ABSTRACT
Studies associate rotavirus vaccination with intussusception. In Germany, a retrospective multicenter matched case–control study was performed to identify risk factors for intussusception with a special focus on rotavirus vaccines. Children with place of birth and residence in Germany who had been treated for intussusception from 2010 to 2014 and who had been less than 1 year old at the time of intussusception were recruited. Case report forms were independently validated by two pediatricians according to the criteria of intussusception defined by the Brighton Collaboration (BC). Cases with the highest diagnostic certainty (level 1) were matched with population-based controls by age, gender, federal state, and place of residence. Information on vaccine exposures originated from vaccination certificates. One hundred and sixteen cases were matched with 272 controls. A significantly increased adjusted odds ratio (aOR) for intussusception (5.74, 95% CI: 1.51–21.79) was detected in individuals immunized with rotavirus vaccine dose 1 prior to symptom onset as compared to non-exposed individuals. Age at the start of the rotavirus immunization series did not modify the risk of intussusception. The odds for intussusception were not increased postdose 2 and 3 as well as any dose. One further risk factor for intussusception, family history of intussusception (aOR 3.26, 95% CI 1.09 − 9.77) was identified. Breastfeeding was found to have a protective effect (aOR 0.54, 95% CI 0.33 − 0.88). Rotavirus vaccine dose 1 was associated with a 5.7-fold increased risk to develop intussusception regardless of age at immunization whereas the overall risk for intussusception in the first year of life was not increased.
Introduction
Incidence
Intussusception primarily affects infants and toddlers. The background incidence rate of intussusception in children under 1 year of age was found to vary considerably between continents and countries (range 9–328/100,000 child-years) with the worldwide average being 74/100,000 child-years.Citation1 In Germany, the background incidence rate of intussusception in children under the age of 1 year was estimated to be 61.7/100,000 child-years, it was lowest in the first 3 months of life (19.2/100,000 child-years) and highest during the 6th to 8th month of life (98.5/100,000 child-years).Citation2 Intussusception occurs more frequently in males with a gender ratio of approximately 2:1.Citation3,Citation4
Etiology
The seasonal pattern of intussusception with the occurrence rate in the northern hemisphere being highest in the winter and lowest in the summer monthsCitation5, as well as the high virus detection rate in the stools of intussusception patientsCitation6, suggest that viral infections like adenovirus,Citation7-11 enterovirus B,Citation9 herpesvirus 6,Citation8,Citation12 cytomegalovirus,Citation13 norovirus,Citation6 astrovirus,Citation14 and respiratory syncytial virusCitation15 may play a role in the development of intussusception. Of note, the patterns of monthly virus detection rate and intussusception occurrence rate were found to correlate.Citation6 Although rotavirus was detected in stool samples of intussusception patients, wildtype rotavirus infection was not considered to carry an increased risk for intussusception.Citation7,Citation16-18
Association with rotavirus vaccination
In 2006, the European Commission approved two live rotavirus vaccines of the second generation, a monovalent live attenuated human strain which is administered orally in 2 doses (RV1; Rotarix, GlaxoSmithKline Biologicals s.a., Rixensart, Belgium) and a pentavalent human bovine reassortant strain containing the antigens G1, G2, G3, G4, and P which is administered orally in 3 doses (RV5; RotaTeq, MSD VACCINS, Lyon, France). None of these two vaccines showed an increased risk for intussusception as compared to placebo in clinical trials prior to marketing authorization.Citation19,Citation20 Also, a systematic review and meta-analysis which was recently updated for the Cochrane Database detailing the risk for intussusception after the administration of rotavirus live vaccine within the scope of clinical trials demonstrated no increased risk.Citation21Postmarketing pharmacoepidemiological studies, however, revealed that these vaccines were nevertheless associated with an increased risk for intussusception.Citation22-36 According to the product information of the two second-generation rotavirus vaccines, up to 6 additional cases per 100,000 infants have been observed in observational studies against a background incidence rate of 25 to 101 per 100,000 infants (less than 1 year of age) per year,Citation37,Citation38 whereas 11 to 21 additional cases per 100,000 infants were expected to occur after vaccination with the first-generation vaccine, a tetravalent rhesus-based rotavirus vaccine (RRV-TV; RotaShield, Wyeth Laboratories, Inc., Marietta, Pennsylvania/US).Citation39
Recommendation of rotavirus vaccination in Germany
Since 2006, both rotavirus vaccines were available on the German market. In 2013, the Standing Committee on Immunization (Ständige Impfkommission, STIKO) recommended rotavirus vaccination as part of the standard vaccination program for children. From the age of 6 weeks, depending on the vaccine chosen, two (RV1) or three (RV5) doses shall be administered with a minimal interval of 4 weeks. Due to the risk of intussusception that is supposed to be increasing with age, the STIKO recommends starting the immunization series early – until the age of 12 weeks at latest and preferably terminating it until the age of 16 weeks (RV1) and 20–22 weeks (RV5), respectively.Citation40 In any case, the immunization series needs to be terminated until the age of 24 weeks (RV1) and 32 weeks (RV5), respectively.Citation40 Even before implementation in the routine vaccination schedule in 2013, rotavirus vaccination was used in Germany and linked to a significant reduction in rotavirus-related hospitalizations in the age-groups 6–23 months of age in Germany.Citation41
The German Intussusception Study
In 2015, the Paul-Ehrlich-Institut (PEI), the German Federal Institute for Vaccines and Biomedicines, initiated the German Intussusception Study, a retrospective multicenter matched case–control study in infants less than 1 year which aimed at identifying risk factors for intussusception with a special focus on rotavirus vaccines. Furthermore, from the present body of knowledge, it remains unclear whether age at the start of the immunization series with rotavirus vaccines modifies the risk for intussusception and whether rotavirus vaccines affect the overall risk for intussusception in the first year of life. Against this background, these two secondary research questions were to be addressed.
Results
Recruitment
A total of 43 pediatric clinics were invited to take part in the German Intussusception Study, 19 of which finally participated in the study (response rate: 44.2%). The participating study centers contacted a total of 325 families with children who experienced intussusception in the first year of life 162 of which gave informed consent (response rate: 49.8%). Forty-six out of the 162 cases were excluded from further analysis for various reasons (see ).
Figure 1. Flowchart of recruitment. BC Brighton Collaboration criteria for intussusception; level 1 highest level of diagnostic certainty
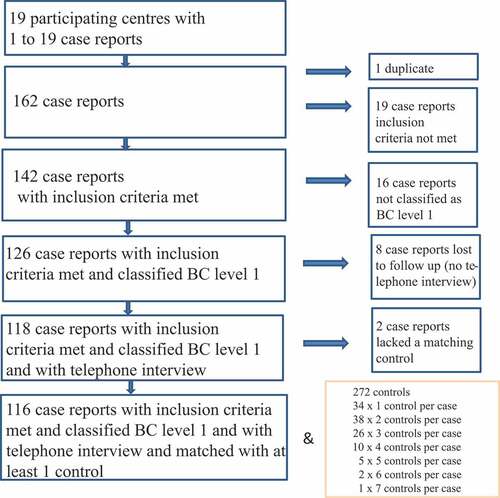
The families of the 116 eligible cases were from 12 out of the 16 German federal states and all first digits (0–9) of the five-digit zip code were represented. For every eligible case, 10 families with children that fulfilled the matching criteria were invited to take part in the study. One to seven families agreed to participate in the study; thus, 116 eligible cases were matched with 272 recruited population-based controls in case–control ratios ranging from 1:1 to 1:7 ().
Demographics
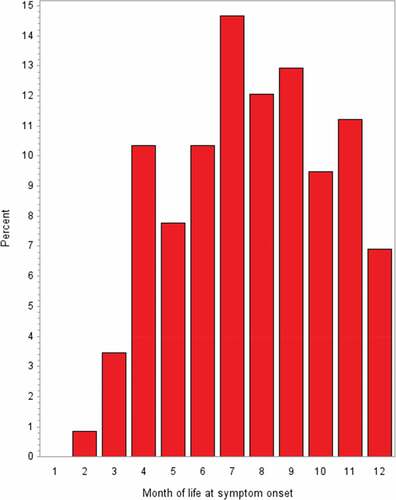
Over 90% of the study participants were Caucasians and about 70% of the participants were males. The variables age, gender (which both belonged to the matching criteria), birthweight, and length at birth were well balanced between the study groups (). During the first 3 months of life, only a small number of intussusceptions occurred whereas the highest frequencies were observed in the 7th, 8th, and 9th months of life ().
Table 1. Demographic characteristics of intussusception cases (BC level 1) and matched controls; comparison among study groups (n = 388)
Clinical characteristics
At hospitalization, 31/116 cases (26.7%) were diagnosed with concomitant gastroenteritis (). In 10 out of these 31 patients with gastroenteritis (32.3%), pathogenic microorganisms [adenovirus (n = 5), rotavirus (n = 1), norovirus (n = 1), Salmonella apeyeme (n = 1), Clostridium difficile (n = 1), and Aeromonas caviae (n = 1)] were detected. Another concomitant acute disease was documented for 21 cases [respiratory tract infection (n = 9), commotio cerebri (n = 1), macular eczema at the abdomen (n = 1), varicella (n = 1), auricular phlegmon (n = 1), hyperglycemia (n = 1), cyanosis (n = 1), conjunctivitis (n = 1), septicemia with Klebsiella pneumoniae (n = 1), septicemia with Staphylococcus aureus (n = 1), convulsion (n = 1), otitis media (n = 1), urinary tract infection with Citrobacter koseri (n = 1)], and a chronic disease was reported in four cases [atrial septal defect II (n = 1), patent foramen ovale (n = 1), persistent airway infection (n = 1), hereditary (primary) lymphedema (n = 1)]. In four children, a malformation was reported [asymmetry of the skull (n = 1), cleft palate (n = 1), hypertrophy of the pylorus (n = 1), and pre-vesical distension of the ureter on the left (n = 1)].
Table 2. Clinical characteristics of intussusception cases (BC level 1) (n = 116)
In five patients, a Meckel’s diverticulum (omphalomesenteric duct) and in two further patients, intestinal polyps were detected. Another potential intestinal predisposition was documented for two children [small inguinal hernia (n = 1), transverseoptosis with loop formation of the colon and unusual high site of the cecum (n = 1)].
Two cases had previously experienced an intussusception. The most frequently reported signs and symptoms were vomiting, abdominal pain, hematochezia, pallor, and reduced food intake ().
Table 3. Signs and symptoms of intussusception in cases (BC level 1) (n = 116)
The ileo-colic type of intussusception accounted for 89 of the 116 cases (76.7%), 3 (2.6%) were of the colo-colic type, 5 (4.3%) of the ileo-ileal type, and in 19 cases (16.4%), localization was not clearly specified.
Management of intussusception and treatment outcomes
In a total of 110 cases (94.8%), non-operative reduction attempts were recorded. The most frequently reported non-operative reduction method was ultrasound-guided hydrostatic reduction with sodium chloride (n = 75, 64.7%) followed by x-ray guided contrast enema reduction (n = 20, 17.2%); 40 of the 116 cases (34.5%) underwent surgery 34 of whom had previously been treated with unsuccessful non-operative reduction (). In 8 patients (6.9%), a partial bowel resection had to be conducted and 3 patients (2.6%) required a revision surgery. In 9 patients (7.8%), recurrent intussusception was observed. All patients recovered, 108 (93.1%) without and 8 (6.9%) with sequelae (partial bowel resection), there were no fatal outcomes.
Table 4. Managementa of intussusception and treatment outcome (BC level 1) (n = 116)
Rotavirus and other vaccine exposures
Copies of the child’s certificate of vaccinations were provided for 114 cases (98.3%) and 266 controls (97.8%). In our study covering the years 2010 to 2014, 48.3% of the cases and 46.3% of the controls received at least one dose of rotavirus vaccine, 42.2% of the cases and 45.6% of the controls completed the immunization series with rotavirus vaccine. Thirty-five cases (30.2%) were vaccinated with RV1 and 21 (18.1%) with RV5. Seventy-eight controls (28.7%) received RV1 and 48 (17.6%) RV5. Prior to intussusception, eleven cases (9.5%) had received rotavirus vaccine dose 1, 30 cases (25.9%) dose 2, and 15 cases (12.9%) dose 3. Absolute and relative frequencies of rotavirus and other relevant vaccine exposures (last dose prior to index date) in cases and controls are shown in .
Table 5. Vaccinations (last dose) prior to index date* as explanatory variables in intussusception cases (BC level 1) and matched controls; comparison among study groups (n = 388)
Explanatory variables other than vaccines
Absolute and relative frequencies for explanatory variables related to pregnancy, delivery, preterm birth, low and high birth weight, weight for age, nutrition, diseases, surgeries, and family history of intussusception are presented in .
Table 6. Explanatory variables in intussusception cases (BC level 1) and matched controls; comparison among study groups (n = 388)
Univariate logistic regression analyses
Regarding vaccine exposures, explanatory variables with p < .25 in the univariate logistic regression analysis included administration of rotavirus life vaccine dose 1, hexavalent (DTPa-IPV-Hib-Hep-B) vaccine dose 1, pneumococcal conjugate vaccine dose 1, and meningococcal C vaccine dose 1 (). Since rotavirus, hexavalent, and pneumococcal conjugate vaccines are usually being administered concomitantly, these variables were highly correlated. This was confirmed by calculating Spearman’s correlation coefficients (r = 0.41485 for exposure to rotavirus vaccine dose 1 and hexavalent vaccine dose 1; r = 0.34263 for exposure to rotavirus vaccine dose 1 and pneumococcal conjugate vaccine dose 1; r = 0.63290 for exposure to hexavalent vaccine dose 1 and pneumococcal conjugate vaccine dose 1). Exposures to pentavalent (DTPa-IPV-Hib), Hep B monovalent, measles, mumps, rubella, and varicella vaccines did not fall below p = .25 in the univariate logistic regression analysis (data not shown).
Explanatory variables with p < .25 in the univariate logistic regression analysis regarding exposures apart from vaccinations comprised nutrition (breastfeeding, formula milk, and supplementary food) in the month of index date, low birthweight (<2500 g), and family history of intussusception ().
Multiple logistic regression analysis
The following variables were entered in the multiple logistic regression model (step 0) with backward elimination as variable selection method: rotavirus life vaccine dose 1, hexavalent vaccine dose 1, pneumococcal conjugate vaccine dose 1, meningococcal C vaccine dose 1, breastfeeding at the month of index date, formula milk at the month of index date, supplementary food at the month of index date, low birth weight (<2500g), and family history of intussusception ().
Table 7. Multiple logistic regression analysis, analysis of conditional maximum likelihood estimates, step 0
The following variables being significantly associated with intussusception (p < .05) stayed in the multiple logistic regression model (step 6) at the end of the backward elimination variable selection procedure ():
rotavirus life vaccine dose 1 (aOR 5.74, 95% CI: 1.51–21.79), p = .0102;
breastfeeding at the month of index date (aOR 0.54, 95% CI 0.33 − 0.88), p = .0139, and
family history of intussusception (aOR 3.26, 95% CI 1.09 − 9.77), p= .0352.
Table 8. Multiple logistic regression analysis, analysis of conditional maximum likelihood estimates, step 6
Interactions between variables were not identified.
Age at rotavirus vaccination and overall risk for intussusception associated with rotavirus vaccines in the first year of life
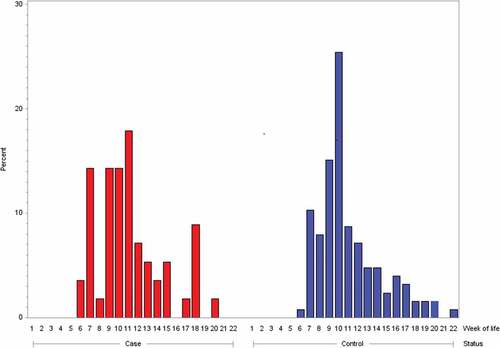
Age at the start of the rotavirus immunization series did not modify the risk for intussusception. In cases, the median age at administration of rotavirus life vaccine dose 1 was 71 days (range 36–139 days) and, in controls, 68 days (range 42–148 days). Age at the start of immunization series with rotavirus life vaccine was not statistically different for cases and controls (two-sample t-test: t value = 0.32, df = 180, p = .7494). The distribution for age at the start of immunization series with rotavirus vaccines is shown in . Irrespective of whether rotavirus vaccine was administered prior to or following index date, 15 of 56 rotavirus-vaccinated cases (26.8%) and 31 of 126 rotavirus vaccinated matched controls (24.6%) received rotavirus life vaccine dose 1 later than day 84 of life, i.e., aged older than 12 completed weeks of life (Chi-square test: χ = 0.0978, p = .7545; OR 1.26; 95% CI: 0.63–2.53). Three of the eleven cases (27.3%) with rotavirus life vaccine administration prior to symptom onset and 4 of the 9 controls (44.4%) with rotavirus life vaccine administration prior to index date (day of life on which the matching case had symptom onset) were older than 84 days at start of the immunization series with rotavirus life vaccine. Rotavirus vaccines did not affect the overall risk for intussusception in the first year of life: 56/116 cases (48.3%) and 119/272 matched controls (43.8%) were vaccinated with rotavirus vaccines (any dose) prior to index date (Chi-square test: χ = 0.4121; OR 1.09; 95% CI: 0.66–1.81).
Sensitivity analyses
The complete case analysis yielded results that were equivalent to the primary analysis in which the missing values were imputed. Multiple regression analysis conducted using two further methods of variable selection, forward and stepwise, revealed identical results.
Median time interval between administration of rotavirus life vaccine dose 1 and symptom onset was 17 days (range 0 to 272 days) with all values but one outlier (272 days) being within a 42-day period. Accounting for risk windows with respect to vaccinations revealed that despite wide confidence intervals the effect for rotavirus dose 1 was stable. The aOR for rotavirus vaccination dose 1 in the 14-, 21-, 28-, 35-, and 42-day risk windows were
14-day risk window: aOR 6.10 (95% CI: 1.08–34.35), p = .0404;
21-day risk window: aOR 5.66 (95% CI: 1.39–23.03); p = .0155;
28-day risk window: aOR 5.68 (95% CI: 1.44–22.43), p = .0132;
35-day risk window: aOR 5.46 (95% CI: 1.37–21.71), p = .0159, and
42-day risk window: aOR 5.89 (95% CI: 1.52–22.83), p = .0103.
Investigating the above-specified risk windows following administration of doses 2 and 3 failed to find associations with intussusception (data not shown).
Capture-recapture analysis
The two largest study centers contacted together a total of 52 families with potentially eligible intussusception cases 34 (64.4%) of whom gave informed consent and participated in the case–control study. All but three of these study cases could successfully be assigned to the chart records of eligible cases in the two largest centers. The independent researcher performing the recapture investigation retrieved a total of 62 patients with diagnosis of intussusception (ICD 10 code K56.1) 54 of whom fulfilled the inclusion criteria. Thirty-one of these 54 eligible cases could successfully be assigned to the cases included in the case–control study. The Chapman estimator for undercount was 2.2 and the estimated total number of eligible cases in these two largest centers was 59.2 (95% CI 57.5–67.0). Completeness of case capture of the two largest study centers was estimated to be 91.0% and 94.0% for MLE and NUE, respectively. Conversely, the completeness of case capture of the case–control study in the two largest centers was estimated to be 57.0% and 59.0% for MLE and NUE, respectively. This finding indicates that whereas case identification in the participating pediatric clinics reached a high level, several cases could not be considered due to the refusal of parents to give informed consent.
Reasons for nonparticipation
Parents of 10 cases and of 33 controls answered our request to specify the reasons for nonparticipation.
Parents of cases stated that they had no interest to take part in a study (n = 2), for personal reasons (n = 6), since diagnosis of intussusception was not confirmed (n = 1), that they were annoyed that the attending physician did not take the parents seriously who suspected an intussusception from the very beginning (n = 1), that they had no time (n = 2), could not speak enough German (n = 1), that the child had no diseases in the first year of life (n = 1), that the child had not been hospitalized in the time period under review (n = 1). Only once the parents noted that they did not participate in the study because the child was not vaccinated against rotavirus gastroenteritis (n = 1). Three of the “missed” cases (30%) were vaccinated against rotavirus gastroenteritis, 4 (40%) were not and for 3 cases (30%) information was not available.
In controls, parents stated that they had no interest to take part in a study (n = 7), they did not understand the study design (n = 6), for personal reasons (n = 8), for lack of time (n = 2), because the child was born abroad (n = 2), because they could not speak enough German (n = 2), they had a healthy child so, there was no need to participate in a study (n = 6), because the family lived in another federal state in the child’s first year of life (n = 2), data in the invitation letter apparently would not match with the data of the own child (n = 8). Five children (15.2%) were vaccinated against rotavirus gastroenteritis, 13 (39.4%) were not, and for 15 subjects (45.5%) information was missing.
Discussion
Main findings
In this case–control study, the administration of rotavirus vaccine dose 1 was associated with a 5.7-fold increased odds ratio for intussusception. This finding is in line with other studies on rotavirus vaccination and intussusceptionCitation22–36 albeit in our study the risk window was not limited to 14 days post-vaccine. This may be linked to the fact that both rotavirus vaccines, RV1 and RV5, contain life viruses, which are excreted into stool after vaccination.Citation19,Citation20 In a study conducted in Malawi, RV1 fecal shedding was detected in 68% of vaccinated infants with proportions of infants with RV1 vaccine virus shedding being 43% and 53% up to day 10 after administration of RV1 dose 1 and dose 2, respectively.Citation42 An Australian study detected RV5 in stool samples from 87%, 57.4%, and 47.3% of children after administration of RV5 doses 1, 2, and 3 and found the median (interquartile range) shedding duration to be 3 (1–8), 1.5 (1–3), and 1 (1–2), weeks, respectively.Citation43 Maximum shedding durations were 13 (dose 1), 9 (dose 2), and 14 (dose 3) weeks.Citation43 Age at the start of immunization series against rotavirus gastroenteritis was not found to modify the association between administration of rotavirus vaccine dose 1 and intussusception. In this study, there was no increased odds ratio for intussusception following administration of doses 2 and 3 whereas some observational studies reported a slightly elevated risk post dose 2.Citation44 Furthermore, the overall risk of intussusception in the first year of life associated with rotavirus vaccines (any dose) was not increased. This supports the notion that the excess intussusception cases that can be ascribed to the administration of dose 1 will be compensated by the end of the first year of life. So, one interpretation would be that dose 1 changes the timing rather than the risk for intussusception by triggering intussusception in vulnerable infantsCitation45 Another interpretation would be that rotavirus vaccination decreases the long-term risk for intussusception by preventing natural rotavirus infection. This assumes that natural rotavirus infection can cause intussusception, as has been suggested by Konno et al. in 1978Citation46 albeit not by others.Citation7,Citation16-18 A not increased long-term risk of intussusception is consistent with recently published research: Based on insurance claims data, the Centers for Disease Control and Prevention found a non-significant decrease in intussusception (hazard ratio, 0.79 [95% CI, 0.57–1.09]) in fully rotavirus-vaccinated US children followed up to the age of 2 years.Citation47
One other independent risk factor, family history of intussusception, was identified. Considering the reports on familial intussusception,Citation48–50 there may be subjects who are disposed to intussusception. Although a family history of intussusception has not been classified as a contraindication for rotavirus vaccination, it may be advisable to ask for a family history of intussusception on a routine base prior to rotavirus vaccination and to recommend immediately seeking medical help when noticing first symptoms.
In our study, breastfeeding was identified as a protective factor. This finding is consistent with a case–control study conducted in Italy where exclusive breastfeeding was linked to halving the odds ratio for intussusception.Citation51
Strengths
We included intussusception cases from all over Germany with residence in 12 of the 16 federal states and across all first digits of the 5-digit zip code and used population-based controls as well as very stringent matching criteria. In addition, we chose as index date the date of symptom onset in cases and the day of life on which the matching case experienced first symptoms in controls. This guarantees that cases and controls were from a common population and, most importantly, takes account of gender- and age-specific background incidence rates. In addition, the age distribution of our intussusception cases () is in line with published data.Citation2 Therefore, the study results are supposed to be generalizable to the German infant population less than 1 year of age.
Cases were independently validated according to the criteria defined by the Brighton Collaboration by two pediatricians blinded for vaccine exposure status and eligibility was restricted to the highest diagnostic certainty (level 1), thus reducing the risks for both selection and ascertainment bias.
Information on tradenames and batch numbers of vaccines as well as vaccination dates originated from the child’s international certificate of vaccination or more precisely, a copy thereof which was obtained from the patient’s parents. In general, this document is up to date and besides entries like the vaccine-preventable disease and vaccination dates, usually, for each vaccination, contains a stuck in label with tradename, batch number, and expiry date. Copies of the certificates of vaccination were available for 380 of the 388 study participants (97.9%). Thus, the information obtained on vaccinations in the first year of life is supposed to be very reliable.
Selection bias may be an issue in pharmacoepidemiological studies especially in case the suspected association between drug and adverse event is known among health professionals and consumers. Since vaccine exposures were not recorded by the study centers, the risk of bias due to preferential inclusion of cases exposed to rotavirus vaccine by investigators is supposed to be low.
To specifically address the risk of selection bias, a second investigation was conducted by an independent researcher in the two largest study centers. It revealed that the case–control study primarily “missed” cases due to the refusal of parents to give informed consent since case capture (prior to seeking written informed consent) of the two largest study centers was estimated to be 91.0% and 94.0% for MLE and NUE, respectively. To find out why parents declined the invitation to participate in the study we asked them to specify the reasons. The parents’ responses (not focusing on rotavirus vaccination) indicated that the risk for selection bias due to unequal nonparticipation was negligible.
Limitations
The level of evidence regarding the results of observational studies is, of course, lower as compared to interventional clinical trials, but as intussusception following rotavirus vaccine is a rare adverse event, randomized controlled clinical trials prior to authorization with sample sizes of more than 60,000 children failed to exclude a risk for intussusception of <1 additional case per 10,000 doses administered.Citation19,Citation20 Against this background, findings originating from pharmacoepidemiological studies add to the body of knowledge, especially regarding vaccine safety. The case–control design was chosen because it is particularly suitable for rare diseases.
Planning for this study was done in 2014, recruitment started in May 2015 including individuals who had been treated for intussusception between January 2010 and December 2014. Five federal states (Bundesländer) had recommended rotavirus vaccination prior to 2013 (therefore federal state was one of the matching criteria). Although several health insurance companies in Germany, already since 2008, reimbursed rotavirus vaccination prior to the national vaccination recommendation, we cannot totally exclude a healthy user bias (infants vaccinated in a time period might be healthier, living in families with higher socioeconomic status/educational level and having a healthier lifestyle). However, the impact of such an effect on the outcome variables remains unknown.
The limited sample size caused confidence intervals to be wide and precluded performing separate analyses for the two rotavirus life vaccines on the European market. This shortcoming may be overcome by taking the results of previous epidemiological studies into consideration. In the meantime, it is well established that both second-generation rotavirus vaccines are associated with intussusception. Of note, in this study, cases and controls had a comparable uptake of RV1 and RV5, respectively.
The information obtained via a standardized telephone interview with the parents of cases and controls may be compromised by recall bias since this was a retrospective study. This is not a big issue unless it affects cases and controls differently. For example, it may be difficult for parents of population-based controls to remember exactly when in the first year of life the child had gastroenteritis (a common pediatric disease) whereas parents of cases usually remember very well that the child had gastroenteritis at the time of hospitalization. Indeed, gastroenteritis prior to hospitalization was found to be a risk factor for intussusception in two other recently published case–control studies on intussusception.Citation51,Citation52
There was clinical information that was only obtained for cases so that several variables, e.g. anatomical features like the presence of Meckel’s diverticulum, due to lacking information for controls, could not be included in the multiple logistic regression analysis, although these may nevertheless represent risk factors.Citation53–55 Since only a limited number of explanatory variables could be considered, chances are that there was unmeasured confounding.Citation56
Due to the fact that hexavalent, pneumococcal conjugate, and rotavirus life vaccines are usually being administered concomitantly, the effects for the respective variables were highly correlated. For this reason, as a method of variable selection, a backward elimination procedure was used. The obtained results were confirmed by using two further variable selection methods (forward and stepwise).
Conclusions
This study conducted in Germany demonstrated in an infant population less than 1 year of age that administration of rotavirus life vaccine dose 1 was associated with a 5.7-fold increased odds ratio for intussusception. Age at the start of immunization series did not modify this risk and rotavirus vaccines did not affect the overall risk for intussusception in the first year of life. One further risk factor for intussusception, family history of intussusception, was identified whereas breastfeeding was found to have a protective effect.
Patients and methods
German pediatric clinics from all over Germany were invited by mail to participate in the German Intussusception Study.
Case identification
Participating centers were asked to identify from their patient population individuals with place of birth and residence in Germany who had been treated for intussusception (ICD 10 code K56.1 at discharge from hospital) between January 2010 and December 2014 in a German pediatric clinic and who had been less than 1 year old at the time of intussusception. Parents of patients fulfilling the inclusion criteria were contacted by the centers to obtain written informed consent to transcribe recorded data (including demographics, medical history, nutrition, examinations, relevant findings, and therapy) to a standardized case report form (CRF).
Case ascertainment and validation
The CRFs were independently validated by two pediatricians (DM and IB) according to the criteria of intussusception as an adverse event following immunization (AEFI) defined by the Brighton CollaborationCitation57 blinded for the patient’s and the center’s identity and the exposures to potential risk factors including vaccination. In case of discrepant expert validations, the pediatricians were asked to reevaluate the case. An agreement was achieved by thoroughly recapitulating the criteria laid down in the Brighton Collaboration case definitionCitation57 and discussing the case. Case reports fulfilling the criteria of BC level 1 (highest diagnostic certainty) were eligible.
Population-based controls
Cases and controls ought to be recruited from a common German pediatric population. For this purpose, addresses of potential population-based controls with place of birth and place of residence in Germany were identified by the regional registration offices and transmitted to the study secretary. Parents of potential controls were contacted by study personnel to obtain written informed consent. Exclusion criterion was treatment for intussusception from January 2010 through December 2014.
Matching
Validated cases of intussusception were matched with population-based controls by date of birth with a tolerance of ±30 calendar days, gender, federal state (due to slightly discrepant local vaccination recommendations in the 16 German federal states), and place of residence (first digit of the zip code) in a ratio of at least 1:2.
Index date
The index date for cases was the date of symptom onset. For controls, the index date was the day of life on which the matching case experienced the first symptoms of intussusception.
Exposures
Information on birth weight, weight for age, preexisting or concomitant medical conditions, predispositions, as well as vaccinations was obtained within the scope of a standardized telephone interview by the study personnel with parents of cases as well as population-based controls. As reference data for small and large for gestational age children, the percentiles of body measurements of newborns in Germany published by Voigt et al.Citation58 were used. To confirm the type and date of vaccinations, parents of cases and controls were asked to send a copy of the international vaccination certificate. In this document, all immunizations are being entered by the attending pediatrician including vaccination date, tradename of the vaccine, batch number, stamp, and seal of the physician who administered the vaccine. Usually, one of the labels supplied by the vaccine manufacturer is inserted for each vaccination. When assessing vaccine exposures, the last dose prior to index date was considered.
Reasons for nonparticipation
Refusal to give informed consent may be associated with selection bias. To find out why parents declined the invitation to participate in the study we asked them to specify the reasons therefore in plain text allowing them to give as many reasons as they desired. In addition, we asked whether the child had been vaccinated against rotavirus gastroenteritis. The nonparticipating families gave consent that the given reasons for nonparticipation may be analyzed for study purposes.
Capture-recapture analysis
In case of a known association between risk factor and outcome, there is always a risk of selection bias. Within the scope of a capture-recapture analysis,Citation59,Citation60 the completeness of case capture was investigated for the two largest participating centers. For this purpose, these were contacted and asked to give consent to have a secondary investigation carried out on-site by an independent researcher. As soon as the written agreement was received, a contract was concluded defining the modalities of study conduct, data protection, as well as rights and obligations of the contracting parties. Upon receipt of written consent, an appointment was made with the contact person of the center. Patients with a confirmed diagnosis of intussusception (ICD 10 code K56.1 at discharge from hospital) from January 2010 through December 2014 who were less than 1 year old at the time of hospitalization were included. Data linkage was performed manually on multiple variables including year of birth, gender, date of hospitalization, concomitant diseases, and therapy.
Based on both independent investigations (centers [capture], an on-site investigation [recapture]) an estimate for “undercount” according to ChapmanCitation61 was determined. In addition, two estimates for the completeness of case capture were determined using the Maximum Likelihood Estimator (MLE)Citation59,Citation62 and the Nearly Unbiased Estimator (NUE).Citation61,Citation63 The two estimators use slightly different methods to calculate “the unobserved cell,” i.e., the number of cases that were captured neither by source 1 nor by source 2. This difference has well been described by Hook and Regal 1995.Citation60
Outcomes
(I) Primary outcome was to identify risk factors for intussusception with a special focus on rotavirus vaccines.
(II) Two secondary research questions were to be addressed:
a. Does age at the start of the immunization series with rotavirus vaccines modify the risk for intussusception?
b. Do rotavirus vaccines affect the overall risk for intussusception in the first year of life?
The outcomes were used for defining and taking risk-minimizing measures regarding rotavirus vaccination.
Data management
In-house monitoring, queries, double independent data entry, as well as computer-assisted plausibility checks were used to guarantee a high data quality.
Statistics
Descriptive analyses
Absolute and relative frequencies were calculated for qualitative variables, median, minimum, and maximum for quantitative variables. Depending on frequencies and distribution characteristics, differences in characteristics between cases and controls were tested by using Chi-square/Fisher’s Exact test for qualitative variables and Student’s t-test/Wilcoxon two-sample test for quantitative variables.
Primary outcome
Univariate and multiple logistic regression analyses with intussusception as dependent variable were performed to identify factors associated with the disease. To quantify the strength of the association, odds ratios (OR) and 95% confidence intervals (CI) were calculated.
Variable selection is a typical exploratory exercise in multiple logistic regression analysis when the investigator is interested in identifying important prognostic factors from a large number of candidate variables. Only covariates that were associated with intussusception (p < .25) in the univariate analyses were entered in the multiple logistic regression model. Multiple imputation was applied to account for missing data in the covariates. Using a backward elimination method, only covariates associated with intussusception (p < .05) in the multiple logistic regression analysis stayed in the final model. Interactions between covariates were assessed as well.
A p value <.05 was considered statistically significant. Due to the exploratory character of the study, α adjustment was not performed.
Secondary outcomes
Within the logistic regression analysis, the interaction between rotavirus vaccination and age at rotavirus vaccination >12 completed weeks of life was assessed. In addition, age at rotavirus vaccination dose 1 was compared between cases and controls using a two-sample Student’s t-test.
In addition to dose-specific analyses, the overall risk for intussusception in the first year of life with respect to rotavirus vaccines was assessed in the logistic regression analysis by combining the exposures to doses 1, 2, and 3 (any dose).
Sensitivity analyses
A complete case analysis was performed to investigate whether the missing data were differential. In addition, the multiple logistic regression analysis was repeated with two other methods of variable selection, forward and stepwise, in order to investigate whether and how the results were influenced by the method of variable selection. Regarding stepwise and forward selection, for all variables not in the model, the one with the smallest p value was entered if the p value was less than or equal .05. Regarding stepwise selection, for all variables in the model, the one with the largest p value was removed if the p value exceeded .05.
To account for risk windows following immunization, the multiple regression analysis was repeated for specified time intervals between vaccination and intussusception.
Statistical software
All statistical analyses were performed using the software package SAS, version 9.4 (SAS Institute Inc. Cary, NC, US).
Abbreviations
| ACIP | = | Advisory Committee on Immunization Practices |
| AEFI | = | Adverse event following immunization |
| aOR | = | Adjusted odds ratio |
| BC | = | Brighton Collaboration criteria for intussusception |
| CI | = | Confidence interval |
| cOR | = | Crude odds ratio |
| CRF | = | Case report form |
| DTPa-IPV-Hib-Hep-B | = | diphtheria, tetanus, acellular pertussis, inactivated polio, Haemophilus influenzae type B, hepatitis B combined vaccine |
| ICD | = | International Classification of Diseases |
| MLE | = | Maximum Likelihood Estimator |
| NUE | = | Nearly Unbiased Estimator |
| OR | = | Odds ratio |
| PEI | = | Paul-Ehrlich-Institut |
| STIKO | = | Ständige Impfkommission |
| RRV-TV | = | Tetravalent rhesus-based rotavirus vaccine |
| RV1 | = | Rotarix |
| RV5 | = | RotaTeq |
| US | = | United States |
| VAERS | = | Vaccine Adverse Event Reporting System. |
Disclosure of Potential Conflicts of Interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The work was financed by institutional resources. DO, MH, JP, DM, IB, UD, and BK have indicated no financial conflicts of interest.
Ethics statement
This observational study was approved by the Ethics Committee of Human Experimentation at the Medical Chamber of the Federal State Hessen (Germany) (ID No. FF 30/2015). The study was conducted in accordance with the Helsinki Declaration 1975. Written informed consent was obtained from the parents of participating cases and controls.
Acknowledgments
We wish to thank all participating study centers as well as parents of patients and controls for their active support.
References
- Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception. PLoS One. 2013;8:e68482. doi:10.1371/journal.pone.0068482.
- Weiß S, Streng A, von Kries R, Liese J, Wirth S, Jenke AC. Incidence of intussusception in early infancy: a capture-recapture estimate for Germany. Klin Padiatr. 2011;223:419–23. doi:10.1055/s-0031-1279735.
- Jenke AC, Klaassen-Mielke R, Zilbauer M, Heininger U, Trampisch H, Wirth S. Intussusception: incidence and treatment-insights from the nationwide German surveillance. J Pediatr Gastroenterol Nutr. 2011;52:446–51. doi:10.1097/MPG.0b013e31820e1bec.
- Samad L, Marven S, El Bashir H, Sutcliffe AG, Cameron JC, Lynn R, Taylor B. Prospective surveillance study of the management of intussusception in UK and Irish infants. Br J Surg. 2012;99:411–15. doi:10.1002/bjs.v99.3.
- European Centre for Disease Prevention and Control. ECDC expert opinion on rotavirus vaccination in infancy; 2017. https://ecdc.europa.eu/sites/portal/files/documents/rotavirus-vaccination-expert%20opinion-september-2017.pdf.
- Lee YW, Yang SI, Kim JM, Kim JY. Clinical features and role of viral isolates from stool samples of intussuception in children. Pediatr Gastroenterol Hepatol Nutr. 2013;16:162–70. doi:10.5223/pghn.2013.16.3.162.
- Bines JE, Liem NT, Justice FA, Son TN, Kirkwood CD, de Campo M, Barnett P, Bishop RF, Robins-Browne R, Carlin JB, et al. Risk factors for intussusception in infants in Vietnam and Australia: adenovirus implicated, but not rotavirus. J Pediatr. 2006;149:452–60. doi:10.1016/j.jpeds.2006.04.010.
- Lappalainen S, Ylitalo S, Arola A, Halkosalo A, Räsänen S, Vesikari T. Simultaneous presence of human herpesvirus 6 and adenovirus infections in intestinal intussusception of young children. Acta Paediatr. 2012;101:663–70. doi:10.1111/apa.2012.101.issue-6.
- Minney-Smith CA, Levy A, Hodge M, Jacoby P, Williams SH, Carcione D, Roczo-Farkas S, Kirkwood CD, Smith DW. Intussusception is associated with the detection of adenovirus C, enterovirus B and rotavirus in a rotavirus vaccinated population. J Clin Virol. 2014;61:579–84. doi:10.1016/j.jcv.2014.10.018.
- Arbizu RA, Aljomah G, Kozielski R, Baker SS, Baker RD. Intussusception associated with adenovirus. J Pediatr Gastroenterol Nutr. 2014;59:e41. doi:10.1097/MPG.0b013e3182868971.
- Ukarapol N, Khamrin P, Khorana J, Singhavejsakul J, Damrongmanee A, Maneekarn N. Adenovirus infection. J Med Virol. 2016;88:1930–35. doi:10.1002/jmv.v88.11.
- Asano Y, Yoshikawa T, Suga S, Hata T, Yamazaki T, Yazaki T. Simultaneous occurrence of human herpesvirus 6 infection and intussusception in three infants. Pediatr Infect Dis J. 1991;10:335–37. doi:10.1097/00006454-199104000-00015.
- Park H, Park S, Hong YJ, Lee SW, Cho M-S. Cytomegalovirus-associated intussusception with florid vascular proliferation in an infant. J Pathol Transl Med. 2015;49:270–73. doi:10.4132/jptm.2015.04.01.
- Aminu M, Ameh EA, Geyer A, Esona MD, Taylor MB, Steele AD. Role of astrovirus in intussusception in Nigerian infants. J Trop Pediatr. 2009;55:192–94. doi:10.1093/tropej/fmn101.
- Moore FO, Berne JD, Slamon NB, Penfil SH, Dunn SP. Intussusception in a child with respiratory syncytial virus. Del Med J. 2006;78:185–87.
- Velázquez FR, Luna G, Cedillo R, Torres J, Muñoz O. Natural rotavirus infection is not associated to intussusception in Mexican children. Pediatr Infect Dis J. 2004;23:S173–8. doi:10.1097/01.inf.0000142467.50724.de.
- El-Hodhod MA, Nassar MF, Ezz El-Arab S, Ahmed EF. Rotavirus fecal antigen retrieval in infantile intussusception. Eur J Clin Microbiol Infect Dis. 2008;27:879–81. doi:10.1007/s10096-008-0506-6.
- Chang EJ, Zangwill KM, Lee H, Ward JI, et al. Lack of association between rotavirus infection and intussusception. Pediatr Infect Dis J. 2002;21:97–102. doi:10.1097/00006454-200202000-00003.
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi:10.1056/NEJMoa052434.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi:10.1056/NEJMoa052664.
- Bergman H, Henschke N, Pitan F, Cunliffe, N, Soares‐Weiser, K, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2019 (10). doi: 10.1002/14651858.CD008521.pub5.
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, Booy R, Bines JE. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the national immunization program in Australia. Vaccine. 2011;29:3061–66. doi:10.1016/j.vaccine.2011.01.088.
- Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Márquez AB, Flannery B, Esparza-Aguilar M, Montenegro Renoiner EI, Luna-Cruz ME, Sato HK, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364:2283–92. doi:10.1056/NEJMoa1012952.
- Velázquez FR, Colindres RE, Grajales C, Hernández MT, Mercadillo MG, Torres FJ, Cervantes-Apolinar M, DeAntonio-Suarez R, Ortega-Barria E, Blum M, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J. 2012;31:736–44. doi:10.1097/INF.0b013e318253add3.
- Yen C, Tate JE, Steiner CA, Cortese MM, Patel MM, Parashar UD. Trends in intussusception hospitalizations among US infants before and after implementation of the rotavirus vaccination program, 2000-2009. J Infect Dis. 2012;206:41–48. doi:10.1093/infdis/jis314.
- Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, Bines J, McIntyre PB. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s national immunization program. Clin Infect Dis. 2013;57:1427–34. doi:10.1093/cid/cit520.
- Haber P, Patel M, Pan Y, Baggs J, Haber M, Museru O, Yue X, Lewis P, DeStefano F, Parashar UD, et al. Intussusception after rotavirus vaccines reported to US VAERS, 2006-2012. Pediatrics. 2013;131:1042–49. doi:10.1542/peds.2012-2554.
- Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, Klein NP, Glanz JM, Jacobsen SJ, Naleway A, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014;370:513–19. doi:10.1056/NEJMoa1311738.
- Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, Selvam N, Selvan M, Lee GM, Nguyen M, et al. Intussusception risk after rotavirus vaccination in U.S. Infants N Engl J Med. 2014;370:503–12. doi:10.1056/NEJMoa1303164.
- Quinn HE, Wood NJ, Cannings KL, Dey A, Wang H, Menzies RI, Moberley S, Reid S, McIntyre PB, Macartney KK, et al. Intussusception after monovalent human rotavirus vaccine in Australia. Pediatr Infect Dis J. 2014;33:959–65. doi:10.1097/INF.0000000000000362.
- Pérez-Vilar S, Díez-Domingo J, Puig-Barberà J, Gil-Prieto R, Romio S. Intussusception following rotavirus vaccination in the Valencia Region, Spain. Hum Vaccin Immunother. 2015;11:1848–52. doi:10.1080/21645515.2015.1049787.
- Bauchau V, van Holle L, Mahaux O, Holl K, Sugiyama K, Buyse H. Post-marketing monitoring of intussusception after rotavirus vaccination in Japan. Pharmacoepidemiol Drug Saf. 2015;24:765–70. doi:10.1002/pds.3800.
- Contopoulos-Ioannidis DG, Halpern MS, Maldonado Y. Trends in hospitalizations for intussusception in California in relationship to the introduction of new rotavirus vaccines, 1985-2010. Pediatr Infect Dis J. 2015;34:712–17. doi:10.1097/INF.0000000000000653.
- Haber P, Parashar UD, Haber M, DeStefano F. Intussusception after monovalent rotavirus vaccine-United States, vaccine adverse event reporting system (VAERS), 2008-2014. Vaccine. 2015;33:4873–77. doi:10.1016/j.vaccine.2015.07.054.
- Stowe J, Andrews N, Ladhani S, Miller E. The risk of intussusception following monovalent rotavirus vaccination in England. Vaccine. 2016;34:3684–89. doi:10.1016/j.vaccine.2016.04.050.
- Leino T, Ollgren J, Strömberg N, Elonsalo U. Evaluation of the intussusception risk after pentavalent rotavirus vaccination in Finnish infants. PLoS One. 2016;11:e0144812. doi:10.1371/journal.pone.0144812.
- Rotarix - Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000639/WC500054789.pdf.
- RotaTeq - summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000669/WC500054185.pdf.
- Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, Zanardi LR, Setia S, Fair E, LeBaron CW, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–72. doi:10.1056/NEJM200102223440804.
- Robert Koch-Institut. Empfehlungen der Ständigen Impfkommission (STIKO) beim Robert Koch-Institut - 2018/2019. Epi Bull; 2018. p. 351.
- Dudareva-Vizule S, Koch J, der Heiden M, Oberle D, Keller-Stanislawski B, Wichmann O. Impact of rotavirus vaccination in regions with low and moderate vaccine uptake in Germany. Hum Vaccin Immunother. 2012;8:1407–15. doi:10.4161/hv.21593.
- Bennett A, Pollock L, Jere KC, Pitzer VE, Lopman B, Parashar U, Everett D, Heyderman RS, Bar-Zeev N, Cunliffe NA, et al. Infrequent transmission of monovalent human rotavirus vaccine virus to household contacts of vaccinated infants in Malawi. J Infect Dis. 2019;219:1730–34. doi:10.1093/infdis/jiz002.
- Ye S, Whiley DM, Ware RS, Kirkwood CD, Lambert SB, Grimwood K. Multivalent rotavirus vaccine and wild-type rotavirus strain shedding in Australian infants: a birth cohort study. Clin Infect Dis. 2018;66:1411–18. doi:10.1093/cid/cix1022.
- Rosillon D, Buyse H, Friedland LR, Ng S-P, Velázquez FR, Breuer T. Risk of intussusception after rotavirus vaccination. Pediatr Infect Dis J. 2015;34:763–68. doi:10.1097/INF.0000000000000715.
- Simonsen L, Morens DM, Elixhauser A, Gerber M, Van Raden M, Blackwelder WC. Effect of rotavirus vaccination programme on trends in admission of infants to hospital for intussusception. The Lancet. 2001;358:1224–29. doi:10.1016/S0140-6736(01)06346-2.
- Konno T, Suzuki H, Kutsuzawa T, Imai A, Katsushima N, Sakamoto M, Kitaoka S, Tsuboi R, Adachi M. Human rotavirus infection in infants and young children with intussusception. J Med Virol. 1978;2:265–69. doi:10.1002/(ISSN)1096-9071.
- Burke RM, Tate JE, Dahl RM, Aliabadi, N and Parashar, UD, et al. Does rotavirus vaccination affect longer-term intussusception risk in US infants? J Pediatric Infect Dis Soc. 2019. doi: 10.1093/jpids/piz035.
- Kurzbart E, Cohen Z, Yerushalmi B, Yulevich A, Newman-Heiman N, Mares AJ. Familial idiopathic intussusception. J Pediatr Surg. 1999;34:493–94. doi:10.1016/S0022-3468(99)90510-9.
- Papadopoulou F, Efremidis SC. Familial intussusception. J Pediatr Surg. 2002;37:1549–51. doi:10.1053/jpsu.2002.36182.
- Oshio T, Ogata H, Takano S, Ishibashi H. Familial intussusception. J Pediatr Surg. 2007;42:1509–14. doi:10.1016/j.jpedsurg.2007.04.012.
- Restivo V, Costantino C, Giorgianni G, Cuccia M, Tramuto F, Corsello G, Casuccio A, Vitale F. Case-control study on intestinal intussusception: implications for anti-rotavirus vaccination. Expert Rev Vaccines. 2018;17:1135–41. doi:10.1080/14760584.2018.1546122.
- Fotso Kamdem A, Vidal C, Pazart L, Leroux F, Pugin A, Savet C, Sainte-Claire Deville G, Guillemot D, Massol J. A case-control study of risk factors for intussusception among infants in eastern France after the introduction of the rotavirus vaccine. Vaccine. 2019;37:4587–93. doi:10.1016/j.vaccine.2019.02.053.
- Ong NT, Beasley SW. The leadpoint in intussusception. J Pediatr Surg. 1990;25:640–43. doi:10.1016/0022-3468(90)90353-B.
- Navarro O, Dugougeat F, Kornecki A, Shuckett B, Alton DJ, Daneman A. The impact of imaging in the management of intussusception owing to pathologic lead points in children. A review of 43 cases. Pediatr Radiol. 2000;30:594–603. doi:10.1007/s002470000261.
- Lin XK, Xia QZ, Huang XZ, Han YJ, He GR, Zheng N. Clinical characteristics of intussusception secondary to pathologic lead points in children. Pediatr Surg Int. 2017;33:793–97. doi:10.1007/s00383-017-4101-8.
- Fewell Z, Davey Smith G, Sterne JAC. The impact of residual and unmeasured confounding in epidemiologic studies. Am J Epidemiol. 2007;166:646–55. doi:10.1093/aje/kwm165.
- Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, Hansen J, Murphy TM, Music S, Niu M, Varricchio F, et al. Acute intussusception in infants and children as an adverse event following immunization. Vaccine. 2004;22:569–74. doi:10.1016/j.vaccine.2003.09.016.
- Voigt M, Rochow N, Schneider KTM, Hagenah HP, Scholz R, Hesse V, Wittwer-Backofen U, Straube S, Olbertz D. Neue Perzentilwerte für die Körpermaße neugeborener Einlinge. Z Geburtshilfe Neonatol. 2014;218:210–17. doi:10.1055/s-00000095.
- Petersen CGJ. The yearly immigration of young plaice into the Limfiord from the German sea. Rep Dan Biol Stn. 1896;6:5–84.
- Hook EB, Regal RR. Capture-recapture methods in epidemiology: methods and limitations. Epidemiol Rev. 1995;17:243–64. doi:10.1093/oxfordjournals.epirev.a036192.
- Chapman DG. Some properties of the hypergeometric distribution with applications to zoological sample censuses. Univ California Publ Stat. 1951;1:1131–60.
- Lincoln FC. Calculating waterfowl abundance on the basis of banding returns. Circular No. 118. Washington (DC): US Department of Agriculture; 1972. p. 1–4.
- Wittes JT. On the bias and estimated variance of Chapman’s two-sample capture-recapture population estimate. Biometrics. 1972;592–97. doi:10.2307/2556173.