ABSTRACT
In July 2017, the Japanese Association for Infectious Diseases issued guidance for the administration of the PPSV23 revaccination. Despite increasing recognition of its protective benefits, levels of PPSV23 revaccination coverage rate in Japanese elderly population are unclear at present. Here, we report the results of a survey to know PPSV23 revaccination rates among elderly patients aged 65 and older. We asked an array of questions related to PPSV23 revaccination to Elderly adults and doctors across Japan via Web-based surveys in June 2018. The sampled population consisted of 5,085 men and women aged 65 and older. The PPSV23 revaccination coverage rate was estimated by survey questions regarded vaccination counts, intervals, and vaccine type. In addition, 400 internal medicine physicians were surveyed and asked about their reasons for recommending PPSV23 revaccination to elderly patients. In total, 1,648 elderly adults had received at least one PPSV23 dose; of these, 58 had received it at least twice (revaccination coverage rate: 3.5%). The most commonly cited justification for revaccination with PPSV23 among the surveyed physicians was that the benefits of revaccination exceed the risks of revaccination. In addition, multivariate analysis showed revaccinated status was most strongly associated with recommendations from peers (e.g. spouse, family, friends) among elderly subjects. This study reports PPSV23 revaccination coverage rate among Japanese adults aged 65 and older for the first time and concludes that the coverage rate is very low.
Introduction
Pneumonia accounts for a large percentage of deaths among elderly adults in Japan, with mortality rates spiking in the aged 65 and older population.Citation1 Streptococcus pneumoniae is the most common causative organism, a bacterium that possesses a polysaccharide capsule. Over 90 capsular serotypes have been identified to date,Citation2 the highly pathogenic among which are responsible for invasive diseases such as pneumonia or bacteremia all over the world.Citation3 Citation4–Citation5
Two types of pneumococcal vaccines containing capsular polysaccharides are available for adults in Japan today. One is the 23-valent capsular polysaccharide pneumococcal vaccine, PPSV23 (Pneumovax®NP Merck & Co., Inc., Kenilworth, NJ, USA), which consists of capsular polysaccharides from 23 serotypes of S. pneumoniae and was approved in 1988 for individuals aged 2 or older, including the elderly, at high risk of serious illness due to pneumococcal infection. Another one is the 13-valent pneumococcal conjugate vaccine, PCV13 (Prevenar13® Pfizer Inc., New York, USA), a pneumococcal conjugate vaccine consisting of 13 capsular polysaccharides covalently bound to detoxified diphtheria toxins. In 2013, PCV13 was approved for children between 2 months and 6 years old and introduced into Japanese routine immunization schedule; the following year (2014), it was approved for adults aged 65 and older. In October 2014, PPSV23 was introduced into Japanese routine immunization schedule. It was recommended for adults aged 65 and older, and for adults aged 60–64 years with cardiac, renal, or respiratory dysfunction equivalent to Level 1 physical disability, and public subsidies were introduced at the same time to partially cover vaccination costs.Citation6
While initially high, pneumococcus-specific antibody titers gradually decrease over time following PPSV23 vaccination. This decline would be occurred more rapidly in the elderly and persons with underlying medical conditions than in healthy adults.Citation7,Citation8 PPSV23 revaccination is widely regarded as an effective means to keep their concentrations high in elderly adults. Hammit et al., Jackson et al. and Remschmidt et al. in the United States have shown that PPSV23 revaccination with a minimum interval of five years between doses can raise pneumococcus-specific antibody titers.Citation9 Citation10–Citation11 In 1997, the U.S. Advisory Committee on Immunization Practices (ACIP) officially recommended that adults who received PPSV23 before 65 years of age get revaccinated five years after their first dose.Citation12
In light of the strong evidence from those studies, the Japanese Association for Infectious Diseases (JAID) established guidelines for pneumococcal revaccination in 2009, encouraged by its potential efficacy in preventing pneumococcal disease in the elderly.Citation13 The same year, together with the Japanese Society of Chemotherapy (JSC), Japanese Respiratory Society (JRS), Japanese Society for Infection Prevention and Control (JSIPC) and JAID submitted a joint request to revise PPSV23’s package insert to permit revaccination, which was granted in October, 2009. Ohshima et al. and Kawakami et al. published the data on the safety and immunogenicity of PPSV23 revaccination in Japanese elderly populations subsequently,Citation14,Citation15 leading the JAID to revise its own revaccination guidelines in July 2017.Citation16 Under the updated guidance, adults who received their initial PPSV23 dose at least five years prior are eligible for revaccination. The PPSV23 revaccination is considered safe and tolerable – local adverse reactions at the injection site are common, but acceptably mild – and induces comparable immunogenicity to levels seen after a prime dose. The accumulated evidence supporting PPSV23 revaccination in adults has convinced most clinicians and elderly of its benefits; this was further reinforced by recent revisions to the vaccine’s package insert and academic societies’ guidance. However, we lack a complete picture of the actual revaccination coverage rate in the Japanese elderly population.
Clinical data on PPSV23 coverage rate, especially revaccination rates, are essential to informed discussions about its cost-effectiveness as a public health measure, and to establish further evidence for its efficacy. In addition, identifying specific factors that encourage or predict PPSV23 revaccination could provide clues to expand the practice’s implementation.
In this paper, we report the findings of a survey investigation to determine PPSV23 revaccination rates among Japanese adults aged 65 and older, along with doctors’ rationales for recommending the practice to elderly patients.
Material & methods
Survey overview
This cross-sectional survey was conducted in June 2018 via Macromill Carenet, a web-based survey company. Separate questionnaires were given to adults 65 and older (“elderly”) and internal medicine physicians (“doctors”) registered in online survey panels managed by the company. Both groups completed the surveys by inputting and clicking relevant responses in an online form via browser.
Elderly survey
The elderly survey was sent to approximately 140,000 Japanese men and women aged 65 and older located all over the country and administered 5,000 who were able to complete the internet survey. Respondents were selected according to age and sex to ensure their ratios in the sample matched 2018 estimates for the Japanese population aged 65 and older. Eligible adults provided consent electronically. The survey asked questions about chronic medical conditions; pneumonia history (yes/no); pneumococcal vaccination history (yes/no), pneumococcal vaccine type (PPSV23/PCV13/Unknown), number of vaccination count(s), and vaccination interval(s) (5 years or more); whether they had been recommended the vaccine by their doctor; whether they had received a vaccine subsidy or notification from their local government; their reasons for getting revaccinated; general knowledge about pneumonia and immunization; influenza vaccination history; and background characteristics (height, weight, and smoking, drinking, and exercise habits).
Outcomes assessed were PPSV23 revaccination coverage rate (primary), first-dose coverage rate, and the interval between primary vaccination and revaccination. Their reasons for getting the revaccination were also analyzed. PPSV23 revaccination odds ratios (ORs) for specific reasons were also calculated.
Doctor survey
The doctor survey was sent to approximately 100,000 Japanese internal medical doctors located all over the country. Four hundred doctors providing care to at least 100 elderly adults (aged 65 and older) per month were enrolled from a nationwide panel to complete the internet survey. Eligible doctors provided consent electronically. Questionnaire assessed whether they perform pneumococcal and influenza vaccinations (2017/2018), the number of pneumococcal vaccines given the previous year, and their reasons for recommending the pneumococcal revaccination to elderly patients.
Statistical analysis
PPSV23 revaccination coverage rate was calculated as the ratio of respondents who had received two or more doses to those who had received only the primary dose. PPSV23 revaccination status was coded for respondents who both affirmed receiving two or more pneumococcal vaccines.
PPSV23 revaccination ORs for specific reasons were calculated as follows: crude ORs and corresponding 95% Confidential Intervals (CIs) were calculated for major revaccination reasons to determine their predictive value for PPSV23-revaccinated status. Reasons identified as significantly associated with PPSV23 revaccination were further explored using multivariate logistic regression, with model selection using the stepwise method, and adjusted odds ratio (AOR) calculated accordingly. Doctors’ reasons for recommending pneumococcal revaccination to elderly patients were analyzed using descriptive statistics. IBM SPSS Statistics 24.0 was used for all analyzes.
Ethics approval
This study was approved by the ethics review board of the Medical Corporation Toukeikai Kitamachi Clinic (June 19, 2018), and registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000033095).
Results
Patients’ demographics and health characteristics
shows the influence of several factors on PPSV23 vaccination and revaccination rates among elderly respondents. Data was analyzed for 5,085 consenting eligible adults who responded to the elderly survey. In total, 1,648 received PPSV23 at least once (mean age: 74.0), while 3,437 have never received it (mean age: 73.7). High health consciousness was evident in the former group, characterized by higher rates of nonsmoking, regular exercise, and influenza vaccinations. Interestingly, history of pneumonia and current underlying medical conditions were more prevalent in this group as well.
Table 1. Demographics and health characteristics of Japanese adults aged 65 and older respondents who vaccinated and unvaccinated the pneumococcal vaccine (n = 5,085).
Fifty-eight of the 1,648 with history of PPSV23 vaccination were vaccinated with PPSV23 at least twice (mean age: 78.2). Revaccinated respondents were significantly more likely to have gotten the influenza vaccine (p < .0001), a recent history of pneumonia at aged ≥50 (p = .0007), and low BMI (<20) (p = .0026).
Noting that many respondents could not identify the type of pneumococcal vaccine they received, we also calculated nonspe-cific coverage rate among chronic patients for each age group for reference: 2,498 respondents had received a pneumococcal vaccine of any type at least once (PPSV23: 1,648, PCV13: 35, unknown: 815), and 320 received pneumococcal vaccines at least twice (PPSV23: 58, PCV13: 18, unknown [second or later dose]: 244).
Revaccination coverage rates among elderly
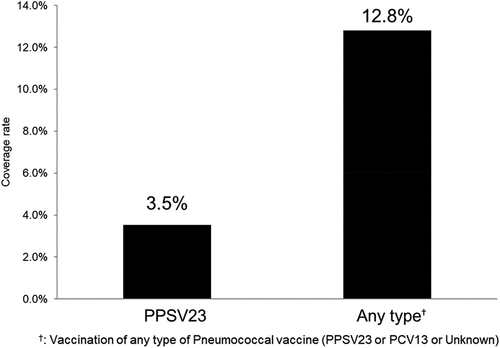
shows the PPSV23 revaccination coverage rate among elderly respondents. shows age-specific (five-year strata: 65–69, 70–74, 75–79, 80–84, 85 and older) coverage rate of PPSV23 vaccination, revaccination, and second-revaccination. The revaccination (≥2 vs ≥1) coverage rate was 3.5% among vaccinated cases, with 58/1,648 receiving the PPSV23 revaccination. The third vaccination (≥3 vs ≥2) rate was 17.2% among revaccinated cases, with 10/58 reporting a third PPSV23 vaccination.
Table 2. PPSV23 coverage rates in Japanese adults aged 65 and older.
Figure 1. Pneumococcal revaccination coverage rates in Japanese adults aged 65 and older (n = 5,085). Any type: vaccination of any type of pneumococcal vaccine (PPSV23 of PCV13 or Unknown).
Revaccination coverage rate was lowest among respondents aged 65–69 (0.7%) and highest among those aged 80–84 (7.4%).
Additionally, nonspecific revaccination coverage rate, including both PCV13 and “unknown” responses, was 12.8%.
Timing for PPSV23 revaccination
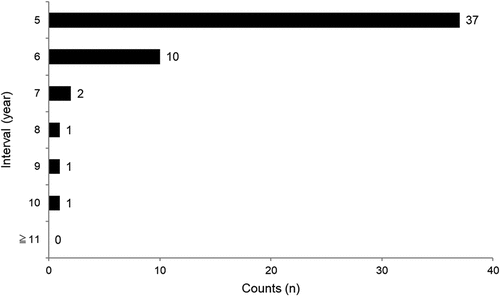
shows the vaccination intervals for PPSV23-revaccinated respondents. Definite intervals were reported by 52/58 revaccinated: the median interval was 5 years (range: 5–10). This was also the most common interval (n = 37/52, 71.1%), followed by 6 years (n = 10/52, 19.2%).
Patients’ reasons for getting PPSV23 revaccination
shows the results of multivariate logistic regression analysis to identify some reasons for affecting the behaviors of PPSV23 revaccination in elderly. A peer recommendations (e.g. from spouse, family, or friends) were most associated with revaccination status (AOR: 38.90), followed by “having knowledge about pneumonia or pneumococcal vaccination” (AOR: 35.76) and “doctors’ recommendation” (AOR: 11.69).
Table 3. Factors associated with PPSV23 revaccination.
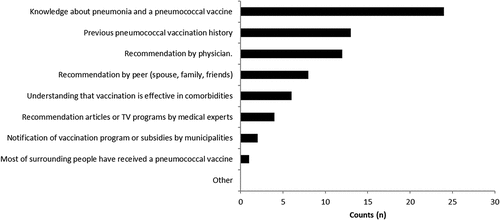
shows patients’ reasons for getting the PPSV23 revaccination. Respondents were allowed to give multiple answers. Elderly adults most often cited “knowledge about pneumonia or pneumococcal vaccination”, followed by “previous pneumococcal vaccination history”, and “doctors’ recommendation” as reasons for revaccination. Relatively few mentioned municipal subsidies/notifications, or high rates of revaccination among their peers.
Doctors’ reasons for recommending PPSV23 revaccination
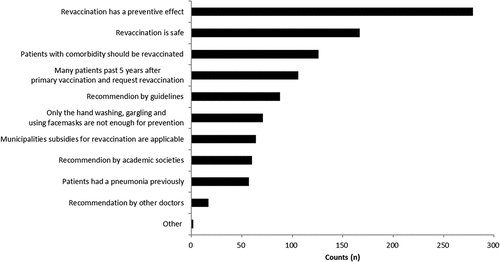
shows doctors’ reasons for recommending pneumococcal revaccination. Most doctors who had given a pneumococcal vaccine before recommended it (354/399: 88.7%). They most often cited “the benefits of the vaccine outweighing its risks”, followed by their “beliefs in the safety of revaccination”, and that “elderly adults with an underlying illness should get revaccinated” as reasons for recommending revaccination.
Discussion
In this paper, we describe the findings of an internet survey aimed to assess PPSV23 revaccination rates and intervals among elderly Japanese adults aged 65 and older, as well as their rationales for pursuing revaccination, and practicing doctors’ reasons for recommending it.
To our knowledge, this is the first report to describe pneumococcal revaccination rates among the Japanese elderly population based on the results of a nationwide survey. Our analysis of first-time and repeat PPSV23 rates did not include “unknown” responses, indicating uncertainty whether the vaccine received was PPSV23 or PCV13. According to these strict parameters, we calculated PPSV23 revaccination coverage rate of 3.5%; however, the nonspecific pneumococcal vaccination coverage rate reached 12.8%. The timing of many immunizations suggested that many uncertain respondents likely received PPSV23 twice, which the revaccination coverage rate in our study would be underestimated. Some cases of second and third vaccination in the 65–69 age group can be shown. It would be speculated that these people had been vaccinated under aged 65, because the patients aged 2 years and older who are at high risk of serious pneumococcal infectious disease can receive the PPSV23 in Japan. Alternatively, our low revaccination coverage rate could be explained by a low baseline coverage rate for the first dose: Naito et al. collected data on cumulative PPSV23 vaccination coverage rates among Japanese adults aged 65 and older: they estimated that only approximately 15% of this population had received PPSV23 by 2013.Citation17 Recently, Shono et al. reported high primary PPSV23 vaccination coverage rates for elderly adults eligible based on Japanese routine immunization program schedule, based on a 2015 national survey of pneumococcal vaccine coverage rate.Citation18 Those eligible for revaccination will only grow in number in 2019 and beyond, with the need for revaccination driving rates even higher.
The results of revaccination intervals suggest that most revaccinated adults got their next dose quite soon after becoming eligible within 5–6 years. Local governments do not send their residents personal reminders about PPSV23 revaccination, suggesting that most revaccinated respondents chose the timing of their next vaccine independently, based on the official standard of a minimum of 5 years between pneumococcal doses. This hypothesis is supported by the high number of respondents who cited having heard “knowledge about pneumonia or pneumococcal vaccination” as motivation for getting PPSV23 revaccination. It would not be cleared that how degree the dosing timing/interval of PPSV23 is related to its effectiveness for preventing pneumonia. However, one British study did find the length of time after PPSV23 vaccination to be associated with pneumococcal pneumonia incidence in an elderly care home: affected residents developed the disease a median of 10.2 years (range: 7.3–17.9) after their last PPSV23 dose, compared with 7.2 years (range: 6.8–12.8) in unaffected residents.Citation19 Moreover, both Kawakami et al.Citation14,Citation20 and Ohshima et al.Citation15 reported persistent immunogenicity following PPSV23 vaccination, lasting approximately seven years, but revaccination can induce comparable immunogenicity to the initial dose. Suzuki et al. also reported that the efficacy of PPSV23 declines beyond 5 years after primary vaccination.Citation21 Thus, encouraging adults to get it soon after becoming eligible should go a long way toward preventing pneumonia.
No studies to date have reported on factors that influence pneumococcal revaccination in the elderly. Sakamoto et al. surveyed elderly club members for vaccination behaviors and primary vaccination rates for the pneumococcal vaccines. They observed vaccinated status to be strongly associated with recommendations from medical professionals, history of influenza immunization, and perceptions of pneumonia severity.Citation22 In overseas reports, one Spanish study found that elderly were encouraged to get preventive vaccinations by recommendations from medical professionals and their peers.Citation23 Another study in an urban area in China reported that elderly residents who were knowledgeable about pneumonia risks and the safety of vaccines were more likely to actively seek PPSV23 vaccination.Citation24 While PPSV23 revaccination is predicted by similar factors as those that motivate the initial dose, it was probably driven more by proactive health attitudes and behaviors in this cohort than medical advice per se. Our revaccinated survey respondents would have received their first dose before revaccination’s official introduction into Japanese routine immunization schedule in 2014. This trend was likely reinforced by doctors’ passive stance toward recommending the revaccination: i.e. mostly knowledgeable patients who inquired received it
Doctors who recommended revaccination most commonly cited the benefits of pneumococcal vaccines exceeding their risks as justification. Due to concerns about adverse reactions, PPSV23 revaccination used to be explicitly discouraged in Japanese official immunization schedule until 2009. In a 2008 survey of JRS and JSIPC officials, however, Oishi et al. found that while most doctors perceived a need for subsequent doses, decisions to revaccinate were usually driven by the patient own request.Citation25 Our survey conducted nearly a decade after the 2008 was conducted, similarly found that most doctors understood the PPSV23 revaccination’s importance, recommending it when curious patients inquired. However, this ‘reactive’ approach was more prevalent than proactively recommending PPSV23 revaccination to eligible patients. Perhaps safety concerns reduce doctors’ willingness to initiate these conversations, an inference supported by the fact that only half of our respondents explicitly cited revaccination’s safety as a justification for recommending it.
Our study has several limitations. Firstly, the elderly subjects were enrolled using stratified sampling by age and gender, to ensure our sample would be statistically representative of Japanese aged 65 and older population. We suspected that ‘adults aged 80 and older might have more difficulty participating in an internet survey; however, we were pleasantly surprised to see that each age stratum in our dataset was close to its ideal size, with plenty of very-old participants (noting, however, that few respondents were 85+). Nonetheless, our methodology still might be a source of bias, since very-old adults capable of using the internet may be healthier overall. Secondly, we were concerned about the inherent bias in surveying only registrants in web-based panels. We ensured statistical representativeness by enrolling eligible elderly and doctors from all over Japan and using stratified sampling to ensure the age and sex ratios among the elderly set would closely approximate corresponding estimates for the general population. Our strategy seems validated by the close concordance between our observed PPSV23 coverage rate (32.4% among adults aged 65 and older) and past data published by the Japanese Ministry of Health, Labor and Welfare (33.5% and 37.8% among eligible adults in 2015 and in 2016, respectively).Citation26 It seems fair to assume that our estimates essentially reflect real coverage rate among Japanese elderly population nationwide. Finally, all analyzed data was self-reported, raising doubts about the reliability of our information on identification of pneumococcal vaccine types (PPSV23 or PCV13) and dosing intervals. Self-reporting is an inherent weakness of internet surveys. Most of elderly people might not accurately recognize the difference of two type of pneumococcal vaccines. Therefore, we designed the survey items carefully and specifically to improve its reliability, for example by explaining in details the setting of routine and voluntary vaccination. However, inadequate data may have affected our investigation given the high number of “unknown” responses for vaccine type, not to mention poor memory among respondents. It should be better to confirm the types of pneumococcal vaccines by any specialists. Thus, while we were able to obtain a value for PPSV23 revaccination coverage rate, this estimate may have been biased by widespread uncertainty among subjects about the type of vaccine received. Accurately identifying pneumococcal vaccines types is a future challenge that needs to be addressed to ensure proper timing of revaccination doses among eligible adults. Japanese status as a super-aging society means that many eligible adults will need not only a second dose of pneumococcal vaccine, but also third and fourth doses every five years. Therefore, it should be monitored continuously the coverage rate and predictive factors for behaviors of revaccination to ensure prevention for pneumococcal pneumonia. In addition, for proceeding like this monitoring smoothly, it will need to establish more accurate vaccination record-keeping system in Japan that does not only depend on respondents’ memory recall about the related information.
In this study, we can estimate that at least 10% or more of elderly people have already been revaccinated with any type of pneumococcal vaccine. Therefore, it is necessary for establishing a public health foundation that a lot of elderly people can understand and accept recommendations of pneumococcal revaccination from doctors through further education on disease awareness raising and prevention to general elderly. It is important to create an awareness of pneumococcal revaccination, because pneumococcal pneumonia incidence and mortality among vaccinated person would be associated with time since the initial dose was administered.Citation19
Conclusion
This study reports PPSV23 revaccination coverage rate among Japanese elderly adults aged 65 and older for the first time. While the healthcare professionals and elderly adults also began to recognize importance of pneumococcal revaccination in currently, our data suggest that the its coverage rate is still very low levels. Creating awareness about revaccination’s importance among the elderly population are essential steps toward preventing pneumococcal infection and improve public health in the future.
Disclosure of potential conflicts of interest
In conjunction with the external investigators, this study was designed, executed, and analysed by the sponsor. The sponsor formally reviewed a penultimate draft. All co-authors approved the final version of the manuscript. KK received payment for lectures from MSD K.K. AN, AW, TI are employees of MSD K.K., Tokyo, Japan, a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. TF is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Employees may hold stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
Acknowledgments
The authors thank, Aya Yano, Tomoko Mizuno, Megumi Yoshinaga, Xiaoqin Yang, Kelly D. Johnson, Shinji Hayashi and Shin Sasaki for contributing to the development of the study concept, protocol and/or manuscript.
Additional information
Funding
References
- Morimoto K, Suzuki M, Ishifuji T, Yaegashi M, Asoh N, Hamashige N, Abe M, Aoshima M, Ariyoshi K. Adult Pneumonia Study Group-Japan (APSG-J). The burden and etiology of community-onset pneumonia in the aging Japanese population: a multicenter prospective study. PLoS One. 2015;10:e0122247. doi:10.1371/journal.pone.0122247. PMID: 25822890.
- Jauneikaite E, Tocheva AS, Jefferies JM, Gladstone RA, Faust SN, Christodoulides M, Hibberd ML, Clarke SC. Current methods for capsular typing of Streptococcus pneumoniae. J Microbiol Methods. 2015;113:41–49. doi:10.1016/j.mimet.2015.03.006. PMID: 25819558.
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67:71–79. doi:10.1136/thx.2009.129502. PMID: 20729232.
- Peto L, Nadjm B, Horby P, Ngan TT, van Doorn R, Van Kinh N, Wertheim HF, Peto L, Nadjm B, Horby P, et al. The bacterial aetiology of adult community-acquired pneumonia in Asia: a systematic review. T Roy Soc Trop Med H. 2014;108:326–37. doi:10.1093/trstmh/tru058. PMID: 24781376.
- Jain S, Self WH, Wunderink RG. CDC EPIC study team. Community-acquired pneumonia requiring hospitalization among US adults. New Engl J Med. 2015;373:415–27. doi:10.1056/NEJMoa1500245. PMID: 26172429.
- Ministry of health, labour and welfare. Pneumococcal infection (elderly person). [accessed 2019 Aug 9]. http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/haienkyukin/index_1.html.
- Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, Gomez-Bertomeu F, Figuerola-Massana E, Raga-Luria X. Hospital-Guardiola. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged ≥ 60 years: 3 years of follow-up in the CAPAMIS study. Clin Infect Dis. 2014;58:909–17. doi:10.1080/21645515.2016.1210744. PMID: 27454779.
- Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30:6802–08. doi:10.1016/j.vaccine.2012.09.019. PMID: 23000122.
- Hammitt LL, Bulkow LR, Singleton RJ, Nuorti JP, Hummel KB, Miernyk KM, Zanis C, Whaley M, Romero-Steiner S, Butler JC, et al. Repeat revaccination with 23-valent pneumococcal polysaccharide vaccine among adults aged 55-74 years living in Alaska: no evidence of hyporesponsiveness. Vaccine. 2011;29:2287–95. doi:10.1016/j.vaccine.2011.01.029. PMID: 21255685.
- Jackson LA, Benson P, Sneller VP, Butler JC, Thompson RS, Chen RT, Lewis LS, Carlone G, DeStefano F, Holder P, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA. 1999;281:243–48. doi:10.1001/jama.281.3.243. PMID: 9918479.
- Remschmidt C, Harder T, Wichmann O, Bogdan C, Falkenhorst G. Effectiveness, immunogenicity and safety of 23-valent pneumococcal polysaccharide vaccine revaccinations in the elderly: a systematic review. BMC Infect Dis. 2016;16:711. doi:10.1186/s12879-016-2040-y. PMID: 27887596.
- Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB. Advisory committee on immunization practices recommended immunization schedule for adults Aged 19 years or older - United States, 2017. Mmwr-Morbid Mortal W. 2017;66:136–38. doi:10.15585/mmwr.mm6605e2. PMID: 28182599.
- The Japanese Association for Infectious Diseases (JAID). Guideline for revaccination of pneumococcal vaccine. [accessed 2019 Aug 9]. http://www.kansensho.or.jp/uploads/files/guidelines/pneumococcus_vaccine.pdf.
- Kawakami K, Kishino H, Kanazu S, Toshimizu N, Takahashi K, Sterling T, Wang M, Musey L. Revaccination with 23-valent pneumococcal polysaccharide vaccine in the Japanese elderly is well tolerated and elicits immune responses. Vaccine. 2016;34:3875–81. doi:10.1016/j.vaccine.2016.05.052. PMID:27265450.
- Ohshima N, Nagai H, Matsui H, Akashi S, Makino T, Akeda Y, Oishi K. Sustained functional serotype-specific antibody after primary and secondary vaccinations with a pneumococcal polysaccharide vaccine in elderly patients with chronic lung disease. Vaccine. 2014;32:1181–86. doi:10.1016/j.vaccine.2013.09.060. PMID: 24120483.
- The Japanese Association for Infectious Diseases (JAID). Guidance for revaccination of pneumococcal vaccine (revised edition). J Jpn Assoc Infect Dis. 2017;91(4):543–52. [accessed 2019 Aug 9]. http://www.kansensho.or.jp/uploads/files/guidelines/pneumococcus_vaccine_re_1707.pdf.
- Naito T, Yokokawa H, Watanabe A. Impact of the national routine vaccination program on 23-valent pneumococcal polysaccharide vaccine vaccination rates in elderly persons in Japan. J Infect Chemother. 2018;24:496–98. doi:10.1016/j.jiac.2018.01.004. PMID: 29398479.
- Shono A, Hoshi SL, Kondo M. The impact on vaccination coverage rate following introduction of a routine pneumococcal vaccination programme for the elderly in Japan. Vaccine. 2018;36:5886–90. doi:10.1016/j.vaccine.2018.08.023. PMID: 30143271.
- Thomas HL, Gajraj R, Slack MP, Sheppard C, Hawkey P, Gossain S, Drew CM, Pebody RG. An explosive outbreak of Streptococcus pneumoniae serotype-8 infection in a highly vaccinated residential care home, England, summer 2012. Epidemiol Infect. 2015;143:1957–63. doi:10.1017/S0950268814002490. PMID: 25298247.
- Kawakami K, Kishino H, Kanazu S, Takahashi K, Iino T, Sawata M, Musey L. Time interval of revaccination with 23-valent pneumococcal polysaccharide vaccine more than 5 years does not affect the immunogenicity and safety in the Japanese elderly. Hum Vacc Immunother. 2018;14:1931–38. doi:10.1080/21645515.2018.1456611. PMID: 29580133.
- Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, Asoh N, Ishida M, Hamaguchi S, Aoshima M, Ariyoshi K, et al. Adult Pneumonia Study Group-Japan (APSG-J). Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17:313–21. doi:10.1016/S1473-3099(17)30049-X. PMID: 28126327.
- Sakamoto A, Chanyasanha C, Sujirarat D, Matsumoto N, Nakazato M. Factors associated with pneumococcal vaccination in elderly people: a cross-sectional study among elderly club members in Miyakonojo City, Japan. BMC Public Health. 2018;18:1172. doi:10.1186/s12889-018-6080-7. PMID: 30314498.
- Vila-Corcoles A, Ochoa-Gondar O, Hospital I, de Diego C, Satué E, Bladé J, Ansa X, Guzmán JA, Salsench E, Ramos F. Pneumococcal vaccination coverage rates among low-, intermediate-, and high-risk adults in Catalonia. Hum Vacc Immunother. 2016;12:2953–58. doi:10.1080/21645515.2016.1210744. PMID: 27454779.
- Liu SJ, Xu EP, Liu Y, Xu Y, Wang J, Du J, Zhang X, Che X, Gu W. Factors associated with pneumococcal vaccination among an urban elderly population in China. Hum Vacc Immunother. 2014;10:2994–99. doi:10.4161/21645515.2014.972155. PMID: 25483646.
- Oishi K, Kawakami K, Nagai H, Sunakawa K, Watanabe A. [A questionnaire study on the necessity of approval for revaccination of the pneumococcal polysaccharide vaccine]. Nihon Kokyuki Gakkai Zasshi. 2010;48:5–9. PMID: 20163014.
- Ministry of Health, Labour and Welfare. Coverage rates of national immunization program published by Japanese ministry of health, labour and welfare. [accessed 2019 Aug 9]. https://www.mhlw.go.jp/topics/bcg/other/5.html.