and disease history.Citation6 On the other hand, PCV13 has seen widespread use in recent years following success in children. It has a well-established tolerability profile among adults, especially >50 years of age, in addition to playing a role in the prevention of pneumococcal diseases in immunocompromised patients and patients with other underlying medical conditions that may have increased their risk of pneumococcal infection.Citation6 Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA), a randomized, double-blind, placebo-controlled trial has demonstrated the efficacy of PCV13 in preventing both IPD and noninvasive pneumonia in older adults aged ≥65 years.Citation8 Since the introduction of PCV13 in the United States in 2010, the vaccine has achieved early success given the reduced incidence of IPD in both children and adults via herd effect.Citation9
Most international guidelines recommend routine pneumococcal vaccination to high-risk individuals aged 2–64 years and older adults aged ≥65 years.Citation6,Citation10 However, recommendations for the high-risk population differ in several aspects such as vaccination intervals and administration of a single dose of PPSV23, PCV13, or both in sequence.Citation11-Citation13 In Hong Kong, a single dose of PCV13 or PPSV23 is recommended for older adults aged ≥65 years without high-risk conditions. A single dose of PCV13 is recommended for previously unvaccinated high-risk individuals aged ≥2 years, followed by a single dose of PPSV23 a year later. For individuals previously vaccinated with PPSV23, a single dose of PCV13 is recommended 1 year after the previous vaccination. Patients are considered high-risk if they have any of the following conditions: a history of invasive pneumococcal diseases, immunocompromised conditions, chronic diseases, and cochlear implants.Citation2 In adult population, sequential vaccination is only recommended for previously unvaccinated high-risk individuals ≥18 years. It is unclear whether extending the sequential vaccination to a wider target population regardless of the risk condition would be cost-effective and reduce the burden of pneumococcal disease in Hong Kong.
Health economic evaluation studies aid decisions in healthcare resource utilization and guide implementation of healthcare policy. Various studies have evaluated the choice between single dose of PPSV23 and sequential vaccination, however, most of these studies were conducted in Western countries and the results are not generalizable to the local Hong Kong population,Citation14-Citation16 particularly as each jurisdiction has a unique healthcare system and economic environment.Citation17 The study aims to evaluate and compare the cost-effectiveness of a single dose of PPSV23 versus a sequential vaccination of PCV13 followed PPSV23 in adults aged ≥65 years and individuals aged 20–64 years with high-risk conditions.
Materials and methods
Model overview
A previously developed sequential deterministic model, which examines the expected clinical and economic impact of PCV13 and PPSV23 in adults ≥20 years in various settings, was used to simulate the occurrence of IPD, all-cause pneumonia (ACP), and disease-specific death with different vaccination strategies. The model aimed to compare the clinical, economic, and cost-effectiveness outcomes after vaccination in its lifetime simulation.
The overall modeled population in the cost-effectiveness analysis (CEA) were adults ≥20 years old. The target population for the vaccination were older adults ≥65 years as well as adults aged 20–64 years with immunocompromising and chronic conditions, defined as high-risk conditions. Two vaccine strategies were evaluated: a single dose of PCV13 followed by a single dose of PPSV23 (PCV13→PPSV23) versus a single dose of PPSV23. Non-vaccinated healthy individuals (20–64 years old with no immunocompromised or chronic conditions) would follow the natural history of disease occurrence in the simulation.
The clinical and economic outcomes were assessed under the scenario that both vaccinations are available. Local epidemiologic and economic data were employed with default model parameters which are based on US settings used when local data were not available. The study was conducted from a societal perspective, with consideration of both direct (medical) and indirect (loss of productivity) costs. We used a lifetime time horizon with both costs and health outcomes discounted at 4% per year, according to health technology assessment guidelines.Citation18
Model inputs
The model population was stratified according to age and risk conditions. The age distribution of patients was based on the 2015 Hong Kong Census as the year of study conduction. Age distribution, risk distribution for the age-group of 20–64 years, incidence, mortality, case-fatality of IPD, and all-cause pneumonia were based on a territory-wide electronic medical database in Hong Kong – Clinical Data Analysis and Reporting System. Details are available from previous publications.Citation19
Estimates of vaccine effectiveness were adjusted to the corresponding levels of serotype coverage and age groups, based on the Hong Kong Center for Health Protection data. The serotype coverage was estimated to be 65% and 76% for PCV13 and PPSV23, respectively, for the age group 20–64 years, and 68% and 77% for PCV13 and PPSV23 for the age group of ≥65 years, respectively.Citation20 Effectiveness of PPSV23 against all-cause non-bacteremic pneumonia was assumed to be zero which was consistent with the assumptions from previous randomized control trials and meta-analysis.Citation21-Citation24 Effectiveness of PCV13 in reducing vaccine-type IPD and non-bacteremia pneumonia was based on the findings from CAPiTA. Due to the lack of evidence on the effectiveness of PCV13 in patients <65 years, and for high-risk persons, effectiveness of PCV13 was assumed to be 75.8% for IPD and 41.1% for vaccine-type nonbacteremic pneumonia, similar to ≥65 years age group.Citation8 The effectiveness of PCV13 was assumed to be the same irrespective of prior vaccination experience with PPSV23 or no vaccination. The rate of decline in PCV13 effectiveness with age was assumed to be equal to 50% of the corresponding values for PPSV23, based on published rates of decay.Citation24-Citation26 We assumed the initial uptake of single-dose PPSV23 to be 40% for adults ≥65 years and zero for adults aged 20–64 years as it is not indicated for this population group. For the sequential strategy, we assumed 10% uptake for moderate to high-risk adults aged 20–64 years with immunocompromising and chronic conditions, and 40% for older adults ≥65 years.
The overall cost included vaccination cost, medical cost, and indirect cost. Vaccination cost included the costs of vaccine and administration from the public sector. Medical cost included hospitalization, outpatient consultation, and inpatient care costs. Indirect cost was defined as productivity loss due to IPD and all-cause pneumonia.
Model outcomes
Clinical outcomes included the cumulative number of cases of IPD, inpatient pneumonia, outpatient pneumonia, number of deaths due to IPD and pneumonia, life years, and quality-adjusted life years (QALYs). Economic outcomes included vaccination costs, direct costs (medical costs and vaccination costs), indirect costs (non-medical cost), and overall costs. Costs were valued in US dollars in 2017 (US$1 to HK$7.84). Strategies with an incremental cost-effective ratio (ICER), defined as incremental cost per QALY gained, less than a willingness-to-pay (WTP) threshold of local GDP per capita ($46,193 GDP per capita in 2017) were considered to be highly cost-effective.
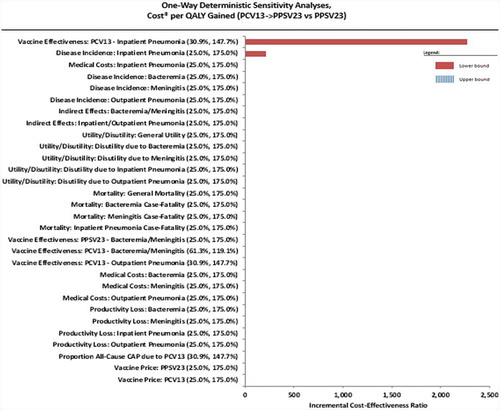
A deterministic sensitivity analysis (SA) was conducted to test the impact of parameter uncertainties on the base-case results. Each of the 30 model inputs was varied according to a predefined range [31%-148% of the base-case input for vaccine efficacy (VE) of vaccine-type nonbacteremic pneumonia, 61%-119% for VE of IPD, and 25% to 175% for all the other base-case values] while the rest of the inputs were kept constant to project its effect on ICER. All tested parameters were ranked according to ICER variation and the most influential parameters were presented in a Tornado diagram.
Ethics
This study was approved by the Institutional Review Board of Hospital Authority/Hong Kong West Cluster (Reference no: UW17-392). Informed consent was waived as data used were anonymized and no patient contact was required.
Results
Clinical outcomes
The total number of adults included in this model was 6,102,500 (age 20–64, 81.7%; age ≥65, 18.3%). Despite a similar number of IPD cases, compared to a single-dose PPSV23, the sequential vaccination strategy had a considerably lower number of inpatient and outpatient pneumonia cases. The sequential vaccination is expected to prevent 2,785 inpatient cases and 8,000 outpatient cases in adults aged 20–64 (); in older adults aged ≥65 years, 5,897 inpatient cases and 7,356 outpatient cases would be prevented (). Additionally, the sequential vaccination strategy would prevent 249 and 879 deaths due to disease in age groups 20–64 years and ≥65 years, respectively ( and ).
Table 1. Clinical outcomes of sequential model for adults aged 20–64 years with immunocompromised and chronic conditions.
Table 2. Clinical outcomes of sequential model for adults age ≥65 years.
Economic outcomes
At a population level, the sequential vaccination strategy would save $28 million direct medical costs and $4 million indirect costs, over a lifetime for adults aged 20–64 years (). It would also save $55 million direct medical costs and $7 million indirect costs for older adults aged ≥65 years (). The total additional investment would be $42 million in vaccination costs for the overall population (adults ≥20 years), resulting in a net overall cost-saving for the sequential vaccination strategy when all age groups are combined.
Table 3. Economic outcomes of sequential model for adults aged 20–64 years with immunocompromised and chronic conditions.
Table 4. Economic outcomes of sequential model for adults age ≥65 years.
Cost-effectiveness analysis
Overall, the sequential administration of PCV13 and PPSV23 was projected to gain 0.004 life years and 0.002 QALY for each individual in all age groups in the base-case analysis. Moreover, ICER was estimated to be dominant (cost-saving) among both age groups (). The sequential vaccination strategy generates greater life years and QALY and prevents substantial cases, deaths, loss of productivity, and medical costs compared with a single-dose administration of PPSV23 in the target population, hence its dominance in the cost-effectiveness analysis. ICER was also estimated according to age and different risk groups (). The sequential vaccination was dominant (less cost and more benefit) in age ≥65 population and age 20–64 year population with chronic and immunocompromised conditions compared to PPV23 alone.
Table 5. Base-case cost-effective analysis.
Table 6. Incremental cost-effective analysis stratified by age and risk group.
Sensitivity analysis
We identified two influential factors that caused variation in the ICER in the deterministic SA (). There was a negative association with ICER in PCV13 vaccine effectiveness in inpatient pneumonia and the incidence of inpatient pneumonia. This indicates higher incidence of inpatient pneumonia and lower VE of PCV13 are associated with an increased ICER when all the other variables were kept constant. However, this variation in ICER remained below the pre-defined WTP threshold hence the cost-effectiveness of the sequential vaccination strategy remains.
Discussion
Results, summary, and comparison with other studies
In this study, sequential administration of PCV13 followed by PPSV23 has proven to be cost-saving compared to a single dose of PPSV23. The analysis incorporated local demographic, clinical, and economic factors to produce results relevant to Hong Kong. The findings were representative for adults aged 20–64 years with high-risk conditions and older adults aged ≥65 years. The ICER was sensitive to PCV13 vaccine effectiveness in the prevention of inpatient pneumonia, the incidence rate of inpatient pneumonia and inpatient medical costs but the baseline conclusion was robust to variations in all the tested parameters. Nevertheless, cost-effectiveness was maintained even with the variations in ICER.
The cost-effectiveness of the sequential vaccination strategy is mainly driven by the reduction of pneumonia. From a disease burden perspective, the competing strategy has a similar preventive effect on the incidence of bacteremia and meningitis. The sequential administration reduced the incidence of inpatient and outpatient pneumonia for adults aged 20–64 years by 56 and 160 per 100,000 persons, respectively. Reductions were also observed in the incidence of inpatient and outpatient pneumonia for older adults ≥65 years by 529 and 656 per 100,000 persons, respectively. Combining the results of both target populations, older adults aged ≥65 years and adults aged 20–64 years with high-risk conditions, the preventive effect of sequential vaccination on the incidence of inpatient and outpatient pneumonia was reduced by 142 per 100,000 persons for inpatient pneumonia and 252 per 100,000 persons for outpatient pneumonia.
The results from this study are consistent with findings from other health economic evaluations conducted in the US, Europe, and other Asian populations.Citation14,Citation16,Citation27-Citation30 In addition, for adults aged 18–64 years with or without immunocompromising conditions, sequential vaccination has been reported to be cost-effective, compared to PPSV23, and without vaccination.Citation14-Citation16 It is generally recognized that the cost-effectiveness of PCV13 is sensitive to VE in non-bacteremic pneumonia as well as the estimated herd effect from childhood pneumococcal vaccination.Citation30,Citation31 However, results from our sensitivity analysis have shown no change to base-case estimate directions due to uncertainties from VE. This could be explained by the high incidence and hospitalization rates of pneumonia in Hong Kong.
In our study, the estimated uptake, based on government reports and market data from industry, was relatively conservative compared to other international studies (60–70% for base-case analysis in general) and the market share of competing vaccines was set to be constant over time. With that in mind, the cost-effectiveness of the sequential vaccination strategy in a dynamic market needs to be interpreted thoughtfully, especially since an increase in vaccine coverage will increase the health benefits and vaccine costs simultaneously.
Clinical and policy implications
Since 2015, IPD has been a notifiable infectious disease in Hong Kong. Identifying a trend in IPD incidence and the benefits of the herd effect from childhood vaccination is challenging due to the short period of active surveillance (2015–2018). Therefore, assessing adult pneumococcal vaccination on the potential reduction of adult pneumonia cases, the most common type of lower respiratory infection and one of the top three leading causes of death worldwide, would be useful in informing policy decisions. In Hong Kong, pneumonia continues to be the second leading cause of death and pneumonia-related admissions increased during the last decade.Citation32 Although Hong Kong enjoys long life expectancy, it faces a demographic challenge due to an increasingly aging population. The percentage of older adults aged ≥65 years increased from 8% to 16% in the last three decades and is expected to double by 2036. This creates an even more pressing need to find efficient and cost-effective preventive measures against pneumonia since the majority of cases occur in older adults.Citation33 In the landmark trial, PCV13 showed prominent efficacy in preventing non-bacteremic and noninvasive vaccine-type CAP by 45% in older adults.Citation8 In a real-world effectiveness study, PCV13 vaccine effectiveness against hospitalized vaccine-type community-acquired pneumonia among US adults aged ≥65 years was found to be 72.8%.Citation34 Moreover, the main advantage of sequential administration is the wider serotype coverage that PPV23 offers for the prevention of IPD and the conjugate vaccine effectiveness against non-bacteremic pneumococcal pneumonia. Taking into account the global and regional disease burden of pneumonia, we anticipate that using PCV13 in addition to PPSV23 will be cost-effective.
Current Hong Kong vaccination guidelines recommend sequential pneumococcal vaccination for adults with high-risk conditions and no history of pneumococcal vaccination. The findings of this study demonstrate the cost-effectiveness of a sequential vaccination strategy compared to PPSV23 single vaccination and support the benefits of extending the vaccination program from older adults over the age of 65 years to a wider population that includes all adults aged 20 years or above. Additionally, in 2009, Hong Kong launched a pneumococcal vaccination program which offers older adults ≥65 years free or subsidized PPSV23. Latterly, in 2017, PCV13 was added to the program; however, government subsidized sequential administration is only offered to older adults ≥65 years with high-risk conditions. The results of this study suggest that extending the vaccination program to adults aged 20–64 years with high-risk conditions, as well as all adults aged ≥65 years, could be cost-effective in lowering the burden of pneumonia. Consistent efforts are needed to ensure the accessibility and affordability of vaccines among the wider population hence the increase of vaccine uptake and the decrease of disease burden.
Study limitations and future directions
There are several limitations to this study. Firstly, due to a lack of local PCV13 and PPSV23 vaccine trials, the VE data was obtained from the international literature, which may not be representative of the local population. The key parameter that determines the cost-effectiveness of competing vaccines is the VE of all-cause pneumonia given its considerable disease burden in all age groups. In this study, we used the VE of vaccine-type nonbacteremic pneumococcal pneumonia from the CAPiTA results and applied it against the sero-epidemiology in Hong Kong to estimate the VE for all-cause pneumonia. This was used instead of the VE against all-cause CAP from the CAPiTA results since the latter reflects the sero-epidemiology of Netherlands at the time the trial was conducted and does not account for the sero-epidemiology in Hong Kong. In view of this, we tested the uncertainty by ranging VE of PCV13 at 31–148% of the base-case input for all-cause pneumonia and 61–119% for IPD according to the 95% confidence intervals of different VE values from the CAPiTA trial. Although VE was sensitive to variations, ICER remained below the WTP threshold. Future clinical and epidemiological studies on the VE of PCV13 and PPSV23, the serotype distribution and the etiology of all-cause pneumonia are recommended to obtain precise and relevant cost-effective evaluation to inform evidence-based decisions. In addition, due to model restrictions, the main analysis included all risk groups for ages 20–64 years old as an overall estimation; however, the risk distribution was considered and adjusted for. In addition, the low-risk population included in the analysis are not vaccinated; hence, they have minimal impact on the difference in disease burden when comparing the vaccination strategies. Furthermore, the subgroup analysis which was performed among the different risk groups shows that the sequential vaccination strategy is cost-saving (dominant) for the high-risk group – the target population of this study. This suggests a minimal impact of this limitation.
Another limitation of this CEA is the one-way sensitivity analysis where the ICER of the sequential vaccination is lower than the predefined WTP in all tested scenarios that primarily demonstrate cost-effectiveness. However, we were only able to run the deterministic SA due to restrictions in the model design. Interpretation of the cost-effectiveness of PCV13 followed by PPSV23 in comparison to only PPSV23 needs to be interpreted cautiously because uncertainties in the parameters were not comprehensively assessed. Advanced SA such as probabilistic SA should be considered for future CEA studies.
Lastly, the indirect cost due to productivity loss was assessed based on the average public monthly salary obtained from the Census. The Census provides a monthly average salary for adults over 55 years as a whole group. In our study, we applied this estimate to the 50–85-year age group and assumed that 100% of the population is in active employment, which is highly unlikely. Hence, indirect and overall costs are likely to be overestimated. However, such overestimates will apply to both competing vaccination strategies; hence, the effect on incremental cost and ICERs will be minimal.
Compared to a single dose of PPSV23, sequential vaccination of PCV13 followed by PPSV23 is cost-saving in older adults aged ≥65 years and adults aged 20–64 years with high-risk conditions. This is based on local epidemiology, disease costs, vaccine price and uptake, as well as the landmark trial vaccine efficacy. The results of this study suggest that implementing a comprehensive sequential vaccination strategy for the prevention of pneumococcal disease would be a cost-effective means of utilizing healthcare resources, especially for older adults aged ≥65 years regardless of risk conditions. Population-specific studies on the effectiveness of pneumococcal vaccines and the epidemiology of pneumococcal diseases are warranted for an informed decision by policymakers.
Disclosure of potential conflicts of interest
Xue Li has received research funding from The Research Fund Secretariat of the Food and Health Bureau of The Government of the Hong Kong Special Administrative Region (HKSAR), Hong Kong; Esther Chan has received research funding from Wellcome Trust, United Kingdom; National Natural Science Fund of China, China; The Hong Kong Research Grants Council, The Research Fund Secretariat of the Food and Health Bureau, Narcotics Division of the Security Bureau of HKSAR, Hong Kong; Bristol-Myers Squibb, Pfizer, Bayer, and Janssen, a Division of Johnson & Johnson, for work unrelated to this study.
Acknowledgments
The authors would like to thank Ms. Lisa Lam for copyediting the manuscript.
Additional information
Funding
References
- World Health Organization. Pneumococcal disease; 2011 [accessed 2015 Nov 27]. http://www.who.int/immunization/topics/pneumococcal_disease/en/.
- Centre for Health Protection, Department of Health, The Government of Hong Kong Special Administrative Region. Scientific committee on vaccine preventable diseases updated recommendations on the use of pneumococcal vaccines for high-risk individuals; 2016 [accessed 2017 Jun 2]. http://www.chp.gov.hk/en/sas6/101/110/106.html.
- Bravo LC. Overview of the disease burden of invasive pneumococcal disease in Asia. Vaccine 2009;27(52):7282–91. doi:10.1016/j.vaccine.2009.04.046.
- Domínguez À, Soldevila N, Toledo D, Torner N, Force L, Pérez MJ, Martín V, Rodríguez-Rojas L, Astray J, Egurrola M, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccination in preventing community-acquired pneumonia hospitalization and severe outcomes in the elderly in Spain. PLoS One 2017;12(2):e0171943–e0171943. doi:10.1371/journal.pone.0171943.
- Mirsaeidi M, Schraufnagel DE. Pneumococcal vaccines: understanding centers for disease control and prevention recommendations. Ann Am Thorac Soc. 2014;11(6):980–85. doi:10.1513/AnnalsATS.201401-042CME.
- Plosker GL. 13-valent pneumococcal conjugate vaccine: a review of its use in adults. Drugs 2015;75(13):1535–46. doi:10.1007/s40265-015-0449-z.
- World Health Organization. Pneumococcal disease: vaccine; 2017 [accessed 2017 Jan 19]. http://www.who.int/ith/vaccines/pneumococcal/en/.
- Bonten MJM, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AMM, Sanders EAM, Verheij TJM, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25. doi:10.1056/NEJMoa1408544.
- Stockmann C, Byington CL. PCV13 in the USA: early successes and potential challenges. Lancet Infect Dis. 2015;15(3):254–56. doi:10.1016/S1473-3099(15)70018-6.
- Pilishvili T, Bennett NM. Pneumococcal disease prevention among adults: strategies for the use of pneumococcal vaccines. Am J Prev Med. 2015;49(6 Suppl 4):S383–390. doi:10.1016/j.amepre.2015.09.008.
- The Advisory Committee on Immunization Practices. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP); 2012 [accessed 2015 Nov 4]. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6140a4.htm#tab.
- National Advisory Committee on Immunization, Public Health Agency of Canada. Statement on the use of conjugate pneumococcal vaccine - 13 valent in adults (Pneu-C-13); 2013 [accessed 2017 Jun 7]. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/13vol39/acs-dcc-5/index-eng.php#rec.
- Castiglia P. Recommendations for pneumococcal immunization outside routine childhood immunization programs in Western Europe. Adv Ther. 2014;31(10):1011–44. doi:10.1007/s12325-014-0157-1.
- Atwood M, Beausoleil L, Breton M-C, Laferriere C, Sato R, Weycker D. Cost-effectiveness of alternative strategies for use of 13-valent pneumococcal conjugate vaccine (PCV13) in Canadian adults. Can J Public Health 2018;109(5–6):756–68. doi:10.17269/s41997-018-0050-9.
- Chen J, O’Brien MA, Yang HK, Grabenstein JD, Dasbach EJ. Cost-effectiveness of pneumococcal vaccines for adults in the United States. Adv Ther. 2014;31(4):392–409. doi:10.1007/s12325-014-0115-y.
- Heo JY, Seo YB, Choi WS, Lee J, Noh JY, Jeong HW, Kim WJ, Kim MJ, Lee HY, Song JY, et al. Cost-effectiveness of pneumococcal vaccination strategies for the elderly in Korea. PLoS One 2017;12(5):e0177342. doi:10.1371/journal.pone.0177342.
- Hospital Authority of Hong Kong. Hospital authority. Drug formulary management manual (Version 0.6); 2015 [accessed 2017 Jan 24]. http://www.ha.org.hk/haho/ho/cad_bnc/AOM-P1108.pdf.
- Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016;316(10):1093–103. doi:10.1001/jama.2016.12195.
- Li X, Blais JE, Wong ICK, Tam AWY, Cowling BJ, Hung IFN, Chan EWY. Population-based estimates of the burden of pneumonia hospitalizations in Hong Kong, 2011–2015. Eur J Clin Microbiol Infect Dis. 2019;38(3):553–61. doi:10.1007/s10096-018-03459-x.
- Center for Health Protection, Department of Health, The Government of HKSAR. Report on IPD in 2017; 2015 [accessed 2018 Nov 25]. https://www.chp.gov.hk/en/resources/29/100015.html.
- Örtqvist Å, Hedlund J, Burman L-Å, Elbel E, Höfer M, Leinonen M, Lindblad I, Sundelöf B, Kalin M. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet 1998;351(9100):399–403. doi:10.1016/S0140-6736(97)07358-3.
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325(21):1453–60. doi:10.1056/NEJM199111213252101.
- Evers SM, Ament AJ, Colombo GL, Konradsen HB, Reinert RR, Sauerland D, Wittrup-Jensen K, Loiseau C, Fedson DS. Cost-effectiveness of pneumococcal vaccination for prevention of invasive pneumococcal disease in the elderly: an update for 10 Western European countries. Eur J Clin Microbiol Infect Dis. 2007;26(8):531–40. doi:10.1007/s10096-007-0327-z.
- Smith KJ, Zimmerman RK, Lin CJ, Nowalk MP, Ko F-S, McEllistrem MC, Roberts MS. Alternative strategies for adult pneumococcal polysaccharide vaccination: a cost-effectiveness analysis. Vaccine 2008;26(11):1420–31. doi:10.1016/j.vaccine.2008.01.007.
- Mufson MA, Krause HE, Schiffman G, Hughey DF. Pneumococcal antibody levels one decade after immunization of healthy adults. Am J Med Sci. 1987;293(5):279–84. doi:10.1097/00000441-198705000-00001.
- Rodriguez-Barradas MC, Musher DM, Lahart C, Lacke C, Groover J, Watson D, Baughn R, Cate T, Crofoot G. Antibody to capsular polysaccharides of Streptococcus pneumoniae after vaccination of human immunodeficiency virus-infected subjects with 23-valent pneumococcal vaccine. J Infect Dis. 1992;165(3):553–56. doi:10.1093/infdis/165.3.553.
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA 2012;307(8):804–12. doi:10.1001/jama.2012.169.
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Lee BY, Zimmerman RK. Modeling of cost effectiveness of pneumococcal conjugate vaccination strategies in U.S. older adults. Am J Prev Med. 2013;44(4):373–81. doi:10.1016/j.amepre.2012.11.035.
- Liguori G, Parlato A, Zamparelli AS, Parlato A, Zamparelli AS, Zamparelli AS, Belfiore P, Belfiore P, Gallé F, Gallé F, et al. Adult immunization with 13-valent pneumococcal vaccine in Campania region, South Italy an economic evaluation. Hum Vaccin Immunother. 2014;10(2):492–97. doi:10.4161/hv.26888.
- Mangen MJ, Rozenbaum MH, Huijts SM, van Werkhoven CH, Postma DF, Atwood M, van Deursen AMM, van der Ende A, Grobbee DE, Sanders EAM, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015;46(5):1407–16. doi:10.1183/13993003.00325-2015.
- Rodriguez Gonzalez-Moro JM, Menendez R, Campins M, Lwoff, N., Oyagüez, I., Echave, M., Rejas, J., Antonanzas, F. Cost effectiveness of the 13-valent pneumococcal conjugate vaccination program in chronic obstructive pulmonary disease patients aged 50+ years in Spain. Clin Drug Investig.
- Centre for Health Protection, Department of Health, HKSAR. Number of deaths by leading causes of death by sex by age in 2017; 2018 [accessed 2019 Jan 7]. https://www.chp.gov.hk/en/statistics/data/10/27/340.html.
- Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–27. doi:10.1056/NEJMoa1500245.
- McLaughlin JM, Jiang Q, Isturiz RE, Sings HL, Swerdlow DL, Gessner BD, Carrico RM, Peyrani P, Wiemken TL, Mattingly WA, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clin Infect Dis. 2018;67(10):1498–506. doi:10.1093/cid/ciy312.