ABSTRACT
Influenza is a viral respiratory disease that causes significant clinical and economic burden globally. Quadrivalent influenza vaccine (QIV) is frequently used to protect people who have a high-risk of developing influenza complications due to comorbidities. QIV offers protection against influenza A (A/H1N1 and H3N2) and B (B/Victoria, and B/Yamagata) strains. The European Medicines Agency has recently approved a cell-based QIV (QIVc) in people aged over 9 years old. QIVc has been shown to be more effective at preventing influenza than traditional egg-based QIV (QIVe). In this study, we use a health economic model adapted to Spain to assess the costs and outcomes associated with using QIVc instead of QIVe in people aged 9–64 at high-risk of complications. Observed vaccine coverage of 32% in the 9–17 age group, 17% in those aged 18–59, and 22% for ages 60–64 was used in the analysis. In total, 2.5 million people were vaccinated in the simulations. Using QIVc instead of QIVe was associated with 16,221fewer symptomatic cases, 4,522 fewer primary care visits, 1,015 fewer emergency room visits and 88 fewer hospitalizations. From a societal perspective, QIVc was more effective and less expensive compared to QIVe, leading to a cost-saving of €3.4 million. From a public payer perspective, the incremental cost-effectiveness ratio for QIVc vs QIVe was €12,852 per QALY gained. In conclusion, QIVc offers a cost-effective alternative to QIVe and should be considered as an alternative vaccine to QIVe for people aged 9–64 at high-risk of influenza complications in Spain.
Introduction
Influenza is a viral respiratory disease characterized by sudden onset of high fever, myalgia, headache and severe malaise, nonproductive cough, sore throat, and rhinitis. During the influenza season, the disease spreads rapidly and results in significant economic burden due to healthcare costs and losses in productivity.Citation1,Citation2 Hospitalization and deaths mainly occur in high-risk groups (elderly, chronically ill).Citation3 Although difficult to assess, these annual worldwide epidemics are estimated to result in approximately 3–5 million cases of severe illness, and around 290,000–650,000 respiratory deaths.Citation3
Whilst there are four types of influenza viruses (A–D), influenza A and B are the main viruses that circulate and cause seasonal disease epidemics in humans.Citation3 Influenza A is further classified into subtypes (strains or lineages) according to the combinations of hemagglutinin and neuraminidase, the proteins on the surface of the virus.Citation3 For influenza A, currently circulating strains in humans are subtypes A/H1N1 (also known as A/H1N1 pdm09) and A/H3N2 viruses; for influenza B, viruses circulating belong to B/Yamagata or B/Victoria linages.Citation3 The influenza type evolves and a different mix of strains is seen every season.
Vaccination is the most effective way to prevent influenza, and the World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC), and other institutions recommend a vaccine composition with specific virus types each year. The WHO Global Influenza Surveillance and Response System, a system of national influenza centers and WHO collaborating centers, continuously monitors the influenza viruses circulating in humans and updates the composition of influenza vaccines twice a year.Citation3 The measure used to reflect how well influenza vaccines prevent influenza is vaccine effectiveness (VE), which depends on the match of the virus composition of the vaccine to the circulating viruses. High VE results in fewer influenza cases, leading to the prevention of a increasednumbers of influenza-related illnesses.Citation4
Different vaccine types exist and the protection they offer varies. Quadrivalent influenza vaccine (QIV) provides protection against A (A/H1N1 and A/H3N2) and B (B/Victoria, and B/Yamagata) strains. Trivalent influenza vaccine (TIV) protects against A strains and only the B strain identified by the WHO as most likely to be dominant the following season. Given the wider protection offered by the QIV, it is more frequently used as it can result in fewer symptomatic cases, hospitalizations and deaths, and decreased productivity and economic losses due to influenza.Citation5
Two types of QIV are available: cell-based vaccine (QIVc) and egg-based vaccine (QIVe). QIVc is not associated with egg adaptions that can occur during the manufacturing of QIVe.Citation6,Citation7 QIVc has been shown to improve VE over traditional QIVe, specifically against the A/H3N2 strain.Citation8 The objective of this study was to determine the cost-effectiveness of QIVc compared with QIVe in a Spanish high-risk population aged 9–64.
Materials and methods
Model structure
A health economic model simulating the costs, benefits, and burden of disease for a high-risk, 9–64-year-old population in Spain vaccinated with QIVc or QIVe over one influenza season was developed. A static, decision-tree model, based on Chit and colleaguesCitation9 and Reed and colleagues,Citation10 was adapted for the Spanish setting. This structure has been used extensively in other influenza cost-effectiveness analyzes,Citation11 Citation12–Citation13 and the analysis was designed in-line with Spanish best practices for health economic modeling.Citation14 A schematic of the economic is shown in .
Figure 1. Schematic of the economic model.
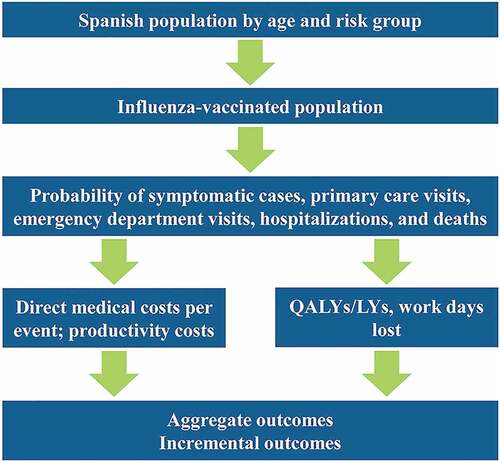
The model is structured around the costs and quality-adjusted life year (QALY) loses associated with five clinical outcomes: symptomatic cases, primary care physician visits, emergency department visits, inpatient hospitalization, and deaths. The analysis includes the public payer and societal perspectives. All costs and outcomes are calculated for an entire influenza season, except for productivity loss due to death and QALY loss due to death. These are calculated over the lifetime of the cohort, and discounted at 3% per year.
Epidemiology
The population size and life expectancy by age group were taken from national Spanish statistics.Citation15,Citation16 The percentage of patients defined as at high-risk of complicationsCitation17 and vaccine coverage by age group were taken from various sources.Citation5,Citation18,Citation19 These are shown in . The rates used are from 2018–19 influenza season, and are considered representative as rates have remained stable since 2010–2011.Citation18
Table 1. Population size, life-expectancy, percentage at high-risk, and vaccine coverage by age group.
Rates of clinical outcomes
The rates per 100,000 for the different clinical outcomes are shown in and are based on surveillance data from the 2014–2015 to the 2017–2018 influenza seasons. Incidence of clinically reported influenza in the Spanish population was taken from surveillance reports from the sentinel general practitioners of the Sistema Centinela de Vigilancia de Gripe in Spain.Citation20–Citation23 This was collected by age group and split by risk group using the data in . The relative risk of developing clinical symptoms is 1.51-times higher for high-risk patients than low-risk patients.Citation19 Patients were split into those that visit a primary care physician (81.67%) and those that visit an emergency department (18.33%).Citation5
Table 2. Rates of different clinical events per 100,000 high-risk patients by age group.
The distribution of inpatient hospitalization by age was taken from the 2017–2018 Centro Nacional de Epidemiologia, Vigilancia en Salud Pública, ISCIII report. However, these were only severe hospitalizations; therefore, they were scaled up to match the overall hospitalization rate of 66 per 100,000 for those aged under 65 years. These adjusted rates were then split by risk as reported by Garcia and colleagues.Citation5 The death rate was also calculated from the hospitalization numbers, and split into risk group by using the rates reported by Garcia and colleaguesCitation5. The symptomatic cases were calculated using the annual probability of developing symptomatic influenza per age group of 19.21% for children and 6.55% for adults.Citation5
Once rates of clinical events per 100,000 were derived, they were split by strain (see Appendix for further details).
Vaccine effectiveness
VE data for QIVc refers to FLUCELVAX® QUADRIVALENT influenza vaccine. The QIVe VE data is based on a range of different vaccines and was derived from a meta-analysis of 56 studies.Citation24 VE for different strains by age group relative to “no vaccination” are provided in . The same VE for strains A/H1N1 and B were used for both QIVc and QIVe.Citation24 The VE for QIVe, shown in the column headed A/H3N2[QIVe] in , is from Belongia and colleagues and derived from egg-based vaccines.Citation24
Table 3. Vaccine effectiveness by strain and age-group relative to “no vaccination”Citation8,24.
However, this could not be used for the cell-based QIVc and therefore the VE for QIVc compared to “no vaccination” was derived using the relative VE of QIVc compared with QIVe calculated by Boikos and colleaguesCitation8 as 26.8%. This was estimated in an observational study of 18–64-year olds. It is used in the model for the 9–64-year-old population, so we assumed that the same rate can be applied to the 9–17-year olds. The VE relative to “no vaccination” for QIVc in A/H3N2 is as A/H3N2[QIVc] in . Effectiveness for high- and low-risk groups was assumed the same and no safety issues were considered for the vaccines.
Utilities
Health-related quality of life (HRQoL) for clinical events was taken from Hollmann and colleagues.Citation25 These values were from a longitudinal study of Spanish patients from major hospitals. Patients reported their HRQoL using the EuroQoL Five Dimensions Three Levels (EQ-5D-3L) instrument for the influenza period and the week before. The disutility was calculated as the difference between EQ-5D-3L prior to the influenza episode and during it. Estimated duration of disutility for inpatients and outpatients was 21 and seven days, respectively. Disutility for symptomatic cases was from Dolk and colleagues.Citation26 Productivity losses were assumed to be five and 15 days to account for a five-day working week.
Costs
Costs for vaccines were €7.50 for QIVc and €6.00 for QIVe.Footnote1 The resource unit costs were taken from official Spanish sources with the cost of a primary care physician visits at €53.75,Citation27 an emergency department visit at €285.75,Citation28 Citation29 Citation30–Citation31 and an inpatient hospitalization, which included an intensive care unit stay for nine days for 7.5% of admissions, at €4,369.66. Patients with symptomatic disease who did not attend a primary care physician visit, emergency department visits, or have an inpatient hospitalization were conservatively assumed to have no public payer or societal costs. A comedication cost of €3.21 for the primary care visit was taken from the publication of Perez-Rubio and Eiros.Citation32,Citation33 Administration and transportation costs were not included as they are the same for QIVc and QIVe.
Productivity losses due to direct illness were based on working days lost multiplied by the probability of being employed, which is 59%,Citation34 multiplied by the daily rate of €117.28.Citation35 Productivity losses were also applied to children as one parent may take time off work to care for them or take them to the hospital. This probability was 59% x 59% = 35% (i.e., the probability that both parents are working and one needs to take time off) ().
Analysis
The outputs from the model include burden of illness, economic cost, and incremental analyses. The burden of illness outcomes included the number of symptomatic cases, primary care physician visits, emergency department visits, and inpatient hospitalizations when QIVe or QIVc is used as part of the vaccination strategy. Public payer costs and discounted societal costs were calculated as well as total discounted QALYs. The public payer costs were not discounted as they were only calculated over one year, whereas societal costs and QALY losses due to death were calculated based on life-expectancy and discounted accordingly. Incremental cost-effectiveness ratios (ICER) were calculated for QIVc vs. QIVe from a medical cost and societal perspective.
A one-way deterministic sensitivity analysis (DSA) was conducted where model parameters were varied by ±10% and the ICERs calculated to assess the sensitivity of individual parameters on cost-effectiveness. A probabilistic sensitivity analysis (PSA) was conducted by varying parameters based on their confidence intervals during 1,000 iterations of the model to assess the effect of uncertainty on the ICERs. The icers were compared against a willingness-to-pay threshold of €22,000–€25,000 per QALY gained. this is the willingness-to-pay threshold recently identified as the range used by the national health service in spain.Citation36
Results
The total number of people vaccinated with QIVe or QIVc in the simulation was 2,514,788. The number vaccinated by age, along with the number of events when QIVe or QIVc was used, is shown in . The results show that using QIVc instead of QIVe results in 16,221 fewer cases. This includes 4,522 fewer primary care visits, 1,015 fewer emergency room visits, 88 fewer hospitalizations, and five fewer deaths.
Table 4. Baseline utility by age and disutility by age and clinical outcome.
Table 5. Number of vaccinated people and clinical events with QIVe or QIVc as part of the vaccination strategy in Spain.
Incremental costs are shown in . The incremental costs for the clinical events are lower for QIVc compared with QIVe. From a societal perspective, using QIVc results in a net saving of €3,392,832. From a public payer perspective, the incremental cost is €2,838,844.
Table 6. Total and incremental costs by cost category when QIVe and QIVc are used as part of the vaccination strategy in Spain, along with QALY loss.
The total QALY loss due to influenza in the high-risk 9–64-year-old population is 33,278 QALYs when QIVe is used and 33,057 when QIVc is used, leading to a total QALY gain of 221 for QIVc. Therefore, from the payer perspective, the ICER is €12,852 per QALY gained. From a societal perspective, QIVc is the dominant strategy, leading to a net saving of €3,392,832.
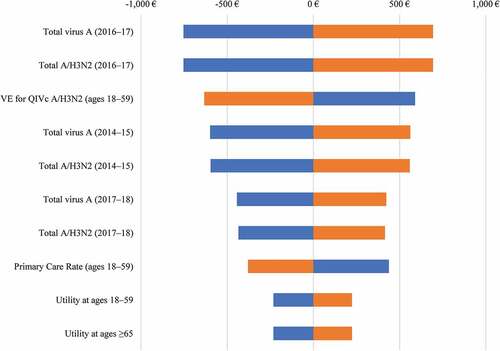
The DSA examining the impact on the ICERs from a public payer perspective for QIVc vs. QIVe is shown in . The most impactful drivers behind cost-effectiveness were the levels of influenza A circulating in the populations.
Figure 2. DSA for QIVc vs. QIVe.
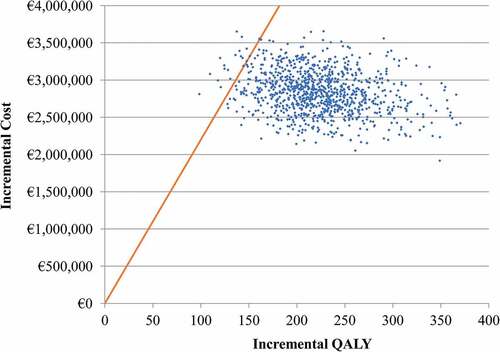
A PSA was also conducted with the scatter plots on the cost-effectiveness plane shown in . For QIVc vs. QIVe, 98% of the model iterations fall below the €22,000 per QALY willingness-to-pay threshold. Ninety-nine percent would be below the €25,000 per QALY willingness-to-pay threshold.
Discussion
Mutation of key epitopes in vaccine-virus strains during the isolation and propagation steps of vaccine production in eggs is likely the key factor that can lead to reduced VE.Citation37 Citation38 Citation39–Citation40 The inactivated QIVc is the first and only available QIVc in Spain. It is supported by extensive clinical data in adults and children. Using mammalian cell culture rather than eggs during the vaccine virus isolation and manufacturing process ensures that the original candidate vaccine virus for the cell-based influenza vaccine is much closer in antigenicity to the circulating wild-type influenza virus strains.Citation41
Cost-effectiveness analysis is increasingly used to assess the value of different vaccines and vaccination strategies. These models calculate costs and outcomes to enable healthcare providers to make informed evidence-based assessment on how to best allocate limited resources. This analysis demonstrates that, with an ICER of €12,852 per QALY gained compared with QIVe from a public payer perspective, QIVc offers a cost-effective alternative in the high-risk, 9–64-year-old population at the €22,000 per QALY threshold used in Spain.Citation36 From a societal perspective, using QIVc instead of QIVe would result in major cost savings, driven by the reduction in symptomatic cases and work-days lost due to sickness. In addition, because the VE from Boikos and colleagues covers all circulating influenza strains whereas the relative VE is only applied to the A/H3N2 strain in this model, the VE value used in the model, and therefore the results, can be considered conservative. The data for the VE is from the 2017–18 season. QIVc was launched in the US in 2016 and in the EU in 2019 so there is limited long-term data to support similar efficacy in different years. This means applicability across seasons is a limitation of the analysis.
The results from Spain reflect those for the United Kingdom (UK) and Italy, which have compared QIVc with QIVe.Citation42,Citation43 In these countries, similar reductions in the burden of disease were observed, with QIVc being cost-effective or dominant compared with QIVe. While cost-effectiveness studies for Spain for QIVc vs. QIVe are not available from other authors, a model by Garcia and colleaguesCitation5 has been published comparing the QIV with TIV in Spain. The QIV was an egg-based vaccine, and outcomes for QIVe were compared between this study and Garcia and colleagues and were found to be similar.
As with all economic models, there are limitations to the study. Not all data are from Spain. Key data sources, such as incidence, general practitioner visits, emergency room visits, hospitalization, demographic data, resource use, costs, vaccine coverage, and some utility data are from Spain. However, influenza mortality rates were from the UK and vaccine effectiveness by age and strain, and some utility data, were from a variety of countries. The model has a one-year horizon, so it only calculates costs and outcomes for a single influenza season. The model assumes no cross immunity or effect across years for the vaccine and therefore may underestimate overall effectiveness. The model is static rather than a dynamic transmission model. This means that herd immunity is not accounted for and therefore will underestimate cost-effectiveness of QIVc, resulting in a conservative approach. Due to the inherent variability in the characteristics of the influenza virus, predicting future effectiveness and incidence is difficult. Therefore, averages from previous years were used. However, the benefits within years may vary considerably. Another limitation is the analyses assume that all seasons have an egg adaptation by manufacturing egg-based vaccines, but this may not always happen, or it may be more severe in some seasons. This seasonal variation adds uncertainty to the results.
This model only considers patients aged 9–64-years old. The impact of influenza on this population is high at a societal level as patients include children whose parents will need to take time off work to care for them and working-age adults who will have to take time off work. This is reflected in the results as having high societal costs. Therefore, switching to QIVc from QIVe would be cost-effective from a public payer perspective, cost saving from a societal perspective, and will considerably reduce the burden of illness in this large segment of the population.
Disclosure of potential conflicts of interest
Ray Gani and Piedad Alvarez are salaried employees of Evidera and are not allowed to accept remuneration from any clients for their services. Jesús Ruiz-Aragón and Sergio Márquez received consultancy fees from Evidera to conduct the study and develop this manuscript.
Acknowledgments
The authors thank the support made by Seqirus for the preparation of this work.
Additional information
Funding
Notes
1. Prices provided by Seqirus, based on regional and central tender prices.
References
- Dabestani NM, Leidner AJ, Seiber EE, Kim H, Graitcer SB, Foppa IM, Bridges CB. A review of the cost-effectiveness of adult influenza vaccination and other preventive services. Prev Med. 2019;126:105734. doi:10.1016/j.ypmed.2019.05.022.
- Puig-Barbera J, Mira-Iglesias A, Burtseva E, Cowling BJ, Serhat U, Ruiz-Palacios GM, Launay O, Kyncl J, Koul P, Siqueira MM, et al. Influenza epidemiology and influenza vaccine effectiveness during the 2015–2016 season: results from the global influenza hospital surveillance network. BMC Infect Dis. 2019;19(1):415. doi:10.1186/s12879-019-4017-0.
- World Heath Organization (WHO). Influenza (Seasonal). 2018 [Accessed 2019 Sep 5. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal).
- World Heath Organization (WHO). Questions and answers: vaccine effectiveness estimates for seasonal influenza vaccines. 2015 Sept 5 [Accessed 2019]. https://www.who.int/influenza/vaccines/virus/recommendations/201502_qanda_vaccineeffectiveness.pdf
- Garcia A, Ortizde Lejarazu R, Reina J, Callejo D, Cuervo J, Morano Larragueta R. Cost-effectiveness analysis of quadrivalent influenza vaccine in Spain. Hum Vaccin Immunother. 2016;12(9):2269–77. doi:10.1080/21645515.2016.1182275.
- DeMarcus L, Shoubaki L, Federinko S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017–2018 influenza season. Vaccine. 2019;37(30):4015–21. doi:10.1016/j.vaccine.2019.06.004.
- Lamb YN. Cell-based quadrivalent inactivated influenza virus vaccine (Flucelvax((R)) tetra/flucelvax quadrivalent((R))): a review in the prevention of influenza. Drugs. 2019;79(12):1337–48. doi:10.1007/s40265-019-01176-z.
- Boikos T Effectiveness of the cell culture- and egg-derived, seasonal influenza vaccine during the 2017–2018 Northern hemisphere influenza season. NFID Clinical Vaccinology Course; November 9–10, 2018; Bethesda, MD.
- Chit A, Roiz J, Aballea S. An assessment of the expected cost-effectiveness of quadrivalent influenza vaccines in Ontario, Canada Using a static model. PLoS One. 2015;10(7):e0133606. doi:10.1371/journal.pone.0133606.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine. 2012;30(11):1993–98. doi:10.1016/j.vaccine.2011.12.098.
- Uhart M, Bricout H, Clay E, Largeron N. Public health and economic impact of seasonal influenza vaccination with quadrivalent influenza vaccines compared to trivalent influenza vaccines in Europe. Hum Vaccin Immunother. 2016;12(9):2259–68. doi:10.1080/21645515.2016.1180490.
- Jamotte A, Chong CF, Manton A, Macabeo B, Toumi M. Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia. BMC Public Health. 2016;16:630. doi:10.1186/s12889-016-3297-1.
- Mennini FS, Bini C, Marcellusi A, Rinaldi A, Franco E. Cost-effectiveness of switching from trivalent to quadrivalent inactivated influenza vaccines for the at-risk population in Italy. Hum Vaccin Immunother. 2018;14(8):1867–73. doi:10.1080/21645515.2018.1469368.
- Lopez-Bastida J, Oliva J, Antonanzas F, García-Altés A, Gisbert R, Mar J, Puig-Junoy J. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513–20. doi:10.1007/s10198-010-0244-4.
- Instituto Nacional de Estadística (INE). Fenómenos demográficos. Tablas de mortalidad. 2018 [Accessed 2019 Sep 5]. https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736177004&menu=ultiDatos&idp=1254735573002.
- Instituto Nacional de Estadística (INE). Censos demográficos. Cifras de población. 2018 [Accessed 2019 Sep 5]. http://www.ine.es/dynt3/inebase/es/index.htm?padre=1894&capsel=1895.
- Ministerio de Sanidad CyBSM. La Gripe. ¿Que es la Gripe? 2018 Accessed 2019 Sep 5]. http://www.mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/gripe/gripe.htm#Prev1.
- Ministerio de Sanidad CyBSM. Tabla 12. Coberturas de vacunación frente a gripe en ≥65 años, personas de 60–64 años. Comunidades autónomas. Campaña 2017–2018. 2018 [Accessed2019 Sep 5]. http://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/docs/CoberturasVacunacion/Todas_las_tablas.pdf.
- Seqirus. Data on file, 9 Sep 2019.
- Instituto de Salud Carlos III (ISCIII). Informe de Vigilancia de la Gripe en España. Temporada 2014–2015 (Desde la semana 40/2014 hasta la semana 20/2015). Sistema de Vigilancia de la Gripe en España. 2014–2015. http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/pdf_2015/Informe_Vigilancia_GRIPE_2014-2015_vf_29092015.pdf.
- Instituto de Salud Carlos III (ISCIII). Informe de Vigilancia de la Gripe en España. Temporada 2015–2016 (Desde la semana 40/2015 hasta la semana 20/2016). Sistema de Vigilancia de la Gripe en España. 2015–2016. http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/gripe.shtml.
- Instituto de Salud Carlos III (ISCIII). Informe de Vigilancia de la Gripe en España. Temporada 2016–2017 (Desde la semana 40/2016 hasta la semana 20/2017). Sistema de Vigilancia de la Gripe en España. 2016–2017. http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/gripe.shtml.
- Instituto de Salud Carlos III (ISCIII). Informe de Vigilancia de la Gripe en España Temporada 2017–2018. Instituto de Salud Carlos III. Sistema de Vigilancia de la Gripe en España. 2017–2018. http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/fd-gripe/fd-informes-semanales-vigilancia-gripe/pdfs_2017-2018/Informe_Vigilancia_GRIPE_2017-2018_27julio2018.pdf.
- Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, McLean HQ. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–51. doi:10.1016/S1473-3099(16)00129-8.
- Hollmann M, Garin O, Galante M, Ferrer M, Dominguez A, Alonso J. Impact of influenza on health-related quality of life among confirmed (H1N1)2009 patients. PLoS One. 2013;8(3):e60477. doi:10.1371/journal.pone.0060477.
- Dolk C, Eichner M, Welte R, Anastassopoulou A, Van Bellinghen L-A, Poulsen Nautrup B, Van Vlaenderen I, Schmidt-Ott R, Schwehm M, Postma M, et al. Cost-utility of quadrivalent versus trivalent influenza vaccine in Germany, using an individual-based dynamic transmission model. Pharmacoeconomics. 2016;34(12):1299–308. doi:10.1007/s40273-016-0443-7.
- Junta DA. Boletin Oficial de la Junta de Andalucia (BOJA), Numero 92 - Martes 15 de Mayo de 2018. Pagina 16 - I.1.1.1. Consulta médica de atención primaria en el centro en horario ordinario. 2018 Accessed 2019 Sep 5. https://www.juntadeandalucia.es/boja/2018/92/BOJA18-092-00003-8350-01_00135732.pdf.
- Región DM. Consejería de Hacienda y Administración Pública. Orden de 3 de Febrero de 2015. Precios Públicos del Servicio Murciano de Salud - A.1.1 Estancia/día Cama Observación de urgencias. 2015 Accessed 2019 Sep 5. https://www.borm.es/borm/documento?obj=anu&id=725195.
- Marin-Corral J, Climent C, Munoz R, Samper M, Dot I, Vilà C, Masclans JR, Rodriguez A, Martin-Loeches I, Álvarez-Lerma F, et al. Patients with influenza A (H1N1)pdm09 admitted to the ICU. Impact of the recommendations of the SEMICYUC. Med Intensiva. 2018;42(8):473–81. doi:10.1016/j.medin.2018.02.002.
- Ministerio de Sanidad CyBSM. Área de Inteligencia de Gestión. Grupos relacionados por el Diagnostico - GRD. NORMA ESTATAL DE LOS GRD AP. AÑO 2015. 2015.
- Ministerio de Sanidad CyBSM. Area de Vigilancia de la Salud Publica ICSIII – reporte 2017–18 con valores para las estaciones 2013–2018. 2019.
- Pérez-Rubio AMEJ. Impacto económico y sanitario de la utilización de vacuna antigripal adyuvada con MF59 en población mayor de 65 años en España. Rev Esp Quimioter. 2018;31:43–52.
- Región DM Consejería de Hacienda y Administración Publica. Orden de 3 de Febrero de 2015. Precios Públicos del Servicio Murciano de Salud - I.3.1.3. Procedimiento de enfermería en el centro de atención primaria. Costo de 2015 aun aplicable en 2018. 2019.
- Instituto Nacional de Estadística (INE). Instituto Nacional de Estadística. Actividad, ocupación y paro. Encuesta de Población Activa - Trimestre 2/2018. 2018.
- Instituto Nacional de Estadística (INE). Encuesta anual de coste laboral. Coste laboral anual por trabajador - Año 2017. 2018 Accessed 2019 Sep 5. https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736060920&menu=ultiDatos&idp=1254735976596.
- Vallejo-Torres L, Garcia-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27(4):746–61. doi:10.1002/hec.3633.
- Blanton L, Wentworth DE, Alabi N, Azziz-Baumgartner E, Barnes J, Brammer L, Burns E, Davis CT, Dugan VG, Fry AM, et al. Update: influenza activity - United States and Worldwide, May 21-September 23, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(39):1043–51. doi:10.15585/mmwr.mm6639a3.
- Wu NC, Zost SJ, Thompson AJ, Oyen D, Nycholat CM, McBride R, Paulson JC, Hensley SE, Wilson IA. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog. 2017;13(10):e1006682. doi:10.1371/journal.ppat.1006682.
- Zost SJ, Parkhouse K, Gumina ME, Kim K, Diaz Perez S, Wilson PC, Treanor JJ, Sant AJ, Cobey S, Hensley SE, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A. 2017;114(47):12578–83. doi:10.1073/pnas.1712377114.
- Francis Crick Institute. Report prepared for the WHO annual consultation on the composition of influenza vaccine for the Northern Hemisphere 2017–2018. 2017.
- Rajaram S, Suphaphiphat P, van Boxmeer J, Leav B, Iheanacho I, Kistler K. Retrospective assessment of the antigenic similarity of egg- vs cell culture-propagated reference A/H3N2 influenza compared with circulating A/H3N2 2003–2018. Paper presented at: ID Week 2018; San Francisco, CA.
- Nguyen VHM-PS, Ruiz-Aragón J, Nasiri M, Mould-Quevedo J, Rajaram S. The economic advantages of a cell-based quadrivalent influenza vaccine in the adult population in Europe. The Cost-Effectiveness Evidence in United Kingdom and Spain. Options for the Control of Influenza; 2019; Singapore.
- Rizzo CTF, Capri S, Merler S. Valutazione economica dell’introduzione del nuovo vaccino antinfluenzale quadrivalente da coltura cellulare (Flucelvax® Tetra) nel contesto di cura italiano. Ital J Public Health. 2019;8:5.
Appendix
Overall, influenza outcome incidence must be split into: A/H1N1, A/H3N2, B/Victoria, and B/Yamagata. The distribution by strain per influenza season is shown in . For the base case, the 2014–2015 through 2017–2018 seasons are included. This distribution was used to calculate the average outcome rates per season per strain.
Table A1. Distribution of cases by strain per influenza season.