ABSTRACT
Objective: To advance the development of an ideal and sustainable framework agreement for the public procurement of vaccines in Spain, and to agree on the desirable award criteria and their relative weight.
Methods: A multidisciplinary committee of seven health-care professionals and managers developed a partial multi-criteria decision analysis to determine the award criteria that should be considered and their specific weights for the public procurement of routine vaccines and seasonal influenza vaccines, considering their legal viability. A re-test of the results was carried out. The current situation was analyzed through 118 tender specifications and compared to the ideal framework.
Results: Price is the prevailing award criterion for the public procurement of both routine (weighting of 60% versus 40% for all other criteria) and influenza (36% versus 64%) vaccines. Ideally, 22 criteria should be considered for routine vaccines, grouped and weighted into five domains: efficacy (weighting of 29%), economic aspects (27%), vaccine characteristics (22%), presentation form and packaging (13%), and others (9%). Per criteria set, price was the most important criterion (22%), followed by effectiveness (9%), and composition/formulation (7%). Regarding influenza vaccines, 20 criteria were selected, grouped, and weighted: efficacy (29%), economic aspects (25%), vaccine characteristics (20%), presentation form and packaging (16%), and others (11%). Per criteria set, price was also the most relevant criterion (19%), followed by composition/formulation (8%), and effectiveness (8%).
Conclusions: Contrary to the current approach, technical award criteria should prevail over economic criteria in an ideal and sustainable framework agreement for the public procurement of vaccines.
Introduction
Except for water purification, no other public health measure has contributed as much as vaccines to the decrease of morbidity and mortality in the human species.Citation1 Health-care authorities in most countries are very aware of this fact and have increased the number of available vaccines and vaccination programs.Citation2 Moreover, centralized public procurement is one of the instruments used to acquire vaccines more efficiently.Citation3
Spain, while having a regionally decentralized health-care system, has operated with a centralized public procurement procedure at a national level since 2012. Regions may opt to adhere to this procedure through a Framework Agreement (FA).Citation4 To date, three FAs have been signed to publicly acquire routine vaccines, in addition to those signed each year to purchase influenza vaccines.Citation5
The FA sets maximum prices and establishes the foundations that regulate the call-off agreements for the public procurement of vaccines included in the immunization schedule as well as for other vaccines. More specifically, the FA includes a list of the economic and technical award criteria that should be used. Subsequently, the autonomous communities adhering to this FA establish the weighting required for each criterion in order to convene the purchase procedure in that specific region.Citation6 This procedure also includes technical specifications describing the necessary technical requirements of each vaccine for the allocation.
The goal of the FA is to optimize the purchase of vaccines, unify the system, and contain expenses.Citation7 Nevertheless, the procedure faces many challenges that may be detrimental to the future sustainability of the purchase of vaccines, hence to public health. Although the weightings of award criteria are highly unrestricted, in practice price is usually the main criterion considered in the decision-making process. Moreover, some authors criticize that the FA does not give enough weight to vaccine characteristics, vaccine health-care goals, and the situation of vaccine manufacturers. These same authors suggest the promotion of other criteria besides price.Citation8,Citation9
Moreover, there are substantial regional differences regarding the award criteria and their weightings. These differences can generate problems associated with regional consistency and equity. In addition, the ambiguity of the standard often leads to discrepancies in the interpretation of the criteria. Therefore, more accurate definitions of the award criteria are deemed necessary. Furthermore, the new public sector contract law, which is applicable to any type of product, introduces new elements that may contribute to adjust the public procurement of vaccines.Citation10 Accordingly, one of the existing challenges when purchasing vaccines in Spain is the incorporation of models with a greater focus on the value that these vaccines can bring to society from a holistic and sustainable point of view for the system as a whole.
Therefore, the purpose of this study was to determine the criteria that should be included in an FA and their relative weightings for the public procurement of vaccines in Spain, to compare the ideal FA to the current situation, and to identify areas in need of improvement. Ultimately, the present study aims to advance the development of an ideal and sustainable FA for the public procurement of vaccines in Spain, contributing to a more cohesive and transparent process, and favoring an efficient, equitable, and sustainable health-care system. For this purpose, the multi-criteria decision analysis (MCDA) tool was used. More general guidelines are also suggested to improve the current FA, specially focusing on the global sustainability of the system.
Methodology
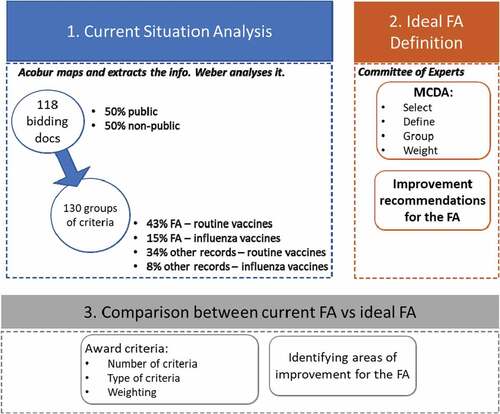
The methodology is comprised of three stages. In the first stage, the current situation of the public procurement of vaccines in Spain was analyzed. In the second stage, an MCDA was performed to determine the ideal FA. Finally, in the third stage, the current FA was compared to the ideal FA, and potential areas for improvement were identified ().
A differentiated approach was applied to routine and influenza vaccines approved by the Interterritorial Council of the Spanish National Health System, given their differential features and coverage rates. Analyses were carried out from a macro perspective for all vaccines in general, i.e., without analyzing the specific characteristics of each vaccine.
Stage 1: current situation
In order to analyze the current situation on the public procurement of vaccines in Spain, we mapped and extracted relevant information from the tender specifications for the public procurement of vaccines performed in Spain between January 2017 and July 2018. These tender specifications were related to the 2016 FA and other procedures not included in such FA. Specifically, the choice of award criteria and their relative weightings were analyzed and used as the basis for the development of the MCDA in the second stage.
A total of 118 tender specifications for the public procurement of vaccines were used for analysis. Of these, 50% were public and available for download (15 of them were downloaded from the State’s procurements online platform,Citation11 and 44 from the websites of autonomous communitiesCitation12-23), whereas the remaining 50% were provided by the governments of autonomous communities and pharma labs. Furthermore, these tender specifications were issued by the 17 Spanish autonomous communities (88% of the analyzed documents), the autonomous cities of Ceuta and Melilla (4 tender specifications or 3.4% of the analyzed documents), and different national public contracting authorities such as the Ministry of Health, Social Services and Equality, the Ministry of Defense, the Prison System, and the National Institute of Healthcare Management (8.5%) (). Madrid was the region with the largest number of tender specifications issued (11), followed by Catalonia (10), and Aragon and Asturias (9 documents each).
Table 1. Distribution of the analyzed 118 tender specifications by autonomous community or public authority
Stage 2: ideal situation
A partial MCDA was performed to determine the agreed upon, specific, and well-defined award criteria that should ideally be included in an FA for the public procurement of routine and influenza vaccines in Spain, including the specific weight each criterion should have in the final decision. This was carried out by an advisory board which comprised seven experts on vaccines with different profiles, with experience in the clinical and/or management areas and representing different regions in Spain. Moreover, this committee was further advised by a health economist and a legal expert on public contracts.
The MCDA was performed in three steps:
Step 1: The experts of the advisory board received online training on the MCDA methodology and were encouraged to think about the potential award criteria that should be considered for an ideal FA.
Step 2: The award criteria for an ideal FA for routine and influenza vaccines were selected, grouped, defined, and weighted during a face-to-face meeting of the advisory board, held in April 2019. Experts were provided with a list of potential award criteria derived from the analysis of the current situation. The selected criteria had to meet certain conditions, such as being non-redundant, independent, complete, operational, and measurable.Citation24 Experts individually reflected on their particular choice of criteria, including the possibility of suggesting additional new criteria. Subsequently, an open discussion was held in which each expert gave his or her opinion based on his or her experience and knowledge, in order to reach a consensus on whether or not to include each criterion. Moreover, the legal feasibility of each criterion was further taken into account based on the advice provided by Acobur Asesores. Thereafter, criteria were grouped into different domains and their definitions were agreed upon in order to avoid ambiguities and differing interpretations. Once experts reached a consensus on which criteria to include, the two sets of resulting criteria (for routine and influenza vaccines) were individually weighted by each expert using the 100 Point Allocation Method. First of all, 100 points were distributed among the different domains. Thereafter, 100 points were distributed among the criteria included in each domain. The weightings assigned by the experts were confidential and based on their knowledge, expertise, and understanding. The mean values are presented in this document.
Step 3: An online validation stage was performed in order to revisit weightings and manage the uncertainty of the model. Additionally, other factors relevant to the ideal process of the public procurement of vaccines, such as pricing, the duration of the contracts, and the number of awardees, are described in the Discussion section of this article.
Stage 3: comparison between the current and ideal situation
The current FA was matched to the ideal FA by comparing criteria and weights, and potential areas for improvement were identified.
Results
Analysis of the current framework agreement
Since some tender specifications made distinctions based on vaccine batch type (with differentiated criteria and/or weightings), it was possible to extract and analyze 130 sets of criteria. Of these, 43% were FAs for routine vaccines (56 procedures), 15% were FAs for influenza vaccines (20 procedures), 35% were other procedures for routine vaccines (44 procedures), and the remaining 8% were other procedures for influenza vaccines (10 procedures).
Criteria set considered
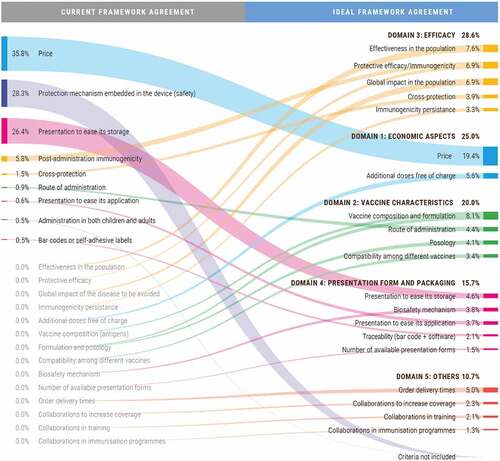
Based on the analyzed tender specifications, a total of 32 award criteria (standardized and restructured by Weber) are being taken into account at the time of analysis for the public procurement of vaccines in Spain. However, there are remarkable differences between routine and influenza vaccines ().
Table 2. Most common award criteria in the tender specifications (% of analyzed procedures, where each criterion was considered), by type of procedure
The FA for routine vaccines currently considers 22 different award criteria. The most frequent criteria are price (included in 100% of the analyzed procedures), vaccine presentation to ease its application (57%), additional doses free of charge (55%), and percentage of free replacement vaccines after cold chain issues (54%). Moreover, the procedures for the purchase of routine vaccines which are not included in the FA consider 23 award criteria. Five of these are not included in the FA (biosafety mechanism, delivery times, number of available presentation forms, global impact of the disease to be avoided, and cross-protection).
On the other hand, the current FA for influenza vaccines considers nine different award criteria. The most frequent criteria are the presentation to ease its application (included in 75% of the analyzed procedures), the protection mechanism embedded in the device (70%), and price (55%). Moreover, the procedures for the purchase of influenza vaccines which are not included in the FA consider 18 award criteria of which 12 are not included in the FA. From these, the most frequently used criteria are additional doses free of charge (included in 60% of the analyzed procedures), collaboration in the immunization programs (40%), and biosafety mechanism (30%).
Mean number of criteria per procedure
The mean number of criteria considered per tender specification varies from 2.7 in the FA for influenza vaccines to 5.5 in the FA for routine vaccines (). With regard to the procedures under the FA for influenza vaccines, the number of criteria varies from one (Galicia) to five (Aragon and Asturias), while for those procedures related to the FA for routine vaccines, the criteria range is wider and varies from one (Asturias, La Rioja, and Madrid only consider price) to 14 criteria (Canary Islands).
Table 3. Number of award criteria per analyzed procedure, by type of procedure
Criteria weighting
Regarding criteria weighting, there are also remarkable differences between autonomous communities, and also between routine and influenza vaccines. Overall, price weighs for 63% of the final decision for the purchase of the vaccine. However, this weight is generally higher for routine vaccines compared to influenza vaccines, and for procedures not included in the FA compared to those under it ().
Table 4. Award criteria mean weights included in the 130 groups of analyzed criteria, by type of procedure
With regard to the 22 analyzed procedures under the FA for routine vaccines, the weight of economic criteria is 70% (60% for price and 10% for additional doses free of charge). The remaining 30% is distributed among a wide range of technical criteria, namely vaccine composition (7%), presentation to ease its application (5%), percentage of free replacement vaccines after cold chain issues (2.8%), and post-administration immunogenicity (2.5%), among others. Outside the FA, the procedures of routine vaccines include 23 different award criteria, in which the weight of economic factors is 85% (79% for price and 6% for additional doses free of charge).
The FA for influenza vaccines takes nine different criteria into account and 90% of the final decision is based on three criteria: price (36%), the protection mechanism embedded in the device (28%), and presentation to ease its storage (26%). Outside the FA, the procedures for the purchase of influenza vaccines include 18 award criteria. Similarly, price is also the most important criterion (70%), followed by additional doses free of charge (7%), collaboration in the immunization programs (5%), and presentation to ease its storage (3.9%).
Results of the MCDA: the ideal framework agreement
The expert committee agreed on an ideal set of 19 award criteria for routine vaccines and a set of 17 award criteria for influenza vaccines to be included in the ideal FA. In both cases, the criteria were grouped into the same five domains. Grouping and definition of each criterion are described in the table included in the supplementary material.
Price was the award criterion with the highest average weight for the public procurement of routine vaccines (29 points out of 100, or 29%), followed by effectiveness in the population (7.1%), additional doses free of charge (7.0%), and global impact on the population (6.7%). Moreover, price showed the highest variation in assigned weight (SD: 9.7), followed by additional doses free of charge (SD: 4.3), and the global impact on the population (SD: 3.8). Similarly, for the public procurement of influenza vaccines, price was the award criterion with the highest average weight (27%), followed by vaccine composition and formulation (9%), the global impact on the population (7.4%), and effectiveness in the population (7.1%).
Thereafter, a re-test was performed given that experts agreed to add three criteria on collaborations to the ideal set. These had initially been excluded upon legal appraisal, but experts later agreed they should be considered award criteria as long as they were related to the object of the contract. These new criteria were included in the ‘Other’ domain. Thus, the final ideal set comprised 22 award criteria for routine vaccines and 20 criteria for influenza vaccines.
According to the weightings of the re-test, price continued to be the criterion with the highest relative weight for the public procurement of routine and influenza vaccines (22% and 19%, respectively), and showed the greatest variation (SD 0.088 and 0.083, respectively). Other important criteria in both cases were effectiveness in the population, vaccine composition and formulation, the global impact on the population, the protective efficacy, and additional doses free of charge ( and ).
Figure 2. Ideal weighting of the selected criteria for routine vaccines, by type of criterion
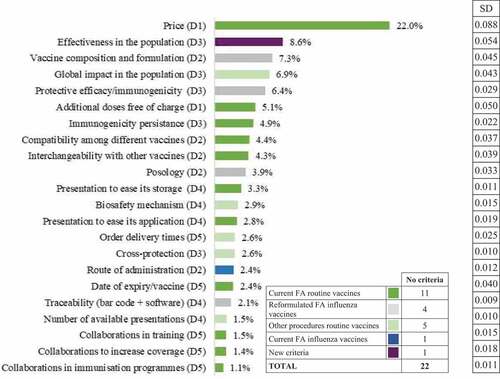
Figure 3. Ideal weighting of the selected criteria for influenza vaccines, by type of criterion
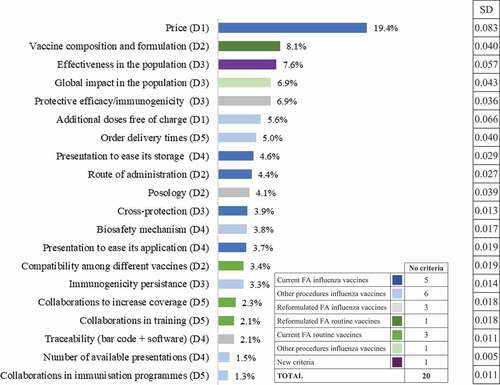
Per domain, the final decision should ideally adhere to the following scheme. For routine vaccines: efficacy (29%), economic aspects (27%), vaccine characteristics (22%), presentation form and packaging (13%), and others (9%) (). For influenza vaccines: efficacy (29%), economic aspects (25%), vaccine characteristics (20%), presentation form and packaging (16%), and others (11%) ().
Figure 4. Comparison between the actual FA and the ideal FA, routine vaccines
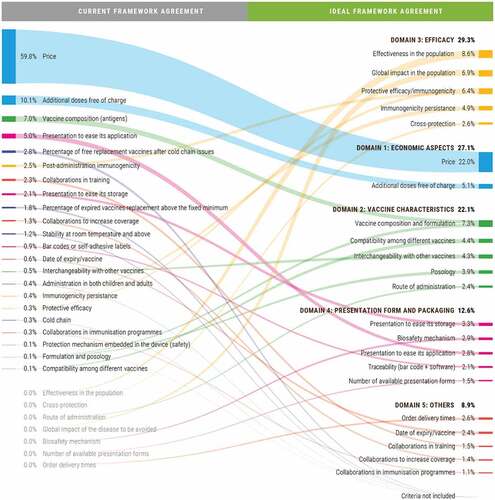
Figure 5. Comparison between the actual FA and the ideal FA, influenza vaccines
Furthermore, the largest differences in the relative weights between influenza vaccines and routine vaccines were observed for order delivery periods (5% vs. 2.6%), routes of administration (4.4% vs. 2.4%), and collaboration in activities to increase coverage (2.3% and 1.4%).
Comparison between the current framework agreement and the ideal framework agreement
For routine vaccines, the main difference between the current and the ideal FA is the relative weight of the economic aspects (price + additional doses free of charge). Nowadays, this accounts for 70% of the final decision and should decrease to 27% according to the ideal FA (). Similarly, the relative weight of the price criterion would decrease almost two-thirds, from 60% to 22%. Moreover, the criterion of additional doses free of charge would move from the second to the sixth position, and its relative weight would be reduced in half, from 10% to 5% (). Furthermore, while having the same number of award criteria, the current FA for routine vaccines accounts for only 50% of the ideal criteria. The 11 remaining criteria have either been reformulated or come from the FA for influenza or from other procedures on routine vaccines. Finally, the expert committee suggested including a new criterion on ‘the effectiveness in the population’ ().
Figure 6. Comparison between the current and the ideal FAs for routine and influenza vaccines: Mean weighting of the economic vs. technical award criteria
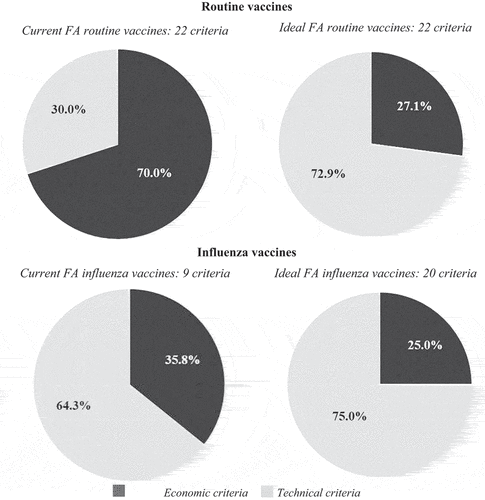
The context for influenza vaccines would be very different compared to that for routine vaccines. In this case, the ideal weight of the economic aspects would also decrease compared to the current situation, but to a lesser extent, from 36% to 25%. Moreover, price would still be the item with the highest relative weight (19%), though much lower than in the current situation (36%) (). The largest difference compared to the current scenario would come from the number of criteria that should be considered. The committee believed that the range should be increased and move from the current set of 9 award criteria to an ideal set of 20 award criteria. Of these, five are already being applied in the current FA for influenza vaccines, three are being applied though needing some reformulation, six are being applied on other procedures for influenza vaccines, five are being applied in for routine vaccines, and one should be created (effectiveness in the population) ().
Discussion
The MCDA methodology is increasingly being used for the prioritization of health-care resources. This methodology allows an explicit assessment of different value items in the assessed interventions, encourages the transparency and the coherence of the process, and favors a multidisciplinary dialogue.Citation25,Citation26 In the field of vaccines, the purchase process plays a crucial role in which specific technical knowledge is required to guarantee a timely, efficient, and high-quality supply. The goal of this study was to contribute to ease and standardize the decision-making process for the public procurement of vaccines in Spain, and ultimately to move toward models of vaccine purchase that may contribute to improve the efficiency, cohesion, and sustainability of the National Healthcare System. Our project is aligned with some of the improvement actions proposed by WHO to benefit from the power of vaccines.Citation27
To our knowledge, this is the first MCDA that has focused on the award criteria that should ideally be used to determine the awardee in the public procurement of vaccines. Previous MCDAs in the field of vaccines have focused on the introduction of new vaccines.Citation28-33 Nevertheless, the WHO’s reference manual on the procurement of vaccines for public-sector programs should also be taken into account. According to this manual, the weights of the four value attributes for the public procurement of measles vaccines are financial criteria (35 points), technical criteria (35 points), contractual criteria (15 points), and commercial criteria (15 points).Citation34 Some of the lessons learned from procurement procedures in other countries refer to follow a predefined evaluation procedure involving key stakeholders using unbiased evaluation system; to create a contract with defined mandatory terms as well as decisions on any flexible terms proposed during the bidding process; and to monitor the ability of both contractual parties to meet the terms of the agreed contract and monitor the performance of the vaccine through appropriate regulatory and programmatic reporting channels.Citation35 Including stakeholder-centered criteria in decision procedures would significantly increase their transparency and accountability, support international capacity building to improve health, and reduce societal costs and inequity resulting from suboptimal health decision-making.Citation28
Overall, the present study has revealed that the current FA is far from the ideal FA for the public procurement of vaccines in Spain. This has not only been shown in terms of the set of award criteria to be considered but also in terms of the relative weights these criteria should have in the final decision. In addition, the present study showed that the ideal framework for the purchase of routine vaccines is different from that of influenza vaccines.
More specifically, the first conclusion that can be drawn from this study is that the ideal FA should back away from purely economic criteria and move toward relevant technical criteria. The latter entails including the actual effectiveness of the vaccine. Furthermore, the weighting that the economic criteria currently have on the final decision is excessive compared to the technical criteria. Accordingly, for the purchase of routine vaccines, the current weight of the economic criteria should decrease from 70% to an ideal 27%, and decision-makers should give more weight to technical aspects related to vaccine characteristics, efficacy, presentation, and delivery process. Similarly, for the purchase of influenza vaccines, the current weighting of economic aspects should decrease from 36% to an ideal 25%. When interpreting the results, the fact that the FA specifications for routine and influenza vaccines are different should be considered. For routine vaccines, the FA states that price should be included as an award criterion in the subsequent call-off agreements. By contrast, the FA for influenza vaccines states that price should not be included, yet several contracting authorities included price as an award criterion.
Another crucial area for the improvement of the current FA is the set of criteria to be considered for the contract award. Accordingly, the present study concluded that a wider range of relevant criteria – up to 20 – should be considered when selecting the awardees. Moreover, while the number of relevant criteria for the purchase of routine vaccines would not vary, the elements themselves would have to change. On the one hand, award criteria should not overlap with the technical specifications included in the technical tender specification. Accordingly, the cold chain, the commitment to supply, or the plan for providing to the immunization points would not be part of the ideal set of criteria. On the other hand, it would be advisable to include other factors that are currently being applied by some autonomous communities outside the current FA. Accordingly, the ideal FA should include criteria such as timeframes for order delivery, global impact on the population, or available presentation forms. Moreover, effectiveness in the population or traceability by an integrated software have been identified as highly recommended criteria to be included in the ideal FA.
Nevertheless, criteria should be accurately defined to ensure these are not subject to interpretation. Moreover, these criteria must be concise, objectively assessed, agreed upon, and clearly bound to the object of the contract in order to avoid its legal recourse. This is especially relevant for those criteria related to collaborations in training or collaborations to increase vaccine coverage.
The ideal FA should also pay special attention to more general considerations. Firstly, any process that could be detrimental to the procurement of vaccines should be avoided; for example, having price as the only award criterion accepted by the contracting authorities or fixing a price well below the market price or second-round price would compromise vaccine supply. Secondly, having only one awardee with a contract duration of 3 or more years could also compromise vaccine supply given the occurrence of problems in their production chain. Furthermore, this would lead to a less competitive and attractive market for other pharma labs to enter. Thirdly, a lack of budget and contract planning, in addition to the short timeframe available from bidding until doses are needed, generate uncertainty regarding production capacity and potential shortages. Therefore, procurement procedures should be planned beforehand taking into account the internal stages of the procedure and the terms needed by the industry to give an answer to the object of the contract. Fourthly, in order to avoid price-based decisions and favor sustainability, each call-off agreement should include a minimum and a maximum number of technical criteria depending on the vaccine (an average of five). Finally, the new Law of Public Procurement should be taken into account as it allows for more flexible and innovative FAs moving toward procurement of services and not only supplies; for example, through risk-sharing contract models.Citation10
The present study has some limitations. First of all, the analysis of the current FA was based on the available tender specifications only. Nevertheless, the information gathered for analysis featured a large amount of public and nonpublic information provided by procurement authorities and tenderers. Secondly, the ideal approach was subjectively addressed, and results depend entirely on the preferences, experiences, and judgments of the advisory board. However, the advisory board comprised experts on vaccines both at national and regional levels from both clinical and management areas. In addition, the present MCDA was partial and did not address the scoring phase of a specific vaccine. Thus, the present MCDA just created a general, ad hoc framework to be used as a basis for the future assessment of specific products.
In conclusion, in order to move toward a more efficient, equitable, and sustainable model for the public procurement of vaccines, the present study suggests standardizing the current common purchase model in Spain. Furthermore, the ideal model would improve transparency, find a balance between the weights assigned to technical and economic aspects, and introduce flexibility to allow for innovation. In turn, these improvements would guarantee vaccine supply at a competitive price. Future studies should delve into this matter so that the health-care system as a whole could be better prepared to face the technical, logistic, economic, political, and social challenges ahead.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (88 KB)Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Andre FE, Booy R, Bock HL, Clemens J, Datta SK Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ [accessed 2019 Feb 26]. https://www.who.int/bulletin/volumes/86/2/07-040089/en/.
- European Commission. Vaccination programmes and health systems in the European Union; 2018 [accessed 2019 Sep 3]. http://dx.publications.europa.eu/10.2875/18503.
- WHO. Challenges and opportunities in improving access to medicines through efficient public procurement in the WHO European region; 2016 [accessed 2019 Sep 10]. http://www.euro.who.int/__data/assets/pdf_file/0003/323598/Challenges-opportunities-improving-access-medicines-efficient-public-procurement.pdf?ua=1.
- European Commission, European Observatory on Health Systems and Policies. The organization and delivery of vaccination services in the European Union; 2018. [accessed 2019 Sep 3]. https://ec.europa.eu/health/sites/health/files/vaccination/docs/2018_vaccine_services_en.pdf.
- Consejo de Ministros. La Moncloa, 25 noviembre 2016. Acuerdo marco para la compra centralizada de más de veinte millones de dosis de vacunas. [accessed 2019 Jun 10]. https://www.lamoncloa.gob.es/consejodeministros/Paginas/enlaces/251116-enlacevacunas.aspx.
- Mestre-Ferrandiz, Misiego A, Martín JI, Calderón JM, Celorrio JM, Estupiñan-Romero F, Hernandez MJ. Vacunas: política y salud pública. Fundación Gaspar Casal; 2019. [accessed 2019 Jun 10]. http://fundaciongasparcasal.org/publicaciones/vacunas-politica-y-salud-publica.pdf.
- Deloitte. Las vacunas en España. Situación actual y perspectivas de futuro; 2017 [accessed 2018 Dec 10]. https://www2.deloitte.com/content/dam/Deloitte/es/Documents/sanidad/Deloitte-ES-sanidad-estudio-vacunas.pdf.
- París G. ¿Es sostenible el modelo actual de adquisición de vacunas para el calendario infantil de vacunación? Rev Esp Econ Salud. 2016;11:672–81.
- Deloitte. El valor social de las vacunas. Elementos de reflexión para facilitar el acceso; 2015. [accessed 2018 Jul 26]. https://www2.deloitte.com/content/dam/Deloitte/es/Documents/sanidad/Deloitte_ES_Sanidad_el-valor-social-de-las-vacunas-informe-completo.pdf.
- Ley 9/2017, de 8 de noviembre, de Contratos del Sector Público, por la que se transponen al ordenamiento jurídico español las Directivas del Parlamento Europeo y del Consejo 2014/23/UE y 2014/24/UE, de 26 de febrero de 2014. BOE Núm. 272. [accessed 2019 Feb 26]. https://www.boe.es/buscar/act.php?id=BOE-A-2017-12902.
- Gobierno de España. MInisterio de Hacienda. Plataforma de Contratación del Sector Público. [accessed 2018 Sep 7]. https://contrataciondelestado.es/wps/portal/plataforma.
- Generalitat de Catalunya. Plataforma electrònica de contractació pública. [accessed 2018 Sep 3]. https://contractaciopublica.gencat.cat/ecofin_pscp/AppJava/search.pscp?reqCode=start&set-locale=ca_ES.
- Consejería de Hacienda de la Ciudad Autónoma de Ceuta. Contratación Pública. [accessed 2018 Sep 3]. http://web.ceuta.es:8080/contratacion/principal/index.jsp;jsessionid=ED4336B2964E8173D48F58726F43591E.
- Junta de Andalucía. Contratación pública Junta de Andalucía. [accessed 2018 Sep 3]. https://www.juntadeandalucia.es/temas/contratacion-publica.html.
- Gobierno de Aragón. Contratación Pública del Gobierno de Aragón. [accessed 2018 Sep 4]. https://www.aragon.es/-/perfil-contratacion.
- Gobierno del Principado de Asturias. Perfil del contratante. [accessed 2018 Sep 5]. https://sede.asturias.es/portal/site/Asturias/menuitem.218089047cf1c2b5cee63510100000f7/?vgnextoid=9224dbe25a232110VgnVCM100000b0030a0aRCRD&i18n.http.lang=es.
- Govern Illes Balears. Plataforma de Contractació de la Comunitat Autònoma de les Illes Balears. [accessed 2018 Sep 5]. http://www.plataformadecontractacio.caib.es/LicitacionesTerminoAbierto.jsp?idi=ca.
- Junta de Castilla y León. Contratación administrativa. Portal Salud Junta Castilla León [accessed 2018 Sep 5]. https://www.saludcastillayleon.es/institucion/es/empresas/contratacion-administrativa.
- Gobierno de La Rioja. Contratación Pública. [accessed 2018 Sep 6]. https://www.larioja.org/contratacion-publica/es.
- Comunidad de Madrid. Portal de la Contratación Pública de la Comunidad de Madrid. [accessed 2018 Sep 6]. http://www.madrid.org/cs/Satellite?cid=1203334374251&language=es&pagename=PortalContratacion%2FPage%2FPCON_contenidoFinal.
- Región de Murcia. Contratación Pública. Anuncios de licitación y adjudicación. [accessed 2018 Sep 6]. https://www.carm.es/web/pagina?IDCONTENIDO=1615&IDTIPO=200&RASTRO=c709$m.
- Gobierno de Navarra. Portal de contratación de Navarra. Navarra.es. [accessed 2018 Sep 6]. http://portalcontratacion.navarra.es/es/.
- Vasco G. Plataforma de contratación Pública en Euskadi. [accessed 2018 Sep 6]. http://www.contratacion.euskadi.eus/w32-1081/es/v79aWar/comunJSP/v79aCambioIdioma.do?idioma=es.
- Marsh K, IJzerman M, Thokala P, Baltussen R, Boysen M, Kaló Z, Lönngren T, Mussen F, Peacock S, Watkins J, et al. Multiple criteria decision analysis for health care decision making—Emerging good practices: report 2 of the ISPOR MCDA emerging good practices task force. Value Health. 2016;19:125–37. doi:10.1016/j.jval.2015.12.016.
- Rappuoli R, Breghi G, Timmis JK. Maximizing the contribution of vaccination to the SDGs and the grand convergence - Integrating multi criteria decision analysis with the broader benefits of vaccination to align on sustainable immunization strategies; 2016.
- Tromp N, Baltussen R. Mapping of multiple criteria for priority setting of health interventions: an aid for decision makers. BMC Health Serv Res. 2012;12:454. doi:10.1186/1472-6963-12-454.
- WHO. Global vaccination summit. Lessons from the day and actions needed towards vaccination for all and elimination of vaccine preventable diseases; 2019 [accessed 2019 Sep 23]. https://ec.europa.eu/health/sites/health/files/vaccination/docs/10actions_en.pdf.
- Timmis JK, Black S, Rappuoli R. Improving accountability in vaccine decision-making. Expert Rev Vaccines. 2017;16:1057–66. doi:10.1080/14760584.2017.1382358.
- Kaslow DC, Kalil J, Bloom D, Breghi G, Colucci AM, De Gregorio E, Madhavan G, Meier G, Seabrook R, Xu X. The role of vaccines and vaccine decision-making to achieve the goals of the grand convergence in public health. Vaccine. 2017;35 Suppl 1:A10–5. doi:10.1016/j.vaccine.2016.10.088.
- Phelps CE, Madhavan G, Gellin B. Planning and priority setting for vaccine development and immunization. Vaccine. 2017;35:A50–6. doi:10.1016/j.vaccine.2016.09.072.
- Saul A, O’Brien KL. Prioritizing vaccines for developing world diseases. Vaccine. 2017;35:A16–9. doi:10.1016/j.vaccine.2016.10.087.
- Knobler S, Bok K, Gellin B. Informing vaccine decision-making: A strategic multi-attribute ranking tool for vaccines-SMART Vaccines 2.0. Vaccine. 2017;35(Suppl 1):A43–5. doi:10.1016/j.vaccine.2016.10.086.
- Pooripussarakul S, Riewpaiboon A, Bishai D, Muangchana C, Tantivess S. What criteria do decision makers in Thailand use to set priorities for vaccine introduction? BMC Public Health. 2016;16:684. doi:10.1186/s12889-016-3382-5.
- WHO. Procurement of vaccine for public-sector programmes; 2003.
- WHO. Procurement mechanisms and systems. Vaccine procurement process; 2019 [accessed 2020 Jan 27]. https://www.who.int/immunization/programmes_systems/procurement/mechanisms_systems/country_procurement/en/index2.html.