ABSTRACT
Recent clinical trials utilizing antigen-pulsed dendritic cells (DCs) have demonstrated increased survival of vaccinated cancer patients. Besides, the cytoplasmic transduction peptide (CTP) not only has an excellent transcellular efficiency but also shows a strong tendency to remain in the cytoplasm after transduction, without migrating into the nucleus. In this study, we investigated the effectiveness of immunotherapy against malignant gliomas using DCs pulsed with CTP-fused protein antigens combined with programmed cell death protein 1 blockade (anti-PD1). The expression of tumor associated antigen (WT1 and BIRC5) and PDL1 on glioblastoma (GBM) target cells was confirmed by western blot. The effect of CTP-fused protein antigens on mature DCs (VaxDCs) was determined. The immunophenotypes of VaxDCs pulsed with CTP-fused protein antigens was confirmed by flow cytometry and the cytokine production levels of T helper polarization were measured by enzyme-linked immunosorbent (ELISA) assay. The IFN-γ-enzyme linked immunospot and lactate dehydrogenase release assays were performed to estimate the cytotoxic activity of antigen-specific cytotoxic T lymphocytes (CTLs), stimulated by VaxDCs pulsed with CTP-fused protein antigens and anti-PD1, against malignant glioma cells expressing target antigens. VaxDCs pulsed with CTP-fused protein antigens showed enhanced expression of major histocompatibility complex (MHC) and co-stimulatory markers of DCs and resulted in Th1 cytokine polarization. The increase in the number of IFN-γ+ effector T cells paralleled with the enhanced percent specific lysis of GBM targets cells by antigen-specific CTLs. Our study suggested that using CTP-fused protein antigens for DC vaccine preparation along with PD1 blockade could be an effective immunotherapy strategy for GBM.
Background
Gliomas are a type of tumors derived from glial or precursor cells. They account for approximately 26.5% and 80.7% of all primary brain and malignant tumors, respectively. Glioblastoma (GBM) accounts for the majority (56.1%) of all gliomas.Citation1 The median survival of GBM patients is approximately14.6 months with radiotherapy plus temozolomide .Citation2 Cancer treatment involving surgery, radiotherapy, or chemotherapy has not shown any markedly improved survival outcome in these patients. Therefore, immunotherapy has become an attractive approach for cancer treatment, especially for patients with aggressive tumors .Citation3 Dendritic cells (DCs) are known as the most potent professional antigen-presenting cells (APCs) to initiate a T cell response. The uptake of tumor antigens by DCs, as anti-tumor vaccines, is considered a potential strategy for cancer treatment by improving clinical outcome of the vaccinated GBM patients .Citation4
DCs induce an adaptive immune response mainly through the presentation and cross-presentation of antigen-derived peptide/MHC complexes to T-lymphocytes .Citation5 The present tumor-associated antigens are mainly endogenous antigens in the cytosol of DCs, enabling the MHC class I-presenting pathway, which is a key pathway for tumor-specific cytotoxic T-lymphocyte (CTL) response .Citation6 The cross-presentation process in which exogenous antigens are displayed by MHC class I molecules of DCs to stimulate CD8+ T cell responses has been clarified in previous research .Citation7 Therefore, developing a method that directly delivers exogenous antigens into the cytosol of DCs, like the endogenous antigens, is critical in DC-based cancer immunotherapy. Using cytoplasmic transduction peptides (CTPs) is a newly designed approach for the effective delivery of polymeric molecules across the cell membrane to the cytoplasmic compartment. This function of CTP is advantageous for the development of class I-associated CTL vaccines without causing any adverse effects on nuclear genetic material .Citation8,Citation9
The CTP-fused protein antigens are able to induce tumor antigens into the cytosol of DCs, and stimulate the activation and maturation of DCs, thus enhancing the presentation of targeting antigens and efficiently inducing antigen-specific CTL immune response .Citation10,Citation11 Moreover, immunity induced by DCs loaded with CTP-fused protein antigens could significantly inhibit tumor growth and metastasis compared to that by CTPs or antigens alone .Citation12 Therefore, DCs pulsed with CTP-fused protein antigens might be a promising vaccine candidate for cancer therapy, providing a new insight into DC-based immunotherapy design and future peptide therapy applications.
Early data indicated that the brain lacked dedicated lymphatic channels and considered that most GBMs are cold tumors .Citation13 The reasons that tumors are unresponsive to immunotherapy are likely multifactorial and include a highly immunosuppressive tumor milieu (PD1/PD-L1, IDO, TGF-β, IL-10, etc.), defects in tumor antigen presentation, and features of the physical microenvironment, such as hypoxia and necrosis. However, current research demonstrated that brain microenvironment can generate a robust immune response through homing tendency of the peripheral effector CD8+ T cells to intracranial tumors and induce an immune response at this site .Citation14,Citation15 Therefore, although the brain is an immunologically distinct site, the immune microenvironment offers adequate opportunities to implement immunotherapy for the treatment of brain tumors .Citation16 Hence, in this study, we used combination approaches between CTP-fused protein antigens and PD1 blockade to enhance the function of antigen-specific CTLs with the aim of transforming these cold tumors into hot ones, thus augmenting current immunotherapy strategies.
Methods
Target cells and CTP-fused protein antigen synthesis, and antibody
Surgical specimens from GBM patients were obtained for research purposes following an approval by the Chonnam National University Hwasun Hospital Ethics Panel. Samples were gathered by the Neurosurgery Department and kept at −80° C until use. The U87 human GBM cell line (Gibco-BRL, Gaithersburg, MD, USA) was obtained for cell culture. These cells were routinely grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS) at 37°C in a humidified atmosphere containing 5% CO2.
The primary GBM cell line was isolated directly from fresh GBM tissue samples of patients following approval by the Chonnam National University Hwasun Hospital Ethics Panel. Firstly, tissues were washed with phosphate-buffered saline (PBS) and were minced into 3–4 mm pieces with a sterile scalpel. Tissue pieces were incubated with collagenase type I (0.4%; Gibco, USA) at 37ºC for 4 h. Samples were observed and suspended at 15 min intervals. Therefore, cells were washed with PBS and filtered through 40 µm cell strainer (Falcon, USA) and single GBM cells were collected. Finally, cells were counted and cryopreserved until use. CTP-fused human Wilm’s tumor gene 1 (CTP-fused WT1) and CTP-fused human survivin (CTP-fused BIRC5) were synthesized at JW CreaGene (Seongnam, Korea). The purity of each protein was confirmed to be >95% by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Synthetic protein was dissolved in dimethyl sulfoxide (DMSO) according to the manufacturer’s recommendations and stored at −70°C until use.
The human anti-PD1 antibody (clone: J110) used for in vitro blockade was purchased from BioXcell (West Lebanon, NH, USA).
Western blotting
The expression of WT1, BIRC5, and PDL1 on GBM target cells was confirmed by western blot. Briefly, the bicinchoninic acid (BCA) assay kit (Thermo Scientific, USA) was used to measure protein concentration. Next, SDS- PAGE was used to separate the protein of interest that was transferred onto a polyvinylidene difluoride (PVDF) membrane and soaked in a blocking solution (5% nonfat dry milk in TBST (tris-buffered saline, Tween 20)) for 1 h. The membrane was then probed with the primary antibodies for Wilm’s tumor gene 1 (WT1; Abcam, Cambridge, United Kingdom), survivin (BIRC5; Santa Cruz Biotechnology, CA, USA), programmed cell death ligand 1 (PDL1; Santa Cruz Biotechnology, CA, USA), and β-actin (Santa Cruz Biotechnology, CA, USA) at 4°C overnight, and then incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse polyclonal IgG secondary antibodies (Ab frontier, Korea). Chemiluminescent detection was performed using immobilon western chemiluminescent HRP substrate (Millipore Corporation, Billerica, USA). β-actin was used as an internal control. The expression levels of WT1, BIRC5 and PDL1 were determined by using Amersham Imager 600 (GE Healthcare).
DC maturation and CTP-fused protein antigen pulsed DCs
To confirm the effect of CTP-fused WT1 and CTP-fused BIRC5 pulsed VaxDCs in combination with anti-PD1 in vitro, DCs were used for checking the function of CTP-fused protein antigens in stimulating CTLs after pulsing with VaxDCs. Human CD14+ monocytes, obtained from the peripheral blood of healthy human donors, were used for the experiment. Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% FBS was used for cell culture with the addition of granulocyte-macrophage colony stimulating factor (GM-CSF; 50 ng/mL) and IL-4 (20 ng/mL). Immature DCs were differentiated from CD14+ monocytes after 6 days into mature DCs (VaxDCs) by using a cocktail of cytokines such IFN-α (3,000 IU/mL), IFN-γ (10 ng/mL) and poly (I:C) (20 µg/mL), LPS (1 µg/mL) and were loaded with CTP-fused WT1 and CTP-fused BIRC5 (5 µg/ml) after 2 h .Citation8,Citation17 On day 8, VaxDCs were harvested and cryopreserved in liquid nitrogen until use.
Immunophenotyping and polarization cytokine production of DCs
VaxDCs characteristic were evaluated by immunophenotyping. The expression of MHC and co-stimulatory markers on DCs was compared using flow cytometry analysis at three stages: immature DCs, DCs after 48 h maturation into VaxDCs with and without pulsing CTP-fused protein antigens (CTP-fused WT1 and CTP-fused BIRC5). Immature DCs were used as a negative control. At day 8, cells were obtained and stained for MHC and co-stimulatory markers. Mainly, cell staining was performed using fluorescein isothiocyanate (FITC) or phycoerythrin (PE) conjugated monoclonal antibodies against CD40, CD80, CD83, CD86, CCR7, MHC I, and MHC II. All antibodies were purchased from eBioscience (San Diego, CA, USA).
The supernatant collected from the co-culture of DCs and CD40 L-transfected J558 cells was subjected to ELISA. To evaluate DC function after pulsing with CTP-fused WT1 and CTP-fused BIRC5, the cytokine levels for polarized helper T cells such as IL-12p40 and IL-10 were estimated. These cytokines were measured using ELISA kits following the manufacturer’s protocols (BD Biosciences) .Citation18
Cytotoxic T lymphocyte (CTL) generation
CTLs were generated as previously described with several modifications .Citation19 in general, the magnetic activated cell sorting system (MACs) was used to separate the CD3 lymphocyte populations. These cells were then stimulated by CTP-fused WT1 and CTP-fused BIRC5 pulsed VaxDCs with or without anti-PD1. On day 3, IL-2 (5 ng/mL) and IL-7 (10 ng/mL) cytokines were added. Thereafter, the CTLs were harvested and re-stimulated with CTP-fused WT1 and CTP-fused BIRC5 pulsed VaxDCs for a second time (day 15) and a third time (day 22). Anti-PD1 (BioXcell, USA) used for PD1 receptor blockade was added during each stimulation. After 2 or 3 days from the last re-stimulation, enzyme-linked immunospot (ELISPOT) and lactate dehydrogenase (LDH) release cytotoxicity assays were performed. The anti-PD1 was used for blockade in both DC-CTL interaction and CTL-target cancer interaction.
The IFN-γ ELISPOT assay
IFN-γ+ effector T cells were analyzed using an IFN-γ ELISPOT assay kit (BD Biosciences) .Citation20 ELISPOT assay was performed as previously described with several modifications. Briefly, 96-well microplates were coated with the capture-purified anti-human IFN-γ antibody overnight at 4° C. Next, RPMI medium supplemented with FBS was added to saturate the treated antibody. CTLs stimulated by VaxDCs pulsed with CTP-fused WT1 and CTP-fused BIRC5 were co-cultured with the target cells (U87 GBM cell line and primary GBM cells) at a ratio 10:1 with or without anti-PD1. Co-cultured cells were added to triplicate wells in 10% FBS-RPMI medium and incubated for 24 h at 37° C under 5%CO2. Thereafter, these cells were incubated for 2 h with the biotinylated detection anti–human IFN-γ antibody, and for 1 h with the streptavidin-HRP. After washing, spots were revealed by using an AEC substrate reagent set (BD Bioscience) and measured with an automatic CTL Immunospot Analyzer (Cellular Technology Ltd., USA).
LDH release cytotoxicity assay
CytoTox 96 nonradioactive cytotoxicity assay (CytoTox 96, Promega, USA) was performed to analyze the killing effects of the effector T cells against target cells according to the manufacturer’s instructions. CTLs stimulated with CTP-fused WT1 and CTP-fused BIRC5 pulsed VaxDCs acted as the effector cells. U87 cell line and primary cells (5 × 103 cells/well) were used as the target cells. W6/32 monoclonal antibody (1 μg/mL) (mAb; a gift from Dr. Bin Gao, ICH, London, United Kingdom) was used to block MHC-A, B, C antigen presentation on the target cells. The stimulated CTLs were co-cultured with the target cells at a ratio 10:1 in the 96-well uncoated plates (Costar, USA) for 4 h in 37°C and 5% CO2. Anti-PD1 (10 µg/mL) was added during co-culture. Then, supernatants were collected for lactate dehydrogenase concentration determination. The mean percentage of specific lysis was calculated as following: % Cytotoxicity = [(Experimental – Effector Spontaneous – Target Spontaneous)/(Target Maximum – Target Spontaneous)] × 100
Statistical analysis
All statistical analyzes were performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) with the least significant difference (LSD) post-hoc test was used to across multiple groups. P < .05 was considered statistically significant.
Results
Expression of tumor associated antigens and PDL1 in GBM cells
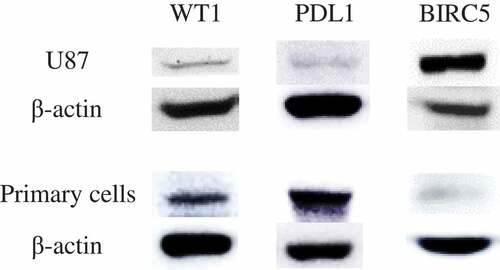
The expression of the two tumor-associated antigens (WT1 and BIRC5) and PDL1 was investigated in the target cells: human GBM U87 cell line and human primary GBM cells. As shown in , these targets cells display a significant signal for the WT1, BIRC5, and PDL1 protein. The U87 cell line showed a high expression of BIRC5 while primary GBM cells showed a strong expression of WT1 and PDL1. The figure comprises multiple gel images. Full-length blots are presented in Figure S1 (for U87 cell line) and Figure S2 (for primary cells).
Figure 1. The expression of WT1, BIRC5 (survivin) and PDL1 on human glioblastoma cells. The expression levels of WT1, BIRC5 and PDL1 on U87 cell line and primary GBM cells were confirmed by western blot. The U87 cell line showed high expression of BIRC5 while primary GBM cells expressed WT1 and PDL1. β-actin was used as an internal control in all western blot experiments. The figure is made up of multiple gel images; full-length blots are presented in supplementary figure. U87 cell line: human glioblastoma cell line; Primary cells: human primary glioblastoma cells
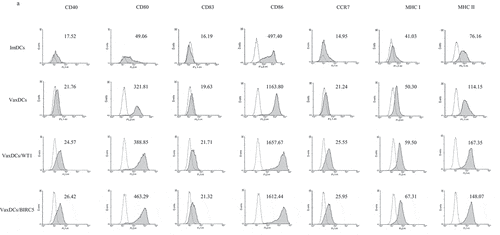
Characterization of CTP-fused protein antigens pulsed DCs
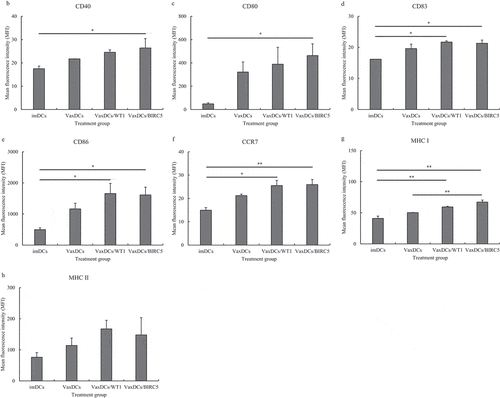
Immunophenotyping characteristics of VaxDCs. The expression of MHC and co-stimulatory molecules such as CD40, CD80, CD83, CD86, CCR7, MHC I, and MHC II on VaxDCs is shown in . . Although there was no significant difference in the expression of these markers between imDCs and VaxDCs, a higher expression tendency of these markers was observed on VaxDCs pulsed with CTP-fused GBM protein antigens compared to that on imDCs. Interestingly, the markers CD83 (p = .012), CD86 (p = .022), CCR7 (p = .011), and MHC I (p = .006) showed enhanced expression on VaxDCs pulsed with CTP-fused WT1 compared to that on imDCs. Moreover, VaxDCs pulsed with CTP-fused BIRC5 also showed increase in CD40 (p = .044), CD80 (p = .041), CD86 (p = .025), CCR7 (p = .01), and MHC I (p = .002) expression, compared to that on imDCs. Both VaxDCs pulsed with CTP-fused WT1 and those pulsed with CTP-fused BIRC5 did not show enhanced levels of MHC II compared to those in imDCs. in general, CTP-fused WT1 and CTP-fused BIRC5-pulsed VaxDCs enhanced the activated markers of VaxDCs compared to those of imDCs, and there was no significant difference observed for the expression of these markers between VaxDCs pulsed CTP-fused WT1 and VaxDCs pulsed with CTP-fused BIRC5.
Figure 2. Immunophenotypes of CTP-fused protein antigen pulsed VaxDCs. (A) Immunophenotypes of DCs after maturation and pulsing with CTP-fused WT1 and CTP-fused BIRC5 were estimated by flow cytometry. (B-H) Data are summarized in a bar chart. Mean fluorescence intensity of MHC and co-stimulation marker expression on DCs such as CD40, CD80, CD83, CD86, CCR7, MHC I, MHC II was estimated. The data represent the mean of triplicate from two independent experiments. ImDCs: immature DCs, VaxDCs: VaxDCs unpulsed with CTP-protein antigens, VaxDCs/WT1: VaxDCs pulsed with CTP-fused WT1, VaxDCs/BIRC5: VaxDCs pulsed with CTP-fused BIRC5. *: p < .05, **: p < .01, ***: p < .001
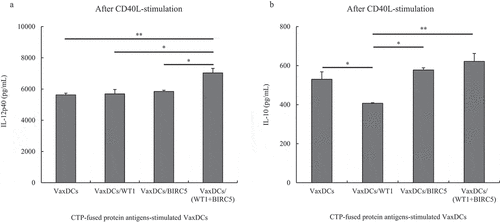
The potential Th1 polarization following cytokine production of DCs pulsed with CTP-fused protein antigens. VaxDCs with and without pulsing by CTP-fused protein antigens were stimulated with CD40 L-transfected J558 cell line and secretion cytokines such as IL-12p40 and IL-10 were estimated. The results are shown in . After the same procedure using VaxDCs pulsed with CTP-fused WT1 or CTP-fused BIRC5, there was no significant difference in IL-12p40 levels when compared to those in VaxDCs. Interestingly, VaxDCs pulsed with the combination of CTP-fused WT1 and CTP-fused BIRC5 showed enhanced IL-12p40 levels compared to those in VaxDCs (p = .01), VaxDCs pulsed with CTP-fused WT1 (p = .011), and VaxDCs pulsed with CTP-fused BIRC5 (p = .017). VaxDCs pulsed with the combination of CTP-fused WT1 and CTP-fused BIRC5 showed stable IL-10 levels compared to those in VaxDCs only, thus indicating that the combination of CTP-fused WT1 and CTP-fused BIRC5 leads to Th1 polarization phenotype of VaxDCs.
Figure 3. The polarization cytokine production of CTP-fused protein antigen pulsed VaxDCs. Cytokines secreted into the culture supernatants after stimulation with CD40 ligand-transfected J558 cell line were measured by ELISA. Secreted cytokine of IL-12p40 (A) and IL-10 (B) are summarized in a bar chart. VaxDCs: VaxDCs unpulsed with CTP-protein antigens, VaxDCs/WT1: VaxDCs pulsed with CTP-fused WT1, VaxDCs/BIRC5: VaxDCs pulsed with CTP-fused BIRC5, VaxDCs/(WT1+ BIRC5): VaxDCs pulsed with CTP-fused WT1 and CTP-fused BIRC5. *: p < .05, **: p < .01, ***: p < .001
The IFN-γ+ effector T cell response against human GBM cells
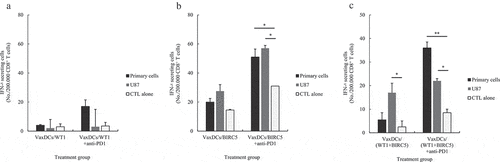
The U87 GBM cell line and primary GBM cells were used as the target cells for ELISPOT assay. The number of IFN-γ+ effector T cells against GBM target cells was studied at 10:1 E/T ratio () and CTL alone was used as the negative control. The WT1-specific CTLs or BIRC5-specific CTLs showed a stable number of IFN-γ+ effector T cells against both U87 cell line and primary GBM cells while a combination of WT1 and BIRC5-specific CTLs showed an enhanced IFN-γ+ effector T cells against the U87 cell line compared to that shown by CTL alone (p = .05). Interestingly, the number of IFN-γ+ effector T cells against both U87 cell line and primary GBM target cells increased after treatment with anti-PD1. While WT1-specific CTLs caused no change in the number IFN-γ+ effector T cells with anti-PD1, BIRC5-specific CTLs and the combination of WT1 and BIRC5-specific CTLs showed an increase in the number IFN-γ+ effector T cells with anti-PD1. With BIRC5-specific CTLs, the number of IFN-γ+ effector T cells enhanced compared to that shown by CTL alone against U87 cell line (p = .012) and primary cells (p = .026) respectively. Moreover, the combination of WT1 and BIRC5-specific CTLs in the presence of anti-PD1 also showed an increase in the number of IFN-γ+ effector T cells compared to that shown by CTL alone with U87 cell line (p = .013) and primary cells (p = .002).
Figure 4. The number of IFN-γ+ effector T cells of protein antigen-specific CTLs with or without anti-PD1 against human glioblastoma cells was evaluated by ELISPOT assay. The number of IFN-γ+ effector T cells of (A) WT1-specific CTLs with and without anti-PD1, (B) BIRC5-specific CTLs with and without anti-PD1, and (C) (WT1 and BIRC5)-specific CTLs with and without anti-PD1 against human primary glioblastoma cells and U87 cell line is summarized. The number of IFN-γ+ effector T cells was increased with CTL stimulated VaxDCs pulsed with CTP-fused BIRC5, and CTL stimulated VaxDCs pulsed with CTP-fused WT1 and CTP-fused BIRC5 against human primary glioblastoma cells and U87 cell line after blocking PD1. Human glioblastoma primary cells and U87 human glioblastoma cell line were used as the target cells. The E/T ratio was 10:1. Primary cell: human glioblastoma primary cells, U87 cell line: human glioblastoma cell line, CTL alone: no target cells; VaxDCs: CTLs stimulated by VaxDCs unpulsed with protein antigens; VaxDCs/WT1: CTLs stimulated by VaxDCs pulsed with CTP-fused WT1; VaxDCs/BIRC5: CTLs stimulated by VaxDCs pulsed with CTP-fused BIRC5; VaxDCs/(WT1+ BIRC5): CTLs stimulated by VaxDCs pulsed with CTP-fused WT1 and CTP-fused BIRC5. *: p < .05, **: p < .01, ***: p < .001
The killing effects of GBM antigen-specific CTLs against human GBM cells
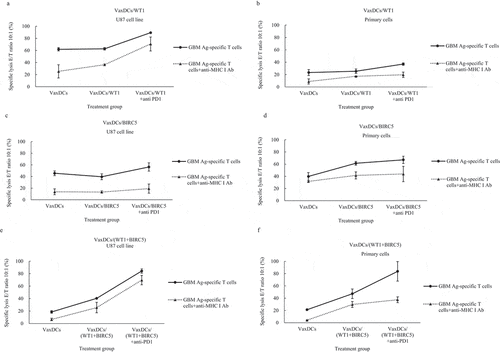
The ability of GBM antigen-specific CTLs to kill the U87 cell line and the primary GBM cells with and without anti-PD1 after stimulation with VaxDCs-pulsed with CTP-fused protein antigens was evaluated by using a standard LDH release assay at E/T ratio of 10:1 (). in general, addition of anti-PD1 presented an increase in the percent specific lysis in WT1-specific CTL group, BIRC5-specific CTL group, and combination of WT1 and BIRC5-specific CTL group. This trend was highest in combination group. Particularly, the percent specific lysis by GBM antigen-specific CTLs increased by 26.63% and 11.6% with WT1-specific CTLs combined anti-PD1 compared to WT1-specific CTLs (, ) and by 16.8% and 5.64% with BIRC5-specific CTLs combined anti-PD1 compared to BIRC5-specific CTLs (, ) against U87 cell line and primary GBM cells, respectively. The percent specific lysis by WT1 and BIRC5-specific CTLs combininganti-PD1 increased by 43.91% for U87 cell line and 36.55% for primary GBM cells (, ). Interestingly, the blocking of MHC I pathway also showed a decrease in all groups.
Figure 5. Lactate dehydrogenase (LDH) release to estimate the killing effects CTLs stimulated by CTP-fused protein antigen pulsed VaxDCs combined with anti-PD1. (A-F) We tested the ability of CTP-fused protein antigen-specific CTLs to kill primary cells and U87 cell line by using a standard LDH release assay. The CTLs stimulated by VaxDCs pulsed with CTP-fused WT1 or CTP-fused BIRC5 or combination of CTP-fused WT1 and CTP-fused BIRC5 along with anti-PD1 exhibited highest cytotoxic activity against both primary cells and U87 cell line. Human glioblastoma primary cells and U87 human glioblastoma cell line were used as the target cells. The E/T ratio was 10:1. Primary cell: human glioblastoma primary cells, U87 cell line: human glioblastoma cell line, CTL alone: no target cells; VaxDCs: CTLs stimulated by VaxDCs unpulsed with protein antigens; VaxDCs/WT1: CTLs stimulated by VaxDCs pulsed with CTP-fused WT1; VaxDCs/BIRC5: CTLs stimulated by VaxDCs pulsed with CTP-fused BIRC5; VaxDCs/(WT1+ BIRC5): CTLs stimulated by VaxDCs pulsed with CTP-fused WT1 and CTP-fused BIRC5. *: p < .05, **: p < .01, ***: p < .001
Discussion
GBMs constitute the most frequent type of brain cancers. Despite the progress in therapies, the mean survival is approximately 15–20 months .Citation2,Citation21 Immunotherapy is a promising therapeutic option for cancer treatments. Many clinical trials for cancer immunotherapies have been performed. in the immunotherapy approach, primary focus is on the development of a method wherein APCs induce CD8+ cytotoxic T cell response through MHC I stimulation because these cells have the ability to lyse antigen-specific tumor cells. The development of a method for the enhancement of T-cell cross-priming by DCs will help improve cancer immunotherapy .Citation22 Developing a new antigen delivery tool based on cross-presentation mechanism for exogenous antigens in DCs by using CTP has been investigated .Citation8,Citation23 The present data also indicated that CTP-fused protein antigens potentially cause antigen-specific CTL function through Th1 immune response.
The antigenic effect of the single tumor-associated antigens (BIRC5 and WT1) has been discussed in previous studies. in a clinical trial, WT1 vaccination was shown to trigger WT1-specific CTLs that suppressed cancer without damaging the normal tissues .Citation24 WT1 was recommended as the most promising cancer antigen .Citation25 Similarly, the functions of BIRC5, such as regulation of apoptosis, cell division, chemo-resistance, and tumor progression have been explored, Citation26 and this antigen has been investigated in malignant glioma in a clinical study .Citation27 Therefore, in this study, these two antigens were chosen and the presentation of WT1 and BIRC5 on target cells (U87 cell line and primary GBM cells) was observed.
VaxDCs before being pulsed with CTP-fused protein antigens remained constant for most of the MHC and co-stimulatory markers such as CD40, CD80, CD83, CD86, CCR7, and MHC I compared to imDCs. However, VaxDCs showed an enhancement in most of these markers after being pulsed with CTP-fused WT1 or CTP-fused BIRC5, thus indicating the potential of CTP-fused WT1 and CTP-fused BIRC5 in stimulating DC activation. Besides, IL-12 has multiple biological activities, and it is a key factor that drives Th1 responses and IFN-γ production. Thus, IL-12 immunotherapy could be of importance in the treatment of diseases where a Th1 response is desirable .Citation28 While IL-12 plays an important role in Th1 polarization, IL-10 acts as an inhibitor of Th1 polarization. An enhanced IL-10 production by antigen-stimulated CD40 L-transfected J558 cell line resulted in the reduction of antigen-specific IFN-γ production .Citation29 Here, the combination of CTP-fused WT1 and CTP-fused BIRC5 showed an increased secretion of Il-12p40 while there was a stable IL-10 level. As per the results, our CTP-fused protein antigens showed a potential differentiation of Th1 effectors.
Immune checkpoint inhibitors played an important role in anti-tumor efficacy of the immune response. The PD1/PD-L1 interaction was shown to be a limitation in T cell reactivity even long after the initial activation, and its blockade can restore immune function .Citation30,Citation31 Importantly, clinical trials using anti-PD1 have suggested that tumor-specific PDL1 expression may be an important biomarker of anti-PD1 efficacy .Citation32,Citation33 A subsequent study demonstrated that the expression of PDL1 is associated with GBM (50–90%) .Citation34 in our study, target GBM cells also showed a high expression of PDL1. Stimulated CTLs showed a significant effect with anti-PD1 after treatment. Especially, anti-PD1 resulted in increased IFN-γ+ effector T cells, leading to a stronger anti-tumor effect with single CTP-fused BIRC5 or combination of CTP-fused WT1 and CTP-fused BIRC5 against U87 cell line and primary GBM cells. Moreover, blocking PD1 receptor in our study also clarified the function of CTP-fused protein antigen-specific CTLs against U87 cell line and primary GBM target cells.
Although our results demonstrated the potential of using CTP-fused protein antigens WT1 and BIRC5 combined with anti-PD1 for GBM therapy, our study has some limitations that may hinder drawing clear conclusions of this treatment application. Firstly, although CTP-fused protein antigens enhanced MHC I expression and presentation of peptides that produced robust CTL immune response compared with CTP or antigens alone in previous studies, Citation8,Citation10,Citation12 the lack of a non-CTP reference control group limits the ability to draw precise conclusions about advanced CTP application in DC vaccine preparation compared to protein antigens only. Therefore, we just confirmed the feasibility of DC-based vaccine against GBM by using CTP-fused protein antigens in combination with anti-PD1. Secondly, the ability of anti-PD1 to promote T-cell responses has been evaluated in previous studies .Citation35 Here, although anti-PD1 enhanced antigen-specific T cell reactivity such as IFN-γ release and T cell proliferation, anti-PD1 had no stimulatory effect in the absence of antigen or T-cell receptor stimulus. Therefore, anti-PD1 did not cause nonspecific lymphocyte activation and therefore, we did not check the effect of anti-PD1 alone. Finally, our data did not show any difference between the U87 and primary cell lines based on different VaxDCs and PD1 blockade in terms of antigens (WT1 and BIRC5) and PDL1 expression. Therefore, more negative and positive target cells should be utilized to reach a clear conclusion in further studies.
Conclusion
In our study, CTP-fused WT1 and BIRC5 used for DC vaccine preparation showed not only an upgraded DC marker phenotype but also a potential in Th1 polarization. There was enhancement in GBM antigen-specific CTL functions against GBM target cells after blocking with anti-PD1 in both VaxDCs pulsed CTP-fused BIRC5 and VaxDCs pulsed with combination of CTP-fused WT1 and CTP-fused BIRC5 group. Our study suggested that using CTP-fused protein antigens for DC vaccine preparation along with anti-PD1 brings a promising effect in boosting DC functions that could be an effective strategy for immunotherapy targeting malignant gliomas.
Abbreviations
| BIRC5: | = | Survivin |
| DCs: | = | Dendritic cells |
| CTLs: | = | Cytotoxic T lymphocytes |
| CTP: | = | Cytoplasmic transduction peptide |
| PD1: | = | Programmed cell death protein 1 |
| PDL1: | = | Programmed cell death ligand 1 |
| WT1: | = | Wilm’s tumor gene 1 |
Declarations
Ethics approval and consent to participate
The blood from heathy donor and surgical specimens from glioblastoma patients were obtained for research purposes following approval by the Chonnam National University Hwasun Hospital Ethics Panel.
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Supplemental Material
Download MS Word (7.4 MB)Acknowledgments
Young-Hee Kim and Thi-Anh-Thuy Tran had worked together and contributed equally to this work. They shared co-first authorship. We would like to express our sincere gratitude to all the staff members, cancer survivors, participants, and others who provided support during the process of this study.
Supplemental Material
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2020.1732165.
Additional information
Funding
References
- Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. doi:10.1093/neuonc/nox158.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi:10.1056/NEJMoa043330.
- Thomas AA, Fisher JL, Ernstoff MS, Fadul CE. Vaccine-based immunotherapy for glioblastoma. CNS Oncol. 2013;2(4):331–49. doi:10.2217/cns.13.29.
- Wen PY, Reardon DA, Armstrong TS, Phuphanich S, Aiken RD, Landolfi JC, Curry WT, Zhu JJ, Glantz M, Peereboom DM. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma. Clin Cancer Res. 2019;25(19):5799–807. doi:10.1158/1078-0432.CCR-19-0261.
- van Montfoort N, van der Aa E, Woltman AM. Understanding MHC class I presentation of viral antigens by human dendritic cells as a basis for rational design of therapeutic vaccines. Frontiers Immunol. 2014. 5.
- Suzuki R, Oda Y, Utoguchi N, Namai E, Taira Y, Okada N, Kadowaki N, Kodama T, Tachibana K, Maruyama K, et al. A novel strategy utilizing ultrasound for antigen delivery in dendritic cell-based cancer immunotherapy. J Control Release. 2009;133(3):198–205. doi:10.1016/j.jconrel.2008.10.015.
- Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol. 2006;18(1):85–91. doi:10.1016/j.coi.2005.11.003.
- Kim D, Jeon C, Kim J, Kim M, Yoon C, Choi I, Kim S, Bae Y. Cytoplasmic transduction peptide (CTP): new approach for the delivery of biomolecules into cytoplasm in vitro and in vivo. Exp Cell Res. 2006;312(8):1277–88. doi:10.1016/j.yexcr.2005.12.029.
- Chauhan A, Tikoo A, Kapur AK, Singh M. The taming of the cell penetrating domain of the HIV Tat: myths and realities. J Control Release. 2007;117(2):148–62. doi:10.1016/j.jconrel.2006.10.031.
- Huang SF, Liu D-B, Zeng J-M, Yuan Y, Xiao Q, Sun C-M, Li C-L, Tao K, Wen J-P, Huang Z-G. Cloning, expression, purification, distribution and kinetics characterization of the bacterial beta-galactosidase fused to the cytoplasmic transduction peptide in vitro and in vivo. Protein Expr Purif. 2009;68(2):167–76. doi:10.1016/j.pep.2009.06.019.
- Song LL, Zhuo M, Tang Y, Chen X, Yu Y, Tang Z, Zang G. Ubiquitin-modified hepatitis B virus core antigen effectively facilitates antigen presentation and enhances cytotoxic T lymphocyte activity via the cytoplasmic transduction peptide in vitro. Mol Med Rep. 2015;12(1):289–96. doi:10.3892/mmr.2015.3352.
- Su HT, Li B, Zheng L, Wang H, Zhang L. Immunotherapy based on dendritic cells pulsed with CTP-FoxM1 fusion protein protects against the development of hepatocellular carcinoma. Oncotarget. 2016;7(30):48401–11. doi:10.18632/oncotarget.10269.
- Platten M, Reardon DA. Concepts for Immunotherapies in Gliomas. Semin Neurol. 2018;38(1):62–72. doi:10.1055/s-0037-1620274.
- Taggart D, Andreou T, Scott KJ, Williams J, Rippaus N, Brownlie RJ, Ilett EJ, Salmond RJ, Melcher A, Lorger M. Anti–PD-1/anti–CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proc Natl Acad Sci U S A. 2018;115(7). E1540-E1549. doi:10.1073/pnas.1714089115.
- Mohammad MG, Tsai VWW, Ruitenberg MJ, Hassanpour M, Li H, Hart PH, Breit SN, Sawchenko PE, Brown DA. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clinl Inves. 2014;124(3):1228–41. doi:10.1172/JCI71544.
- Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15(7):422–42. doi:10.1038/s41571-018-0003-5.
- Jung S-H, Lee H-J, Lee Y-K, Yang D-H, Kim H-J, Rhee JH, Emmrich F, Lee JJ. A phase I clinical study of autologous dendritic cell therapy in patients with relapsed or refractory multiple myeloma. Oncotarget. 2017;8(25):41538–48. doi:10.18632/oncotarget.14582.
- Yang D-H, Kim M-H, Hong CY, Lee Y-K, Jin C-J, Pham TNN, Ahn J-S, Bae W-K, Kim Y-K, Chung I-J, et al. Alpha-type 1-polarized dendritic cells loaded with apoptotic allogeneic myeloma cell line induce strong CTL responses against autologous myeloma cells. Ann Hematol. 2010;89(8):795–801. doi:10.1007/s00277-010-0931-3.
- Lee JJ, Choi B-H, Kang H-K, Park M-S, Park J-S, Kim S-K, Pham TNN, Cho D, Nam J-H, Kim Y-J, et al. Induction of multiple myeloma-specific cytotoxic T lymphocyte stimulation by dendritic cell pulsing with purified and optimized myeloma cell lysates. Leuk Lymphoma. 2007;48(10):2022–31. doi:10.1080/10428190701583975.
- Ikuta Y, Katayama N, Wang L, Okugawa T, Takahashi Y, Schmitt M, Gu X, Watanabe M, Akiyoshi K, Nakamura H, et al. Presentation of a major histocompatibility complex class 1-binding peptide by monocyte-derived dendritic cells incorporating hydrophobized polysaccharide-truncated HER2 protein complex: implications for a polyvalent immuno-cell therapy. Blood. 2002;99(10):3717–24. doi:10.1182/blood.V99.10.3717.
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–18.
- Sanchez-Paulete AR, Teijeira A, Cueto FJ, Garasa S, Pérez-Gracia JL, Sánchez-Arráez A, Sancho D, Melero I. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann Oncol. 2017;28(suppl_12):xii74. doi:10.1093/annonc/mdx727.
- Wu S, Chen X, Tang Y, Zhang Y, Li D, Chen J, Wang J, Tang Z, Zang G, Yu Y. Delivery of Tapasin-modified CTL epitope peptide via cytoplasmic transduction peptide induces CTLs by JAK/STAT signaling pathway in vivo. Acta Biochim Biophys Sin (Shanghai). 2018;50(2):181–90. doi:10.1093/abbs/gmx133.
- Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T, Nakajima H, Elisseeva OA, Oji Y, Kawakami M, Ikegame K, et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. The National Academy of Sciences of the USA; 2004; 101(38):13885–90. doi:10.1073/pnas.04058841010.
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–37. doi:10.1158/1078-0432.CCR-09-0737.
- Garg H, Suri P, Gupta JC, Talwar GP, Dubey S. Survivin: a unique target for tumor therapy. Cancer Cell Int. 2016;16(1):49. doi:10.1186/s12935-016-0326-1.
- Fenstermaker RA, Ciesielski MJ, Qiu J, Yang N, Frank CL, Lee KP, Mechtler LR, Belal A, Ahluwalia MS, Hutson AD. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol Immunother. 2016;65(11):1339–52. doi:10.1007/s00262-016-1890-x.
- Hamza T, Barnett JB, Li B. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int J Mol Sci. 2010;11(3):789–806. doi:10.3390/ijms11030789.
- Tuettenberg A, Fondel S, Steinbrink K, Enk AH, Jonuleit H. CD40 signalling induces IL-10-producing, tolerogenic dendritic cells. Exp Dermatol. 2010;19(1):44–53. doi:10.1111/j.1600-0625.2009.00975.x.
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–87. doi:10.1038/nature04444.
- Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2011;1217:45–59. doi:10.1111/j.1749-6632.2010.05919.x.
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74. doi:10.1158/1078-0432.CCR-13-3271.
- Wang X, Guo G, Guan H, Yu Y, Lu J, Yu J. Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. J Expe Clin Cancer Re. 2019;38(1):87. doi:10.1186/s13046-019-1085-3.
- Lou Y, Shi J, Guo D, Qureshi AK, Song L. Function of PD-L1 in antitumor immunity of glioma cells Saudi J Biol Sci. 201724(4):803–07. doi:10.1016/j.sjbs.2015.06.025.
- Wang CY, Thudium KB, Han M, Wang X-T, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2(9):846–56. doi:10.1158/2326-6066.CIR-14-0040.