ABSTRACT
An observational study to assess immunogenicity before and after the first, second, and third vaccinations with the 23-valent pneumococcal polysaccharide vaccine in a cohort of 16 elderly patients with chronic lung diseases was conducted. The safety of this vaccine was also compared between the first, second, and third vaccinations. Serotype-specific immunoglobulin G (IgG) and the opsonization index (OI) for serotypes 6B, 14, 19F, and 23F were analyzed, and adverse local and systemic reactions were compared. The levels of serotype-specific IgG and OI increased significantly 1 month after the first, second, and third vaccinations. Peak IgG levels were higher after the third vaccination than after the second vaccination, but the levels of serotypes 6B, 14, and 19F were not higher than after the first vaccination. Serotype-specific OIs did not differ after the third vaccination compared with the first and second vaccinations. The level of serotype-specific IgG required for killing 50% of bacteria decreased significantly 1 month after the second vaccination. This level was slightly elevated immediately before the third vaccination but decreased after the third vaccination. Although self-limited local and systemic reactions were more frequent after the second and third vaccinations than after the first vaccination, no serious systemic reactions were seen after any vaccination. These data suggest that sustained functional serotype-specific IgG is produced after the first, second, and third vaccinations and they confirm the safety of the second and third vaccinations in elderly people with chronic lung disease.
Introduction
Despite current advances in medicine, Streptococcus pneumoniae continues to be a major human pathogen that causes infectious diseases in adults worldwide.Citation1 Pneumonia is still one of the most common causes of death in adults, and S. pneumoniae is the leading causative agent of pneumonia in Japan.Citation2
As a result of introduction of 13-valent pneumococcal conjugate vaccine (PCV13) in the national immunization program among children in Japan, the current vaccination rate of the PCV13 is as high as 95% among children aged 5 years and younger.Citation3 Consequently, the incidence of invasive pneumococcal disease (IPD) caused by the S. pneumoniae serotypes included in PCV13 is low in both children and adults, and there is a decreasing trend in the prevalence of penicillin-resistant pneumococcal strains.Citation4 In some other countries, implementation of the seven-valent pneumococcal conjugate vaccine (PCV7) and PCV13 in children has reportedly induced serotype replacement, leading to an elevated incidence of IPD caused by the non-vaccine serotype 12F.Citation5,Citation6 Recently, an increase in the incidence of IPD caused by serotype 12F among adults was reported in Japan.Citation7 Because serotype 12F is included in the 23-valent pneumococcal polysaccharide vaccine (PPSV23), this vaccine is highly effective against serotype 12F IPD.Citation8,Citation9
The PPSV23 comprises a mixture of capsular polysaccharides from 23 serotypes of S. pneumoniae. Several epidemiological and clinical studies have shown that this vaccine is particularly effective in preventing IPD.Citation10,Citation11 A recent study of community-onset pneumonia in adults aged 65 years or older demonstrated that the effectiveness of PPSV23 was 27.4% against all pneumococcal pneumonia, and 33.5% against PPSV23 serotypes using the test-negative design.Citation12 Given these observations, improving the coverage rate of PPSV23 in high-risk populations (such as the elderly and patients with respiratory disorders) should now be a high priority.
In recent years, Japan has become a “super-aged” society, and a growing number of people who received their first dose of pneumococcal vaccine 5 or more years ago have been requesting a second dose. The practice of revaccination with pneumococcal polysaccharide vaccine has also gained support in many other countries following the publication of Jackson et al.Citation13 Although local reactions occur more frequently following revaccination compared with the first vaccination, these reactions resolve within 3 days, and revaccination is not associated with serious adverse reactions. Previously, we obtained similar results demonstrating that a second vaccination with PPSV23 is safe and immunogenic in Japanese patients with chronic lung disease (CLD).Citation14
As the demand for a second dose of pneumococcal vaccine increases, an increase in the number of people requesting a third dose 5 or more years after their second vaccination is expected. Several studies have shown that repeated doses of pneumococcal vaccine are immunogenic and well tolerated.Citation15,Citation16 However, no research to date has assessed the clinical course through their first, second, and third immunizations with this vaccine in the same elderly patients with chronic respiratory diseases.
In this study, we assessed the immunogenicity and safety of repeated doses of PPSV23 by following the same group of patients over time, from their first to third vaccination.
Materials and methods
Study participants
Between October 2001 and November 2002, 151 patients with CLD who were aged 65 years or older received their first vaccination with PPSV23 at our outpatient clinic. Serum samples from these patients had been acquired before and 1 month after the first vaccination and had been preserved for antibody titer analyzes.Citation17 Forty of the 151 patients were enrolled between September 2009 and January 2010 in a study to investigate the safety and immunogenicity of the second vaccination with PPSV23.Citation14
Of these 40 patients, 24 patients died or were lost to follow-up at our outpatient clinic by November 2014, giving 16 patients who were enrolled between December 2014 and February 2015 in our study of the safety and immunogenicity of the third vaccination with PPSV23.
All patients provided written, informed consent. This study was reviewed and approved by the ethics committee of the National Hospital Organization, Tokyo National Hospital, and was conducted according to the principles expressed in the Declaration of Helsinki.
Samples
Blood samples were drawn from 16 study participants before and 1 month after the third vaccination with PPSV23. Serum samples were separated by centrifugation and stored at – 80°C until used. The levels of serotype-specific immunoglobulin G (IgG) and the OI were measured in the serum samples obtained before and 1 month after the first vaccination, before and 1 month after the second vaccination, and before and 1 month after the third vaccination.
Enzyme-Linked Immunosorbent Assay (ELISA)
The concentrations of antipneumococcal IgG antibodies were measured using the World Health Organization (WHO)-approved ELISA methodology with standard reference serum (89-SF or 007sp) and C-polysaccharide and 22F polysaccharide absorption, as previously described.Citation18,Citation19 The concentrations of serotype-specific IgG for the four serotypes, 6B, 14, 19F, and 23F, were measured according to the WHO protocol. A detailed protocol is available at https://www.vaccine.uab.edu/uploads/mdocs/ELISAProtocol(007sp).pdf
Multiplexed Opsonophagocytic Killing Assay (MOPA)
A MOPA for the four serotypes based on antibiotic-resistant target bacteria was performed at the Research Institute for Microbial Diseases, Osaka University, as previously described.Citation20 The quality control serum was prepared from the pooled sera of adults vaccinated with PPSV23 and was used in each assay. The OI was defined as the serum dilution that killed 50% of bacteria, and the OI was determined for the four serotypes 6B, 14, 19F and 23F using opsotiter3 software (www.vaccine.uab.edu/UAB-MOPA) according to the WHO protocol. The functional activity of serotype-specific IgG was expressed as the concentration of IgG required for 50% killing of the pneumococcal strain by dividing the IgG concentration of a test sample by the OI. The serotype-specific IgG required for 50% killing was applicable to adult subjects as previously published.Citation14,Citation21 A lower serotype-specific IgG required for 50% killing indicates a better functional antibody.
The serotype-specific IgG and the opsonization index (OI) were determined for serotypes 6B,14, 19F, and 23F, because S. pneumoniae with these serotypes were prevalent as the causative agents of community-acquired pneumonia when the study patients received the first vaccination with PPSV23 between October 2001 and November 2002.Citation22
Adverse reactions
Participants were provided a diary to record their temperature and any local or systemic reactions that occurred from the day of the third vaccination to day 14 after this vaccination. They were instructed to assess the maximal diameter of any redness or swelling at the site of injection. This was classified as mild for diameters ≥ 1 cm and < 5 cm, as moderate for diameters ≥ 5 cm and < 10 cm, and as severe for diameters ≥ 10 cm. A systemic symptom was considered mild when the participant felt a certain symptom but had no difficulties in daily life. A physical examination with an interview was conducted to record the participant’s condition on the day of the third vaccination and 14 days later. The attending physician assessed the presence of adverse reactions based on daily records submitted by the patient. Data for adverse reactions after the third vaccination were compared with those after the first and second vaccinations.Citation14,Citation17
Statistical analysis
Average antibody concentrations and increases are expressed as geometric means. Differences in the geometric mean concentrations (GMCs) of serotype-specific IgG, OIs, or IgG concentration required for 50% killing were assessed using the Wilcoxon matched-pairs signed-rank test. The relationships between GMCs before the third vaccination and local adverse reactions after the third vaccination were compared using the Mann–Whitney U test. The frequencies of adverse reactions were compared between the first, second, and third vaccinations using Student’s t test. Differences with a P value < .05 were considered to be significant.
Results
Patient characteristics
The group of patients comprised 7 men and 9 women whose mean age was 80 years; 10 participants were in their 70s and 6 were in their 80s. The mean interval between the second and third vaccinations was 5 years and 3 months. Their comorbid illnesses included pulmonary tuberculosis sequelae (44%), bronchial asthma (19%), bronchiectasis (13%), and other conditions (24%). Six patients (38%) were on home oxygen therapy ().
Table 1. Baseline characteristics of 16 patients with chronic pulmonary diseases.
Immunogenicity
The GMCs of serotype-specific IgG for serotypes 14, 19F, 6B, and 23F were significantly elevated 1 month after the first vaccination (P < .01) and remained slightly higher than before the first vaccination, even at the time of the second vaccination. The GMCs of serotype-specific IgG for all serotypes increased significantly by the second vaccination, but the GMCs of serotype-specific IgG for serotypes 14 and 23F were lower 1 month after the second vaccination than 1 month after the first vaccination. Importantly, the GMCs of serotype-specific IgG for all serotypes increased significantly by the third vaccination, and the GMCs of serotype-specific IgG for serotypes 14, 19F, and 23F were higher after the third vaccination than after the second vaccination ().
Table 2. Comparison of geometric mean concentrations (GMCs) and geometric increases (n-fold) in levels of serotype-specific IgG and the opsonization index (OI) for serum samples from the 16 participants before and after the first, second, and third vaccinations.
The GMCs of serotype-specific OIs for all serotypes increased significantly 1 month after the first vaccination (). For all serotypes, the GMCs of serotype-specific OIs were higher 1 month after the second vaccination than 1 month after the first vaccination. The GMCs of serotype-specific OIs for all serotypes increased significantly 1 month after the third vaccination and were higher than before the first vaccination (). The GMCs of serotype-specific OIs for all serotypes decreased thereafter, but the second and third vaccination values were higher or similar to those 1 month after the first vaccination ().
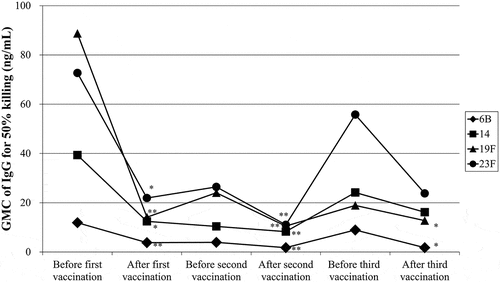
The GMCs of serotype-specific IgG required for 50% killing for all serotypes decreased significantly after the first vaccination (6B, 19F: P < .01, 14, 23F: P < .05; ). Between the first and second vaccinations, the GMCs of serotype-specific IgG required for 50% killing were significantly increased for serotypes 6B and 23F but did not change for serotypes 14 and 19F. The GMCs of serotype-specific IgG required for 50% killing for all serotypes decreased significantly after the second vaccination (P < .01; ). The GMCs of serotype-specific IgG required for 50% killing were significantly increased for all serotypes from before to after the third vaccination. The GMCs of serotype-specific IgG required for 50% killing for serotypes 6B and 19F were significantly decreased after the third vaccination (P < .05; ), but those of serotypes 14 and 23F increased.
Figure 1. The GMCs of serotype-specific IgG required for 50% killing of bacteria for serotypes 6B (diamonds), 14 (squares), 19F (triangles), and 23F (circles) before and after the first, second, and third vaccinations with 23-valent pneumococcal polysaccharide vaccine. **P < .01 and *P < .05 versus before the first vaccination.
Safety
No serious adverse reactions, such as anaphylactic shock, occurred after the third vaccination. The frequencies of local reactions, including local swelling, redness, and pain, peaked 1 day after the third vaccination and then gradually disappeared within 1 week (). Several participants developed systemic symptoms, including nausea, headache, muscle pain, joint pain, or malaise. These symptoms were found more frequently during the first 2 days after the third vaccination and improved slowly thereafter. All adverse reactions disappeared within 2 weeks after the third vaccination.
Table 3. Percentages of participants with local and systemic reactions by day after the third vaccination.
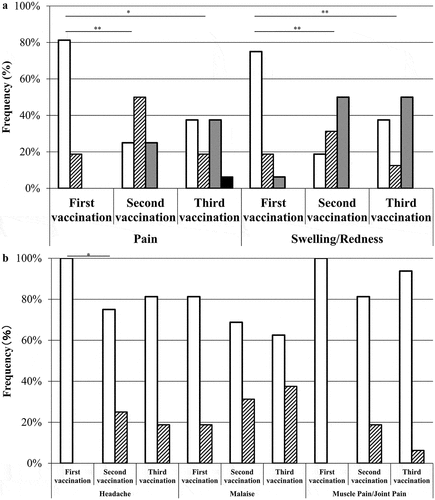
The frequency of local reactions, including pain and swelling or redness, was significantly higher after the third vaccination than after the first vaccination but did not differ significantly between the second and third vaccinations (). The frequency of systemic reactions, especially headache, was significantly higher in subjects after the third vaccination than after the first vaccination ().
Figure 2. Comparison of adverse reactions between the first, second, and third vaccinations in the study participants. Local adverse reactions (A) and systemic symptoms (B) were compared between the first, second, and third vaccinations. Data are shown as the frequencies of participants who received the first, second, and third vaccinations (n = 16). The grade of the finding or symptom is expressed as none (open bar), mild (slash bars), moderate (gray bars), or severe (closed bars). **P < .01 and *P < .05 versus the primary vaccination.
There was no relationship between the GMCs before the third vaccination and local adverse reactions after the third vaccination.
Discussion
This study shows that the third dose of PPSV23 was well tolerated and that serotype-specific IgG levels and serum opsonic activity were higher after than before the third vaccination.
Musher et al.Citation15 and Hammitt et al.Citation16 have shown that the immunogenicity of PPSV23 is preserved after repeated administration. The present study also showed that, for all four serotypes tested, serotype-specific IgG levels were significantly higher after the third vaccination than before the first vaccination. These observations provide a rationale for the use of repeated administration of the pneumococcal vaccine to maintain high concentrations of antibodies against this pathogen. After the second vaccination, the GMCs of IgG antibodies specific for serotypes 14 and 23F did not reach the levels observed after the first vaccination. However, the GMC of serotype-specific IgG for serotype 14 was higher after the third dose than after the first dose. Furthermore, the GMC of serotype-specific IgG for serotype 23F was similar after the third dose to that after the first dose. The mean interval between the second and third doses (63 months) was shorter than that between the first and second doses (91 months).Citation14 This may explain, at least in part, why the third immunization was more effective than the second in producing serotype-specific IgG for serotypes 14 and 23F.
In the present study, opsonic activity against serotypes 6B and 19F was higher after the third dose than after the first dose. We also found that the opsonic activities for serotypes 14 and 23F were similar after the third dose to those after the first dose, although the activities following the third dose were slightly lower. What is particularly noteworthy is that, for all four serotypes tested, the opsonic activities were higher before the third dose than before the first dose. The capsular polysaccharides included in PPSV23 are T-cell-independent antigens. Therefore, this vaccine has traditionally been considered to lack the ability to generate long-lasting immunological memory. However, the present study demonstrates that opsonic activity was still present in the serum of patients who had received their initial dose of PPSV23 at least 10 years earlier. This suggests that PPSV23 vaccination is capable of generating T-cell-independent B-cell memory.Citation23
In the present study, for serotypes 6B and 19F, the GMCs of serotype-specific IgG required for 50% killing were significantly lower after the third dose than before the first dose.Citation14 Similar results were obtained for serotypes 14 and 23F, although the decreases in IgG levels required for 50% killing were not significant. These findings imply that the immunological effects of PPSV23 can be restored by repeated administration of this vaccine at regular intervals.
Our study demonstrated that both local and systemic adverse reactions occurred more frequently after the third dose than after the first dose (), as previously reported by Jackson et al.Citation24 However, these adverse reactions were resolved within 1 week after the third vaccination (), and the frequency of adverse reactions after the third dose was similar to that detected after the second dose (). These results were interpreted as follows. Repeated vaccination with PPSV23 can be performed safely, without serious adverse reactions, and repeated vaccination does not always result in increased adverse reactions.
We as well as other researchers have addressed the important question of whether one could predict the risk of adverse reactions in each individual before vaccination with PPSV23. Jackson et al. showed that higher concentrations of serotype-specific prevaccination antibodies correlate with an increased risk of local adverse reactions (such as redness or swelling) within 2 days of vaccination.Citation13 By contrast, Törling et al. found no correlation between local adverse reactions and prevaccination antibody levels.Citation25 We found that the risk of adverse reactions did not correlate with the concentration of pneumococcal antibodies measured before the third dose (). These findings suggest that prevaccination antibody levels are unlikely to be a strong predictor of adverse reactions associated with administration of PPSV23.
Table 4. Relationship between geometric mean concentrations (GMCs) before the third vaccination and local adverse reactions after the third vaccination in the 16 participant.
There are several limitations to this study. First, it included a small number of participants. The clinical course of patients vaccinated with PPSV23 was followed for an extended period of time (10 years or longer), starting from their first dose. Because these patients were elderly even at the time of the first vaccination, many of them dropped out of the study because of difficulties visiting our hospital. Nevertheless, it was possible to collect long-term follow-up data from the same group of patients. This is unique to our research and has provided valuable insights into how repeated administration of PPSV23 is capable of inducing immunogenicity. Second, only four serotypes were included in the analysis of pneumococcal IgG levels and serum opsonic activity. Third, changes in the concentrations of serotype-specific IgG antibodies were investigated only up to 1 month after the third dose. However, the concentrations of these antibodies were measured before the third dose, as well as after the second dose. Therefore, we were able to show that, in elderly patients with chronic lung disease, the immunogenicity of PPSV23 was preserved over a long period of time, even after multiple doses. Finally, we note that the in vitro assays used to measure serotype-specific IgG levels and serum opsonic activity may not always provide data that can be translated to the in vivo protective effects of vaccination.
In conclusion, the present research demonstrated the immunogenicity and safety of repeated administration of PPSV23 by assessing the clinical course of the same patients through their first, second, and third immunizations. To our knowledge, no other studies have followed the same group of vaccinated patients for an extended period of time, from the first dose to the third dose. In the present study, the third dose was administered at least 10 years after the first dose. It is striking that serotype-specific IgG levels and serum opsonic activity were higher (albeit only slightly) before the third dose than before the first dose.
Japan is experiencing profound aging of its population, and an increase in the number of older adults who request a third dose of pneumococcal vaccine is expected. Our results provide valuable information about the safety and effectiveness of repeated administration of PPSV23.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med. 1995;333(24):1618–24. PMID:7477199. doi:10.1056/NEJM199512143332408.
- Miyashita N, Matsushima T, Oka M. Japanese respiratory society. The JRS guidelines for the management of community-acquired pneumonia in adults: an update and new recommendations. Intern Med. 2006;45(7):419–28. PMID: 16679695. doi:10.2169/internalmedicine.45.1691.
- Sakiyama H, Joh A, Umemoto T, Shimizu H, Oishi K. Childhood vaccination rates in Japan based on national survey. J Ambulatory Gen Pediatr. 2017;20(3):271–82. (in Japanese).
- Ubukata K, Takata M, Morozumi M, Chiba N, Wajima T, Hanada S, Shouji M, Sakuma M, Iwata S. Effects of pneumococcal conjugate vaccine on genotypic penicillin resistance and serotype changes, Japan, 2010–2017. Emerg Infect Dis. 2018;24(11):2010–20. PMID:30334707. doi:10.3201/eid2411.180326.
- Rokney A, Ben-Shimol S, Korenman Z, Porat N, Gorodnitzky Z, Givon-Lavi N, Ron M, Agmon V, Dagan R, Valinsky L. Emergence of Streptococcus pneumoniae serotype 12F after sequential introduction of 7- and 13-valent vaccines, Israel. Emerg Infect Dis. 2018;24(3):453–61. PMID: 29460732. doi:10.3201/eid2403.170769.
- Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51. PMID: 29395999. doi:10.1016/S1473-3099(18)30052-5.
- Shimbashi R, Chang B, Tanabe Y, Takeda H, Watanabe H, Kubota T, Kasahara K, Oshima K, Nishi J, Maruyama T, et al. Epidemiological and clinical features of invasive pneumococcal disease caused by serotype 12F in adults, Japan. PLoS One. 2019;14(2):e0212418. PMID: 30789928. doi:10.1371/journal.pone.0212418.
- Gutiérrez Rodríguez MA, Ordobas Gavin MA, Garcia-Comas L, Sanz Moreno JC, Cordoba Deorador E, Lasheras Carbajo MD, Taveira Jimenez JA, Martin Martinez F, Iniesta Fornies D, Arce Arnaez A. Effectiveness of 23-valent pneumococcal polysaccharide vaccine in adults aged 60 years and over in the region of Madrid, Spain, 2008–2011. Euro Surveill. 2014;19(40):20922. PMID: 25323079. doi:10.2807/1560-7917.es2014.19.40.20922.
- Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30(48):6802–08. doi:10.1016/j.vaccine.2012.09.019. PMID: 23000122.
- Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348(18):1747–55. PMID: 12724480. doi:10.1056/NEJMoa022678.
- Mangtani P, Cutts F, Hall AJ. Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: the state of the evidence. Lancet Infect Dis. 2003;3(2):71–78. PMID: 12560191. doi:10.1016/s1473-3099(03)00514-0.
- Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, Asoh N, Ishida M, Hamaguchi S, Aoshima M, Ariyoshi K, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17(3):313–21. PMID: 28126327. doi:10.1016/S1473-3099(17)30049-X.
- Jackson LA, Benson P, Sneller VP, Butler JC, Thompson RS, Chen RT, Lewis LS, Carlone G, DeStefano F, Holder P, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA. 1999;281(3):243–48. PMID: 9918479. doi:10.1001/jama.281.3.243.
- Ohshima N, Nagai H, Matsui H, Akashi S, Makino T, Akeda Y, Oishi K. Sustained functional serotype-specific antibody after primary and secondary vaccinations with a pneumococcal polysaccharide vaccine in elderly patients with chronic lung disease. Vaccine. 2014;32(10):1181–86. PMID: 24120483. doi:10.1016/j.vaccine.2013.09.060.
- Musher DM, Manoff SB, McFetridge RD, Liss CL, Marchese RD, Raab J, Rueda AM, Walker ML, Hoover PA. Antibody persistence ten years after first and second doses of 23-valent pneumococcal polysaccharide vaccine, and immunogenicity and safety of second and third doses in older adults. Hum Vaccin. 2011;7(9):919–28. PMID: 21860256. doi:10.4161/hv.7.9.15996.
- Hammitt LL, Bulkow LR, Singleton RJ, Nuorti JP, Hummel KB, Miernyk KM, Zanis C, Whaley M, Romero-Steiner S, Butler JC, et al. Repeat revaccination with 23-valent pneumococcal polysaccharide vaccine among adults aged 55–74 years living in Alaska: no evidence of hyporesponsiveness. Vaccine. 2011;29(12):2287–95. PMID: 21255685. doi:10.1016/j.vaccine.2011.01.029.
- Saito W, Nagai H, Suzuki J, Masuda K, Tamura A, Nagayama N, Akagawa S, Kawabe Y, Machida K, Kurashima A, et al. Capsular polysaccharide antibodies after pneumococcal polysaccharide vaccination in patients with chronic respiratory disease. Nihon Kokyuki Gakkai Zasshi. 2005;43(5):277–82. (in Japanese). PMID: 15969208.
- Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2001;8(2):266–72. PMID: 11238206. doi:10.1128/CDLI.8.2.266-272.2001.
- Wernette CM, Frasch CE, Madore D, Carlone G, Goldblatt D, Plikaytis B, Benjamin W, Quataert SA, Hildreth S, Sikkema DJ, et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol. 2003;10(4):514–19. PMID: 12853378. doi:10.1128/cdli.10.4.514-519.2003.
- Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006;13(9):1004–09. PMID: 16960111. doi:10.1128/CVI.00112-06.
- Chen M, Ssali F, Mulungi M, Awio P, Yoshimine H, Kuroki R, Furumoto A, Tanimura S, Kityo C, Nagatake T, et al. Induction of opsonophagocytic killing activity with pneumococcal conjugate vaccine in human immunodeficiency virus-infected Ugandan adults. Vaccine. 2008;26(38):4962–68. PMID: 18639599. doi:10.1016/j.vaccine.2008.06.093.
- Oishi K, Yoshimine H, Watanabe H, Watanabe K, Tanimura S, Kawakami K, Iwagaki A, Nagai H, Goto H, Kudoh S, et al. Drug-resistant genes and serotypes of pneumococcal strains of community-acquired pneumonia among adults in Japan. Respirology. 2006;11(4):429–36. PMID:16771912. doi:10.1111/j.1440-1843.2006.00867.x.
- Defrance T, Taillardet M, Genestier L. T cell-independent B cell memory. Curr Opin Immunol. 2011;23(3):330–36. PMID: 21482090. doi:10.1016/j.coi.2011.03.004.
- Jackson LA, Nelson JC, Whitney CG, Neuzil KM, Benson P, Malais D, Baggs J, Mullooly J, Black S, Shay DK. Assessment of the safety of a third dose of pneumococcal polysaccharide vaccine in the vaccine safety datalink population. Vaccine. 2006;24(2):151–56. PMID: 16122845. doi:10.1016/j.vaccine.2005.07.066.
- Törling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine. 2003;22(1):96–103. PMID: 14604576. doi:10.1016/s0264-410x(03)00521-8.
