ABSTRACT
In France, the incidence of invasive meningococcal disease (IMD) is around 1/100,000, with the following trends over the 2011–2018 period: a leading role of group B in subjects <15 years, a decrease of group C among <1 year since 2017, an increase of group W in all age groups including subjects <1 year since 2014 and a positive correlation between group Y and age group.
In Europe, vaccination progressed with conjugate ACWY vaccines and proteins-based B vaccines. Their benefit-risk-cost balance is however not so obvious for area at low incidence (<2/100,000), explaining tremendous variations between countries, from no recommendation to recommend all available vaccines. In France, the calendar still includes only C with a good adhesion in infants but a fiasco of the catch-up campaign in adolescents and young adults.
In Europe, it is time to consider not only national epidemiology but also trends in the neighborhood. The increase of group W cases encourages switching C to ACWY vaccine both in infants and adolescents. It is also time to protect infants with B vaccine. Large pedagogy on the disease is required to increase the adhesion to the vaccination and to recognize and treat earlier the residual cases.
1. Introduction
Neisseria meningitidis is a highly diverse bacterial species only encountered in humans. The meningococcus is most often present as a commensal bacterial species of the rhino-oropharynx, with an overall carriage rate around 10% in the general population but markedly variable with age: it is low in young children and increase to reach its peak among teenagers and young adults.Citation1 Meningococcal transmission occurs mainly by air droplets through close inter-human contacts. Sporadic sexual transmission has also been reported.Citation2 Rarely, the acquisition of a virulent clone is followed in the absence of natural- or vaccine-induced immunity by an invasive meningococcal disease (IMD) that is dominated by meningitis and meningococcemia (septicemia). Invasive isolates are surrounded by a polysaccharide capsule that determines the serogroup. Among the 12 known serogroups, 6 are responsible for virtually all IMD worldwide: A, B, C, W, Y and X.Citation3 The potential epidemic spread of such invasive meningococcal isolates implies the implementation of preventive measures among contact subjects of IMD cases (antibioprophylaxis ± vaccination). The susceptibility of the host also plays a role in the attack rate after acquisition (e.g., higher incidence in subjects with deficits in the late components of complement).Citation4 IMD has a high fatality rate (10%) and sequelae (around 30%, this value remains very approximate because of a lack of data on the long-term consequences).Citation5
2. Surveillance of meningococcal disease in France
The surveillance of IMD in France relies on two parts: (i) mandatory notification of cases to Public Health France according to a standardized national case definition and (ii) typing of isolates at the National Reference Center for Meningococci (NRCM). Case definition is based mainly on biological criteria, i.e.,, the detection of N. meningitidis (by culture and/or PCR) from a sterile site (such as blood, cerebrospinal fluid, other sterile sites) or purpuric skin lesions. Thus, culture and/or PCR are responsible for the confirmation of >96% of notified cases in France.Citation6
The incidence of IMD in France is around 1 cases per 100,000 inhabitants during the period 2006–2015 and the completeness of mandatory notification system is >90% since 2005.Citation6
3. Evolution of the biologically confirmed cases of invasive meningococcal disease in France since 2011
The NRCM analyzed 3619 IMD cases during the period 2011–2018. Serogroup B predominates for this period but with a significant (p = .0009) decreasing trend. This trend remains significant after excluding IMDB cases from Normandy (i.e., Seine Maritime) where a clonal B outbreak had been observed between 2003 and 2013 that was controlled by MenBvac, an OMV-based vaccineCitation7 (). Serogroup C stagnated at the second rank (23% of cases for the total period). As otherwise in EuropeCitation8 serogroup Y increased in France from 8.2% in 2011 to 15% in 2017. However, IMDY lost its third rank in 2018 (13.9%) due to the emergence of serogroup W from 2.6% in 2011 to 14.3% in 2018 (p = .0036). The total number of IMD cases was rather stable among the infants <1 year while it was decreasing among subjects between 1 and 24 years old. The follow-up of the number of cases by serogroup and age () indicated the following trends over the 2011–2018 period: (i) persistence of a leading role of group B in subjects <16 years; (ii) decrease of group C among <1 year; (iii) increase of group W in all age groups including subjects <1 year since 2014; (iv) association of group Y and age (one-third of total cases in subjects >64 years).
Figure 1. (A) Distribution of French cases of invasive meningococcal disease by serogroup for the period 2011–2018 period (Institut Pasteur data). (B) The decreasing trend of IMDB between 2011 and 2018. Linear regression of cases of IMDB at the country level (black square) or for France after exclusion of a Norman outbreak controlled in 2013 (red circle). Data are shown with solid lines and 95% IC with dashed lines. Note that the slopes are not significantly different
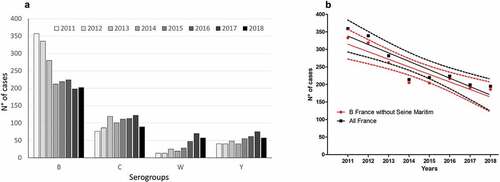
Figure 2. Distribution of French cases of invasive meningococcal disease by serogroups and groups of age for the 2011–2018 period (Institut Pasteur data)
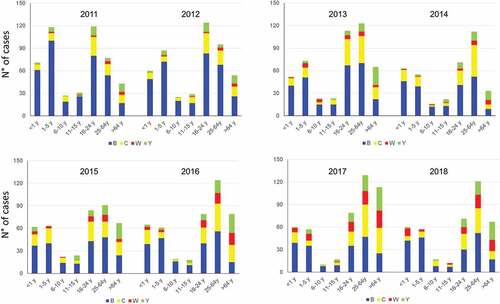
The decrease of cases of IMDC among <1 year is most likely due to the introduction of a dose of MenC conjugate (MCC) vaccine at the age of 5 months in 2017 and the implementation in 2018 of mandatory vaccinations. The increase of IMDW is due to the recent expansion of isolates derivatives of the South American-UK MenW strain.Citation9 Cases of IMD due to these isolates increased mainly among adolescents and young adults since 2015 and have a high mortality rate and frequent atypical initial symptoms of IMD such as the abdominal presentations.Citation9 Citation10 The emergence and expansion of isolates of serogroup W derived from the South America UK strain were also observed in other European countries.Citation11 These isolates first emerged in the UK since 2009 with a subsequent emergence in the Netherlands since 2012–2013.Citation12
4. Available vaccines and historical evolution of the recommendations in France
Capsular polysaccharide conjugate vaccines either monovalent against serogroup C meningococci (MCC) or tetravalent against ACWY (MCV4) are available in France since 2000 and 2010, respectively. They made obsolete the previous non-conjugate polysaccharides vaccines (against C, AC and ACWY) because of the persistence of the immune response and their dual action, not only to confer direct protection against IMD of the matching serogroup (as the previous vaccines) but also to prevent carriage acquisition of the isolates expressing the corresponding serogroup. Therefore, the transmission of invasive clones is prevented provided high vaccination coverage in the targeted population.Citation13
In France, MCC vaccine has been recommended during the period 2000–2010 only for at risk groups. In 2010, the indications for MCC vaccine have been enlarged for the following reasons: (i) an increasing number of alerts and outbreaks; (ii) the emergence of a new C clone (from the clonal complex ST-11) provoking high mortality and high proportion of purpura fulminans; (iii) the impressive success of mass MCC campaigns in United Kingdom and then the Netherlands having so demonstrated the absence of switch to other serogroup (it was initially a theoretical concern); (iv) the occurrence of a pandemic flu (H1N1nv) exposing to a potential increase of IMD (the flu infection favors subsequent infection by N. meningitidis); (v) a favorable evaluation of the cost-effectiveness of the new strategy.Citation14 Thus, MCC was recommended as1 dose to all infants at 12 months age and as a “catch-up” program with also 1 dose for each subject <25 years during the implementation phase of the new strategy and until group immunity (“herd effect”) is obtained. With this strategy, infants <1 year old were supposed to be protected by the group immunity that was aimed to be established through high coverage of vaccination particularly among 16–24 years old (subjects having a driven role in the transmission due to their high carriage rate). Unfortunately, the incidence of IMDC did not decrease but rather increased between 2010 and 2016 among the <1 year oldCitation6 due to low coverage among teenagers and young adults (22.5% for 15–19 years old and 9.4% for 20–24 years old subjects in 2015). The coverage reached 68.2% in 2015 among the 2 years old children. Vaccination hesitation with no active efforts to apply and explain the vaccination strategy seems to be a major reason of this low vaccine uptake, particularly among the 16–24 years old.Citation15 This hesitancy arises from the loss of collective awareness of the benefits of vaccination and the risks of infectious diseases that have become less prevalent. Thus since 2017, the French vaccination schedule includes an additional injection of MCC vaccine at the age of 5 months to directly protect infants (i.e., 2 doses schedule, at M5M12) while the catch-up until the age of 24 years is maintained. Since 2018, the two MCC vaccine doses in infancy have been moved to the mandatory vaccinations (beside tetanus and others). Subsequently, the MCC vaccine coverage among infants at the age of 5 months increased from 39% at the end of 2017 to 76% at the end of 2018 and that was associated with a decrease in the number of cases among <1 year of age from19 cases in 2016 to 14 cases in 2017 and in 6 cases 2018 according to the data from the NRCM (and only one case in a non-vaccinated infant for the first 11 months of 2019). Moreover, a reduction of IMDC cases among children between 1 and 5 years old was also observed (). However, IMCD cases remained stable in older subjects.
Since it was made available, MCV4 vaccine is recommended in France only for at risk subjects or for the control of covered outbreaks. At risk subjects are close contacts, laboratory staff working of meningococci, subjects with terminal pathway complement deficiencies, properdin deficiency, subjects with asplenia, subjects who received a hematopoietic stem cell transplantation and travelers such as pilgrims to the Hajj. Outbreak of IMDC remained targeted by MCC vaccine. Several small outbreaks of IMDW have recently occurred in France, mainly due to derivatives of the South America UK strain, leading to MCV4 campaigns for the affected population, mainly university campuses.Citation16 Citation17 The most important was at the University of Bourgogne with unfortunately a rather low vaccination coverage rate (41%).Citation17
Until 2013 IMDB prevention has been orphaned of effective vaccines, because the conventional polysaccharide approach was not appropriate for that serogroup. Two MenB protein-based vaccines are now licensed in some countries, the 4CMenB (the only one currently available in France) and the bivalent (rLP2086). They are potentially active on non-B isolates as the proteins components of these vaccines are shared by other serogroups but also inactive against few MenB isolates (for each of these two vaccines, around 15% of the current European B isolates are not covered).Citation18 Citation19 Furthermore, these protein-based vaccines do not seem to have a significant impact on the acquisition of meningococcal B carriage, require multi-dose regimens (2 to 3 primary vaccination according to the age of the subject) and have a short durability.Citation20–23 The United Kingdom has been also here pioneer, introducing the 4CMenB in their infant immunization schedule in 2015 because of a high prevalence of IMDB at this age, with a high adhesion (>90%) and a significant decrease of incidence acquired in the first year, although there are still many unknowns about sustainability.Citation24 Other countries have more recently introduced 4CMenB or rLP2086 to their immunization program.
In France, 4CMenB is currently recommended only for risk subjects or in case of IMDB outbreak due to a covered strain. Of note, the “at risk subjects” did not include the close contacts of a B case because it was considered that the delay to obtain a protective immunity is too long.Citation25
5. Current meningococcal vaccine program in other countries with close epidemiology
Different national meningococcal vaccination programs are used wordwide, even in countries facing similar conditions like in Europe.
All countries agree on the WHO vaccination strategy in case of high incidence (>10 cases per 100,000 inhabitants per year) or medium-incidence (2–10 cases/100,000) or frequent epidemics: unanimity in favor of mass vaccination program, targeting preferably young people (9 months – 18 years) using if possible a vaccine active against carriage acquisition. The vision is also unanimous in case of clonal epidemic: vaccination for the exposed population (a barracks, a university, a district …) also if possible with a vaccine active against carriage acquisition. All experts, finally, recommend vaccination for at “high risk subjects”, even if their definition varies discretely from one country to another (in general: immunodeficiency, asplenia, and laboratory personnel working specifically on meningococcal disease).
The divergences begin for the vaccination strategy around one IMD case recommended, for example, in only 24 out of 33 European countries in 2013Citation26 or before a trip in a country of high or moderate IMD incidence with the exception of the Hajj for which an ACWY vaccine is mandatory for all pilgrims.Citation3
Divergences are huge for mass meningococcal vaccination in countries with low endemicity (<2/100,000) which are today the majority. The strategy is indeed much more difficult to define, because of a less obvious benefit-risk-cost balance, resulting in a great variability of recommendations according to the countries even at close socio-economic levels. Thus in July 2019 among the 31 European countries of ECDC 10 did not propose any meningococcal vaccination on the calendar of the general population (i.e., all serogroups and all ages combined), 15 covered the C risk (8 exclusively by MCC, 2 exclusively by MCV4, 5 with a mix of MCC for infants and MCV4 for teenagers) and 6 covered the B risk [ECDC website]. Only one of these 31 countries has opted for an obligation to vaccine: France for C coverage of infants and toddlers as detailed above. Such marked differences are not supported by tremendous epidemiological variations between countries. Same divergences are observed in other continents. In North America, where the overall incidence is very low (0.5/100 000), the United States recommend a MCV4 in adolescence while Canada offers MCC in infancy before MCC or MCV4 at age of 12 years. In Australasia where the incidence is higher (1.5/100 000), New Zealand recommends any meningococcal vaccine while Australia offers coverage against B and ACWY to infants and teenagers.Citation27
6. Suggestions for an evolution of the French meningococcal prevention policy
We would like to make here few suggestions on the basis of our involvement in the epidemiological and microbiological surveillance of meningococci at the country level (MKT, AED) and in clinical care (JG) including management of alert a one decade IMDB outbreak in Normandy (FC, MKT)Citation7
There are a lot of demographic interactions in Europe. For example, each year 12 millions of French subjects travel to immediate neighbors (Benelux, Germany, Italy, Spain and UK), while 54 millions of subjects from these countries visit France. The Erasmus program for student mobility involves annually about 40,000 young adults in France for a mean duration of 6 months. A possible spread of meningococcal isolates was described.Citation28 Symbolically, it is estimated that one million of “Erasmus babies” have borne over 30 years in all Europe. Thus, we appeal to a European harmonization of meningococcal vaccination, at least for teenagers and young adults.
The recent increase of IMDW in Europe, affecting now all groups of age in France, argues for introducing MCV4. The adolescents and young adults would be the first target because of the number of IMDW and also IMDY and the high rate of meningococcal transmission. Such an introduction requires active advocating as the previous MCC catch up without campaign in adolescents and young adults remains a fiasco as detailed above. The better age to apply MCV4 would be probably 15–18 years before leaving family home for university or job. Such a strategy would require high uptake to rapidly achieve both direct but also indirect protection. The benefit of MCV4 against carriage is however somewhat controversial with one study having shown no reduction of MenW carriage in adolescents.Citation29 Such strategy might also decrease incidence of IMDCWY in adults from middle age, thanks to the durability of the immunogenicity at individual level and also to a herd effect (at least for C and Y). In elderly IMDY needs more attention, particularly because it is frequently observed as invasive pneumoniaCitation30-32 implying that its burden may be underestimated.
In infancy, two strategies might be discussed in view of the lower immunogenicity at this age of current available MCV4 compared to MCC against C isolates.Citation33 However, French infants <1year are now affected by W and Y isolates (), while IMDC cases have recently decreased. This is in favor to opt for MCV4 in infants.
Finally, it seems also time to introduce MenB in infants since serogroup B remains, by far, the leading cause of IMD cases in young children. The lack of herd effect is not a barrier to such a policy, particularly at this age rank in which the meningococcal carriage rate is proportionally low. The UK experience offers goods arguments not only about effectiveness but also in a lesser extent about tolerance: 30% of local effects or fever which is high for a meningococcal vaccine but comparable to other well-accepted vaccine such as mumps in infancy; very low risk of serious effect such as seizuresCitation34
Vaccination is the key element to prevent IMD but it is not universal and sometime fails. Thus, it is important to not forget the “secondary prevention”, which is based on diagnosis and treatment of each case as soon as possible. To achieve this goal we would recommend first to more explain IMD to public as well as caregivers, particularly to inform not only about signs of meningitis and risk of mortality, both globally well known, but also about atypical symptoms (see below) and sequelae largely ignored albeit common and severe (amputation, deafness, break of curriculum, prolonged depression …). This might help to the vaccine adhesion particularly in adolescents, a more reluctant group than infants. It is also necessary to explain the two-stage of numerous IMDs with initial nonspecific symptoms (fever, asthenia) mimicking banal virosis (especially since IMDs have the same seasonality). Facing an acute fever without definite diagnosis it is critical to explain to each subject the importance of a surveillance, and this by someone else because the aggravation can be so brutal that the patient will not give the alert; it is the place of the family, but also of peers as it is organized in Anglo-Saxon campus to ensure, even in the middle of the night, that the patient is “not worse” and that it is still a banal virosis, not a beginning IMD. Lastly, it is necessary to teach the “glass test” which in event of an eruption allows the public to identify a purpura and help regulation of health care to prioritize such case for a first antibiotic dose within 20 min, saving so life and limbs. All this was done in Normandy, having saved situations, as had been previously demonstrated in UK and Scandinavia.
7. Conclusions
IMD is difficult to diagnose at early stages with unpredictable epidemiology that is constantly changing. Surveillance to detect changes in incidence, serogroup and age distribution as well as the emergence of new genotypes is critical to adapt vaccination strategies.
Vaccination had make marked progress with now both conjugate capsular polysaccharides vaccines against ACWY and proteins-based vaccines against B.
Facing epidemiological changes in France and in neighboring countries it might be time to implement coverage of ACWY and B in infancy and against ACWY in adolescence.
Large pedagogy on the disease is also required to increase compliance with the vaccination.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–61. doi:10.1016/S1473-3099(10)70251-6.
- Bazan JA, Turner AN, Kirkcaldy RD, Retchless AC, Kretz CB, Briere E, Tzeng Y-L, Stephens DS, Maierhofer C, Del Rio C, et al. Large cluster of neisseria meningitidis urethritis in columbus, Ohio, 2015. Clin Infect Dis. 2017;65:92–99. doi:10.1093/cid/cix215.
- Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, Christensen H, Climent Y, De Wals P, Dinleyici EC, et al. The global meningococcal initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. 2019;18:15–30. doi:10.1080/14760584.2019.1557520.
- Rosain J, Hong E, Fieschi C, Martins PV, El Sissy C, Deghmane AE, Ouachée M, Thomas C, Launay D, de Pontual L, et al. Strains responsible for invasive meningococcal disease in patients with terminal complement pathway deficiencies. J Infect Dis. 2017;215:1331–38. doi:10.1093/infdis/jix143.
- Viner RM, Booy R, Johnson H, Edmunds WJ, Hudson L, Bedford H, Kaczmarski E, Rajput K, Ramsay M, Christie D. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol. 2012;11:774–83. doi:10.1016/S1474-4422(12)70180-1.
- Parent du Chatelet I, Deghmane AE, Antona D, Hong E, Fonteneau L, Taha MK, Levy-Bruhl D. Characteristics and changes in invasive meningococcal disease epidemiology in France, 2006–2015. J Infect. 2017;74:564–74.
- Caron F, du Chatelet IP, Leroy JP, Ruckly C, Blanchard M, Bohic N, Massy N, Morer I, Floret D, Delbos V, et al. From tailor-made to ready-to-wear meningococcal B vaccines: longitudinal study of a clonal meningococcal B outbreak. Lancet Infect Dis. 2011;11:455–63. doi:10.1016/S1473-3099(11)70027-5.
- Broker M, Jacobsson S, Kuusi M, Pace D, Simoes MJ, Skoczynska A, Taha M-K, Toropainen M, Tzanakaki G. Meningococcal serogroup Y emergence in Europe: update 2011. Hum Vaccin Immunother. 2012;8:1907–11. doi:10.4161/hv.21794.
- Hong E, Barret AS, Terrade A, Denizon M, Antona D, Aouiti-Trabelsi M, Deghmane AE, Parent du Chatelet I, Levy-Bruhl D, Taha MK. Clonal replacement and expansion among invasive meningococcal isolates of serogroup W in France. J Infect. 2018;76:149–58. doi:10.1016/j.jinf.2017.10.015.
- Guiddir T, Gros M, Hong E, Terrade A, Denizon M, Deghmane AE, Taha M-K. Unusual initial abdominal presentations of invasive meningococcal disease. Clin Infect Dis. 2018;67:1220–27. doi:10.1093/cid/ciy257.
- Krone M, Gray S, Abad R, Skoczynska A, Stefanelli P, van der Ende A, Tzanakaki G, Mölling P, João Simões M, Křížová P, et al. Increase of invasive meningococcal serogroup W disease in Europe, 2013 to 2017. Euro Surveill. 2019;24. doi:10.2807/1560-7917.ES.2019.24.14.1800245
- Knol MJ, Hahne SJM, Lucidarme J, Campbell H, de Melker HE, Gray SJ, Borrow R, Ladhani SN, Ramsay ME, van der Ende A, et al. Temporal associations between national outbreaks of meningococcal serogroup W and C disease in the Netherlands and England: an observational cohort study. Lancet Public Health. 2017;2:e473–e82. doi:10.1016/S2468-2667(17)30157-3.
- Khatami A, Snape MD, Davis E, Layton H, John T, Yu LM, Dull PM, Gill CJ, Odrjlin T, Dobson S, et al. Persistence of the immune response at 5 years of age following infant immunisation with investigational quadrivalent MenACWY conjugate vaccine formulations. Vaccine. 2012;30:2831–38. doi:10.1016/j.vaccine.2012.02.046.
- HCSP. Avis relatif à la vaccination par le vaccin méningococcique conjugué de sérogroupe C. 2009. [accessed 2019 Dec 15]. www.hcsp.fr/explore.cgi/hcspa20090424_meningC.pdf.
- Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, Cook AR, Jones NS. The State of Vaccine Confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301. doi:10.1016/j.ebiom.2016.08.042.
- Bassi C, Taha MK, Merle C, Hong E, Levy-Bruhl D, Barret AS, Mounchetrou Njoya I. A cluster of invasive meningococcal disease (IMD) caused by Neisseria meningitidis serogroup W among university students, France, February to May 2017. Euro Surveill. 2017;22. doi:10.2807/1560-7917.ES.2017.22.28.30574.
- Barret AS, Clinard F, Taha MK, Girard I, Hong E, Tessier S, Zurbaran M, de Bort C, Antona D, Deghmane AE, et al. Cluster of serogroup W invasive meningococcal disease in a university campus. Med Mal Infect. 2019. doi:10.1016/j.medmal.2019.10.003.
- McNeil LK, Donald RGK, Gribenko A, French R, Lambert N, Harris SL, Jones TR, Li S, Zlotnick G, Vogel U, et al. Predicting the susceptibility of meningococcal serogroup B isolates to bactericidal antibodies elicited by bivalent rLP2086, a novel prophylactic vaccine. MBio. 2018;9. doi:10.1128/mBio.00036-18
- Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, Stefanelli P, Caugant DA, Kriz P, Abad R, Bambini S, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013;13:416–25. doi:10.1016/S1473-3099(13)70006-9.
- Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, Heath PT, Lewis DJM, Pollard AJ, Turner DPJ, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384:2123–31. doi:10.1016/S0140-6736(14)60842-4.
- Soeters HM, Whaley M, Alexander-Scott N, Kanadanian KV, MacNeil JR, Martin SW, McNamara LA, Sicard K, Vanner C, Vuong J, et al. Meningococcal carriage evaluation in response to a serogroup B meningococcal disease outbreak and mass vaccination campaign at a College-Rhode Island, 2015–2016. Clin Infect Dis. 2017;64:1115–22. doi:10.1093/cid/cix091.
- McQuaid F, Snape MD, John TM, Kelly S, Robinson H, Yu LM, Toneatto D, D’Agostino D, Dull PM, Pollard AJ, et al. Persistence of specific bactericidal antibodies at 5 years of age after vaccination against serogroup B meningococcus in infancy and at 40 months. CMAJ. 2015;187:E215–23. doi:10.1503/cmaj.141200.
- Marshall HS, Richmond PC, Beeslaar J, Jiang Q, Jansen KU, Garces-Sanchez M, Martinón-Torres F, Szenborn L, Wysocki J, Eiden J, et al. Meningococcal serogroup B-specific responses after vaccination with bivalent rLP2086: 4 year follow-up of a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2017;17:58–67. doi:10.1016/S1473-3099(16)30314-0.
- Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White JM, Borrow R, Ramsay ME, Ladhani SN, et al. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388:2775–82. doi:10.1016/S0140-6736(16)31921-3.
- HCSP. Avis du HCSP relatif à l’utilisation du vaccin Bexsero (Novartis Vaccines and Diagnostics). 2013. [accessed 2019 Dec 15]. http://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=hcspa20131025_vaccmeningocoqueBBexsero.pdf.
- Vygen S, Hellenbrand W, Stefanoff P, Hanquet G, Heuberger S, Stuart J. European public health policies for managing contacts of invasive meningococcal disease cases better harmonised in 2013 than in 2007. Euro Surveill. 2016;21:23–31. doi:10.2807/1560-7917.ES.2016.21.5.30125.
- Sharma K, Chiu C, Wood N. Meningococcal vaccines in Australia: a 2019 update. Aust Prescr. 2019;42:131–35. doi:10.18773/austprescr.2019.042.
- Taha MK, Deghmane AE, Knol M, van der Ende A. Whole genome sequencing reveals Trans-European spread of an epidemic Neisseria meningitidis serogroup W clone. Clin Microbiol Infect. 2019;25:765–67. doi:10.1016/j.cmi.2018.12.030.
- Oldfield NJ, Green LR, Parkhill J, Bayliss CD, Turner DPJ. Limited impact of adolescent meningococcal ACWY vaccination on neisseria meningitidis serogroup W carriage in University Students. J Infect Dis. 2018;217:608–16. doi:10.1093/infdis/jix596.
- Sall O, Stenmark B, Glimaker M, Jacobsson S, Molling P, Olcen P, FREDLUND H. Clinical presentation of invasive disease caused by Neisseria meningitidis serogroup Y in Sweden, 1995 to 2012. Epidemiol Infect. 2017;145:2137–43. doi:10.1017/S0950268817000929.
- Vienne P, Ducos-Galand M, Guiyoule A, Pires R, Giorgini D, Taha MK, Alonso J-M. The role of particular strains of Neisseria meningitidis in meningococcal arthritis, pericarditis, and pneumonia. Clin Infect Dis. 2003;37:1639–42. doi:10.1086/379719.
- Feldman C, Anderson R. Meningococcal pneumonia: a review. Pneumonia. 2019;11:3. doi:10.1186/s41479-019-0062-0.
- Knuf M, Romain O, Kindler K, Walther U, Tran PM, Pankow-Culot H, Fischbach T, Kieninger-Baum D, Bianco V, Baine Y, et al. Immunogenicity and safety of the quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT) in 2-10-year-old children: results of an open, randomised, controlled study. Eur J Pediatr. 2013;172:601–12. doi:10.1007/s00431-012-1924-0.
- Bryan P, Seabroke S, Wong J, Donegan K, Webb E, Goldsmith C, Vipond C, Feavers I. Safety of multicomponent meningococcal group B vaccine (4CMenB) in routine infant immunisation in the UK: a prospective surveillance study. Lancet Child Adolesc Health. 2018;2:395–403. doi:10.1016/S2352-4642(18)30103-2.
