ABSTRACT
Background: Hepatitis A vaccine has been used in mass and routine public vaccination programs in China. Long-term follow-up studies are required to determine the duration of protection and the need for booster vaccinations.
Methods: A prospective, randomized, open-label clinical trial was performed to compare the geometric mean concentration (GMC) and seroprotection rates of anti-Hepatitis A virus (HAV) antibodies elicited by the inactivated vaccines Healive and Havrix. 400 healthy children were randomly assigned 3:1 ratio to receive two doses of Healive or Havrix at 0 and 6 months. Persistence of anti-HAV antibodies for 5 years post immunization has been reported The current study reports new data at 11 years post immunization for the purpose of showing antibody persistence. Sensitivity analyzes were performed to assess the results. In addition, predictions for long-term antibody persistence were performed using a statistical model. Two different serological assays were used that were shown to be 98.3% concordant for detecting anit-HAV antibody.
Results: GMCs were significantly higher following Healive compared to Havrix at 1, 6, 7, 66, 112 and 138 months post-vaccination. In addition, the GMCs obtained using sensitivity analysis were very similar to those obtained using the original models. Prediction analysis indicated that the duration of protection for both vaccines was at least 30 years after immunization, with a lower limit of the 95% confidence interval for GMC of greater than 20mIU/mL.
Conclusions: Healive is more immunogenic than Havrix in children at 11 years post full immunization. Prediction analysis indicated at least 30 years of antibody persistence for both vaccines.
Introduction
Hepatitis A is a contagious virus that could induce liver disease.Citation2–4 HAV is usually transmitted person-to-person through the fecal-oral route or through the consumption of contaminated food or water.Citation5,Citation6 HAV infection can induce illness with fever, nausea, abdominal pain and jaundice. Although infection in young children is typically asymptomatic, older children and adults generally develop symptoms that can last for several months.Citation7
Hepatitis A continues to be a cause of considerable morbidity and mortality worldwide. Hepatitis A vaccinations have been proven to be effective for prevention. The availability of Hepatitis A vaccines has substantially lowered disease incidence and even potentially eliminated infections. Several countries have recommended routine vaccinations for children and those at a higher risk of infection who have not been previously vaccinated. The implementation of routine childhood hepatitis A vaccinations has resulted in a dramatic reduction in reported hepatitis A rates in several countries including the United States, Israel and Argentina.Citation7 The incidence of Hepatitis A has also been reduced dramatically in China, where the vaccine has been used in mass and routine public immunization programs.Citation8,Citation9
In 2006, a double-blind, randomized and controlled clinical trial was performed in healthy children aged 1–8 years. This was to compare the immunogenicity as assessed by level of anti-HAV antibodies among recipients of three consecutive production lots of Healive® and Havrix® (GlaxoSmithKline Biologicals). A total of 400 children were enrolled and assigned into four groups with 100 children per group. Each group received one of the three lots of Healive or Havrix. The vaccination was a two-dose regimen administered at 0 and 6 months. The three lots of Healive had statistically indistinguishable clinical performance with 100% seroprotection rates (SRs). The geometric mean concentration (GMC) was 3237–3814 mIU/mL at one-month post-second dose.Citation10
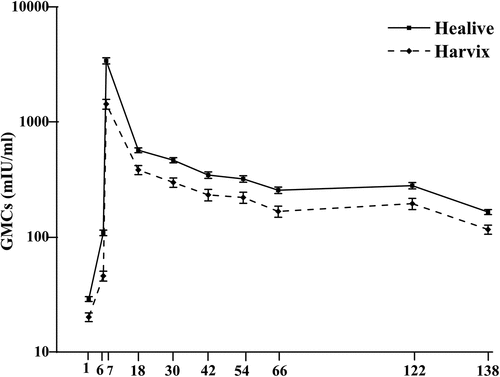
A previous publication that analyzed the 5 year GMC after a full-course immunization demonstrated higher antibody levels after vaccination with Healive compared to Havrix at 1, 6, 7, 18, 30, 42, 54 and 66 months (P < .01) with the peak levels at 7 months (3427.2 mIU/mL for Healive versus 1441.9 mIU/mL for Havrix). The SRs for both groups reached 100% at 7 months post-vaccination and was stable until 66 months (99.1% for Healive and 97.5% for Havrix). Anti-HAV antibody persistence for 5 years post-immunization has been previously reported.Citation1 The aim of the present study was to compare antibody persistence in individuals vaccinated with Healive and Havrix up to 11 years after completion of the vaccination series. In addition, we evaluated the impact of follow-up loss and performed prediction analysis for long-term antibody persistence.
Materials and methods
Clinical trial methodology
This study was a prospective, randomized and controlled, open-label follow-up study to evaluate GMCs and SRs of anti-HAV antibodies elicited by the inactivated vaccines Healive and Havrix for 11 years post-immunization. In the original primary vaccination study that was performed, 400 healthy children were randomly assigned using a 3:1 ratio to receive two doses of vaccine at 0 and 6 months. Healive (0.5 mL/dose) contained 250 U (1IU = 13U) of antigen and 0.25mg of alum without preservatives.Citation9 The Havrix vaccine (0.5 mL/dose) contained 720 ELISA units (El.U) of antigen and 0.25 mg alum with 2-phenoxyethanol as the preservative.
Eligible study subjects who completed their full-course of immunization were followed-up for 11 years. Blood samples were collected at 0, 1, 6, 7, 18, 30, 42, 54, 66, 112 and 138 months, with screening blood samples collected prior to immunization at 0 and 6 months. The data generated from 0 to 66 months were previously reported in Yu et al.Citation1 This study will include the previous generated data for additional analysis to show long-term trends for anti-HAV antibody levels. Over the 138 months of the follow-up period, assay changes were required. Anti-HAV antibody concentrations were assessed initially using a microparticle enzyme immunoassay (MEIA) until the 66 months post-vaccination. From 112 to 138 months post-vaccination, measurements were performed using electro chemiluminescence immunoassays (ECLIA). We used two commercially available kits for measuring anti-HAV antibodies, i.e., from Abbott Laboratories (HAVAB2.0, MEIA method) and Roche Diagnostics (Elecsys Anti-HAV, ECLIA method). For MEIA method of HAVAB2.0, the upper limit of quantification (ULOQ) is 100mIU/mL and lower limit of quantification (LLOQ) is 5mIU/mL. For ECLIA method of Elecsys, the ULOQ of ELICA is 60 mIU/mL and LLOQ is 3 mIU/mL. Other parameters of the two assays are detailed in the kit protocols. A previous publication demonstrated 98.3% agreement between these two assays for detecting HAV antibody.Citation11 In the study, they evaluated for two methods’ performance in order to prove their consistency. Using the serum samples from 476 patients, they analyzed the accuracy and specificity for both methods. The results showed that the positive and negative coincident rate between MEIA and ECLIA were 98.7% and 97.0% respectively, and the total coincident rate was 98.3%. In addition, both methods have quite high clinical sensitivity and specificity. The good analytical performance and correlation between the MEIA assays and ELICA assays for anti-HAV measurements supported our rationale for combining the data analysis. The comparability of the two assays was necessary to enable the two data sets to be merged and combined for modeling long-term persistence. Anti-HAV antibody levels≥20 mIU/mL were defined as seroprotection.Citation2,Citation4,Citation12,Citation13
Consent and study approval
This study was performed in accordance with the Declaration of Helsinki. The Ethics Review Committee of the Changzhou Center for Disease Control and Prevention approved this study. Written informed consents were obtained from the parents (or guardians) prior to study enrollment. This trial was registered at Clinicaltrials.gov (NCT00534885).
Statistical methods
The main analysis of GMCs and SRs of anti-HAV antibodies were calculated based on the actual observed data without missing data handling. Logarithmic transformation (log10) was performed prior to GMC calculation and 95% CI. Student’s t-test or Mann-Whitney U test was used for GMC comparisons, and the Chi-square test or Fisher’s exact test was used for SR comparisons. 2-tailed with P < .05 was considered statistically significant.
Sensitivity analyzes using the given statistical methods were performed to assess robustness. Both multiple imputations (MI) and mixed-effects model for repeated measurements (MMRM) approaches were considered for sensitivity analyses.Citation14,Citation15 For MI analysis, Markov chain Monte Carlo (MCMC) method was used for imputing missing data. We performed multiple imputations for missing log anti-HAV concentration data points. The imputation model included the child’s age at first vaccination, gender, mean of the available anti-HAV concentration data points in the first three follow-ups (1 month, 6 months and 7 months) and the mean of the available anti-HAV concentration data points obtained between 1 and 11 years of follow-up. We used the mean of the early time points to avoid missing data for the imputed data. We imputed 50 datasets and seeded for reproducibility.
Long-term estimates of antibody persistence were obtained based on published methods by using a trend model with one change point during the antibody concentrations decline phase.Citation1,Citation12,Citation14
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
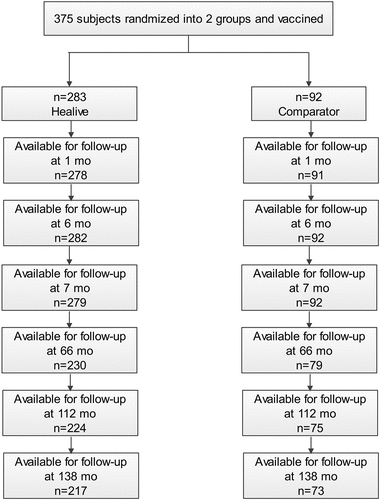
A total of 400 subjects were enrolled in the original vaccination study, with 300 in the Healive vaccinated group and 100 in the control group (Havrix). 375 subjects who completed their full-course of immunization were enrolled in the follow-up study with 283 subjects in the Healive vaccinated group and 92 in the control group. There were 290 subjects who were present in the 11-year post-vaccination follow up visit, with 217 in Healive group and 73 in the control group ().
Of the 375 subjects included in the analysis, the mean age for the Healive group was 3.8 years in Healive and 3.7 years for the control group, 141 out of the 283 subjects in the Healive group and 44 out of 92 subjects in the control group were females. Of the 290 subjects included in the analysis at the 138 month follow-up period, the mean age in the Healive group was 13.7 years and 13.6 years in the control group, 113 out of 217 subjects in the Healive group and 29 out of 73 subjects in the control group were females. The demographic characteristics of the two groups were balanced and comparable at baseline, 112 and 138 months. Demographic characteristics are presented in .
Table 1. Demographic characteristics of study population
GMCs and SRs for the anti-HAV antibodies in the two groups at 1, 6, 7, 66, 112 and 138 months are shown in and . The results at 1, 6, 7 and 66 months in have been previously published.Citation1 At 112 months, GMCs in the Healive and control group were 281.7 and 196.4 mIU/mL respectively. At 138 months, GMCs in the Healive and Control group were 166.2 and 117.1 mIU/mL respectively. The GMCs were significantly higher in the Healive group compared to the control group at each time point from 1 to 138 months post-vaccination (P < .01). At 112 months, SRs in the Healive and Control group were 99.1% and 97.1% respectively. SRs were higher in the Healive group compared to the control group at each time point from 66 to 112 months post-vaccination. At 138 months, the SRs in both groups was 100%.
Table 2. Geometric mean concentrations and seroprotection rates over time
summarizes the sensitivity analyses for GMCs obtained from the MI and MMRM methods. With regards to MI analyses at 66 months post-vaccination, GMCs in the Healive and Control group were 262.2 and 176.3 mIU/mL respectively. At 112 months, GMCs in the Healive and Control group were 269.0 and 200.00 mIU/mL respectively. At 138 months, GMCs in the Healive and Control group were 161.8 and 119.5mIU/mL respectively. With regard to MMRM analyses at 66 months post-vaccination, GMCs in the Healive and Control group were 260.7 and 177.8 mIU/mL respectively. At 112 months, GMCs of Healive and Comparator were 277.9 and 200.0 mIU/mL respectively. At 138 months, GMCs in the Healive and Control group were 159.1 and 118.2 mIU/mL respectively. The results of the two different sensitivity analysis methods were very similar to those of the raw data models.
Table 3. Geometric mean concentrations for sensitivity analyses over time
The predicted GMCs and SRs from 138 to 426 months (based on the 5-year follow up) and from 186 to 426 months (based on the 11-year follow up) derived from the mixed model are shown in . Yu et al.Citation1 used the same model to predict immunogenic persistence but was based on a shorter follow-up period from the identical population (). The same model had been used for long-term evaluation and prediction of immunogenic persistence in previous studies.Citation12,Citation14
Table 4. Comparison of Predicted GMC and Seroprotection Rates based on 5-year and 11-year follow up periods
At 138 months post-vaccination, the observed and predicted GMCs in the Healive group were 166.2 and 138.2 mIU/mL respectively. The observed and predicted GMCs in the Havrix (control) group were 117.1 and 92.4 mIU/mL respectively. Observed values are shown in . These values reflected a high level of agreement between the observed and predicted GMCs. GMCs (95% CI) were predicted to be 29.6 (24.9–35.3) mIU/mL at 366 months and 18.7 (15.4–22.8) mIU/mL at 426 months for the Healive group. GMCs (95% CI) were predicted to be 28.5 (20.5–39.5) mIU/mL at 366 months and 19 (13.1–27.5) mIU/mL at 426 months h for the Havrix group. At 366 months, GMCs for both the Healive and Havrix groups were estimated to have a lower limit of no less than 20 mIU/mL.
The observed and predicted SRs were also consistent at 138 months post-vaccination for the two groups. The predicted SRs are shown in and the SRs in the Healive group was higher compared to the Havrix group up to 420 months post-second dose vaccination.
Discussion
The aim of this follow up study was to compare GMCs and SRs of anti-HAV antibodies induced by the inactivated vaccines Healive and Havrix for 11 years post immunization. In addition, the study evaluated the impact of follow-up loss and predicted the long-term immunogenic persistence.
GMCs were significantly higher in the Healive group compared to the control group at each time point from 1 to 138 months post-vaccination (P < .01). The SRs for both groups reached 100% at 7 months post-vaccination and remained stable for 66 months. There were no significant differences in SRs between the two groups at each time point except at 1 and 6 months post-vaccination. This indicated that seroprotective anti-HAV levels were maintained for at least 11 years after vaccination. Hepatitis A booster doses after completion of the initial vaccination series do not appear to be warranted and are not currently recommended.
Because of the 11 years follow up period, it was not surprising that some of the study subjects were lost during follow up. Both MI and MMRM methods evaluated the impact of follow-up loss. With regards to sensitivity analyzes, the GMCs were very similar compared to the raw dataset results.
At 138 months post-vaccination, the observed GMC levels in the Healive and Havrix group were similar to the predicted GMCs based on the 5-year follow-up data. This confirms that a suitable and robust statistical model was used by Yu et al.Citation1 In our study, antibody persistence in the Healive and Havrix group was predicted to last at least 30 years. The results for the Havrix group were in line with results obtained from some previous trials.Citation14,Citation16,Citation17
There are several limitations to this study. First, this was a long-term study, and several participants were lost on follow-up. The reduced sample size resulting from follow-up loss may have affected the ability to measure differences between the two vaccinated groups. Second, anti-HAV antibody concentrations were assessed using microparticle enzyme immunoassay (MEIA) until the 66 months post-vaccination, and then by electro chemiluminescence immunoassay (ECLIA) from 112 to 138 months post-vaccination. Since the two methods adopted the same International Biological reference preparations provided by WHO and the high agreement between MEIA and ECLIA for detecting anti-HAV antibody, using the two methods did not impact the final results and conclusion. Antibody levels do decline over time and ongoing monitoring is required to evaluate immunity beyond 11 years after vaccination. This is to assess whether children who are vaccinated will be protected throughout adulthood. Finally, the percentage of seroprotected as the correlate of protection is the key parameter of immunogenicity and not the GMC level per se.
In conclusion, the new inactivated hepatitis A vaccine, Healive, with a 0–6 month vaccination schedule, is more immunogenic than Havrix after 11 years post full-course immunization in children. That may be due to different antigen levels and production processes of the two vaccines. The new inactivated vaccine provides long-term persistence in healthy Chinese children. Prediction analysis for immunogenic persistence indicates the vaccine will be efficacious for at least 30 years.
Disclosure of potential conflicts of interest
Yuansheng Hu and Gang Zeng are employees of Sinovac Biotech. All other authors have no conflicts of interest in the publication of this manuscript. All authors listed approved the final submitted version of the manuscript.
Supplemental Material
Download MS Word (149.3 KB)Acknowledgments
The authors would like to acknowledge the parents and their children for participating in this study.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Yu CK, Song YF, Qi YY, Li CJ, Jiang ZW, Li C, Zhang W, Wang L, Xia JL. Comparison of immunogenicity and persistence between inactivated hepatitis AvaccineHealive and Havrix among children: a 5-year follow-up study. Hum Vaccin Immunother. 2016;12(10):2595–602. PMID: 27385349. doi:10.1080/21645515.2016.1197450.
- Rao S, Mao JS, Motlekar S, Zhuang FC, Kadhe G. A review of immunogenicity and tolerability of live attenuated hepatitis A vaccine in children. Hum Vaccin Immunother. 2016;12(12):3160–65. PMID: 27532370. doi:10.1080/21645515.2016.1216286.
- Van Damme P, Banatvala J, Fay O, Iwarson S, McMahon B, Van Herck K, Shouval D, Bonanni P, Connor B, Cooksley G, et al. Hepatitis A booster vaccination: is there a need? Lancet. 2003;362(9389):1065–71. PMID: 14522539. doi:10.1016/S0140-6736(03)14418-2.
- Monjori M, Shah N, Faridi M, Ghosh A, Sankaranarayanan VS, Aggarwal A, Chatterjee S, Bhattacharyya N, Kadhe G, Vishnoi G, et al. Long term follow-up study to evaluate immunogenicity and safety of a single dose of live attenuated hepatitis a vaccine in children. Hum Vaccin Immunother. 2015;11(5):1145–52. PMID: 26018443. doi:10.4161/21645515.2014.979646.
- Lin YL, Chen GJ, Lee YL, Huang YC, Cheng A, Sun HY, Chang SY, Liu CE, Hung CC. Hepatitis A virus infection and hepatitis A vaccination in human immunodeficiency virus-positive patients: a review. World J Gastroenterol. 2017;23(20):3589–606. doi:10.3748/wjg.v23.i20.3589.
- Stuurman AL, Marano C, Bunge EM, De Moerlooze L, Shouval D. Impact of universal mass vaccination with monovalent inactivated hepatitisA vaccines - A systematic review. Hum Vaccin Immunother. 2017;13(3):724–36. PMID: 27786671. doi:10.1080/21645515.2016.1242539.
- Advisory Committee on Immunization Practices, Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55:1–23. PMID: 16708058.
- Cui F, Liang X, Wang F, Zheng H, Hutin YJ, Yang W. Development, production, and post marketing surveillance of hepatitis A vaccines in China. J Epidemiol. 2014;24(3):169–77. PMID: 24481843. doi:10.2188/jea.JE20130022.
- Bhave S, Bavdekar A, Sapru A, Bawangade S, Pandit A. Immunogenicity of single dose live attenuated hepatitis a vaccine. Indian Pediatr. 2011;48(2):135–37. PMID: 21169655. doi:10.1007/s13312-011-0039-4.
- Jiang WP, Chen JT, Wang X, Wang YL, Liu Y, Chen WY, Xu WG, Qiu YZ, Yin WD. Immunogenicity and safety of three consecutive lots of a new preservative-free inactivated hepatitis A vaccine (Healive): a double-blind, randomized and controlled trial. Vaccine. 2008;26(18):2297–301. PMID: 18395305. doi:10.1016/j.vaccine.2007.11.008.
- Bu XY, Jin YP, Liu N, Deng GF, Xi Q, Yan SK. Assessment of microparticle enzyme immunoassay and electrochemiluminescence immunoassay for detection of HAV antibody. Labeled Immunoassays & Clin Med. 2010;17(1):34–36. CNKI:SUN:BJMY.0.2010-01-012.
- Wiedermann G, Kundi M, Ambrosch F, Safary A, D’Hondt E, Delem A. Inactivated hepatitis A vaccine: long-term antibody persistence. Vaccine. 1997;15(6–7):612–15. PMID: 9178459. doi:10.1016/s0264-410x(96)00242-3.
- Loutan L, Bovier P, Althaus B, Glück R. Persistence of antibody to hepatitis A virus 10 years after vaccination among children and adults. Lancet. 1994 5;343(8893):322–24. PMID: 7905144. doi:10.1016/s0140-6736(94)91162-2.
- Hens N, Habteab Ghebretinsae A, Hardt K, Van Damme P, Van Herck K. Model based estimates of long-term persistence of inactivated hepatitis A vaccine-induced antibodies in adults. Vaccine. 2014;32(13):1507–13. PMID: 24508042. doi:10.1016/j.vaccine.2013.10.088.
- DeSouza CM, Legedza AT, Sankoh AJ. An overview of practical approaches for handling missing data in clinical trials. J Biopharm Stat. 2009;19(6):1055–73. PMID: 20183464. doi:10.1080/10543400903242795.
- Siddiqui O. MMRM versus MI in dealing with missing data-A comparison based on 25 NDA data sets. J Biopharm Stat. 2011;21(3):423–36. PMID: 21442517. doi:10.1080/10543401003777995.
- Hammitt LL, Bulkow L, Hennessy TW, Zanis C, Snowball M, Williams JL, Bell BP, McMahon BJ. Persistence of antibody to hepatitis A virus 10 years after vaccination among children and adults. J Infect Dis. 2008 15;198(12):1776–82. PMID: 18976095. doi:10.1086/593335.