ABSTRACT
Incidence of food allergy (FA) during nursing period is 6–8% globally and It is reported %5,7 in Turkey. In our study, the aim is to determine whether the prevalence of food allergy (FA) increases in children vaccinated against rotavirus. The files of 681 infants who are still followed-up were retrospectively evaluated. Children who did not come to our clinic for all of their well-child follow-up visits were excluded from the study. Moreover, children diagnosed with allergy before vaccination and children with known gastrointestinal system disease were excluded from the study. The number of patients diagnosed with food allergy after being vaccinated against rotavirus was 12 (1.76%). Three children had a family history of allergy. Of 12 patients who were diagnosed after vaccination, 3 (n:104) were vaccinated with pentavalent vaccine and 9 (n:507) with monovalent vaccine. In the monovalent vaccination group, food allergy was found in 9 children (1.55%), and in the pentavalent vaccination group, food allergy was found in 3 children (2.88%). The difference between the two vaccination groups in terms of food allergy prevalence was not significant (p > .05). Although it is believed that food allergy, and even cow’s milk protein allergy (CMPA) prevalence increases in infants vaccinated against rotavirus, in this study, no significant increase was observed in the prevalence of food allergy after rotavirus vaccination. Both types of vaccine had similar rates to each other.
Introduction
Rotavirus was first defined in the feces of children with diarrhea in 1973 and is one of the main causes of acute gastroenteritis under 5 years old around the world. The clinical reflection of rotavirus has a broad range. In addition to the supportive fluid treatment for the regulation of the clinical condition in patients, a specific treatment against rotavirus is yet to be found. Every year worldwide approximately 25 million patients are admitted to outpatient clinics, 2 million children are hospitalized, and unfortunately, more than 400,000 children are lost due to rotavirus diarrhea.Citation1 The vaccine developed to prevent rotavirus gastroenteritis was first licensed in 2006.Citation2
Currently, two types of licensed vaccines are used, which are monovalent and pentavalent.Citation3 These vaccines, which are found in the national immunization schedule of some countries, are not found in the immunization schedule in Turkey. Among the complications of both vaccines are food/breast refusal, blood in stool, mucus in stool, dysphagia, regurgitation, nausea, and colic.Citation4
Food allergy (FA) is an IgE and/or non-IgE-mediated reaction that causes symptoms by affecting one or more systems at various degrees. Food allergy in children can occur with conditions such as urticaria, enterocolitis, allergic eosinophilic gastroenteritis, and proctocolitis. Moreover, food allergy can accompany 30% of the infants with moderate-severe atopic dermatitis. The first and most important step in the diagnosis of FA is a good anamnesia, detailed inquiry of personal history-family history and physical examination. The diagnosis must be confirmed by allergen-specific IgE, skin prick test, allergen elimination, and oral provocation test, and other causes must be excluded.Citation5 The most common cause of food allergy among infants is cow’s milk.CMPA is the most common food allergy among infants and young children.Citation6 Incidence of FA during nursing period is 6–8%.Citation7 In the meta-analysis, food allergy varied from 1.2% to 17% for milk and 3% to 35% for any food.Citation6 Although there is no article of food allergy prevalence in this age from Turkey, the prevalence of food allergy in the Black Sea region in Turkey, with questionnaire at school children between 6 and 9 years of age was found 5.7%.Citation8 The resemblance of symptoms and complications that arise following rotavirus vaccination to food allergy symptoms may lead to false positivity in diagnosis, and the doctors may have to ask mothers to discontinue breastfeeding. The question whether the symptoms that arise after rotavirus vaccination are due to the vaccine or FA remains unanswered. Thus, in our study, the aim is to determine whether the prevalence of food allergy increases in children vaccinated against rotavirus and compared with the FA prevalence of general population.
Materials and methods
In this study, the first year of children who received rotavirus vaccination in our clinic between 2012 and 2017 was evaluated. The files of 681 infants who are still followed-up from the birth and first dose were retrospectively evaluated. The control group was not taken because it was a retrospective study and a comparison was made with the general population.
Children who did not come to our clinic for all of their well-child follow-up visits were excluded from the study. Moreover, children diagnosed with allergy before vaccination and children with known gastrointestinal system disease were excluded from the study. Between January 2018 and July 2018, families were interviewed when they visited the clinic for follow-up, or those who could not come to the clinic (n:35) were interviewed on the phone. Demographic characteristics of children, type of delivery, birthweight and gestational week, type of the vaccine used, vaccination months, medical history, whether or not diagnosed with history of atopic dermatitis, month of transition to supplementary food, duration of breastfeeding period, presence of food allergy, and if present, its symptoms, were inquired. Laboratory findings (eosinophile percentage and count, hemoglobin) were obtained from the hospital electronic database. Diagnosis of food allergy was made by the pediatric allergy specialist to whom the child was referred by the following physician. IgE specific to cow’s milk and hen’s egg, skin prick tests (SPT) for cow’s milk, hen’s egg, wheat, peanut, walnut, hazelnut, soy, negative and histamine (Stallergenes, France) were performed for all infants with suspected food allergy. SPTs were considered positive if the mean diameter of wheal was ≥3 mm higher than negative control and specific IgE was considered positive if the value was ≥0.34 ku/L. In addition to a positive test result, if the children also had a positive challenge test for the suspected food, they were diagnosed with food allergy.
Diagnosis of non-IgE-mediated allergic reaction such as allergic proctocolitis was made if the symptoms disappeared after removing the food which is considered responsible following anamnesia and physical examination and recurrence of the complaints following the re-introduction of the food after 2–4 weeks.
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 20.0. (Armonk, NY: IBM Corp.). Descriptive statistics for continuous variables were presented as mean ± standard deviation. The Shapiro-Wilk and Levene tests were used to analyze normality and homogeneity of variances. Categorical variables were tested using Fisher’s exact test and chi-square test. The universe of the study consist of 1500 rotavirus vaccinated children. No reference study on the prevalence of food allergy has been found in this population. Therefore, the sample size of the study was calculated with 50% unknown frequency. The power analysis of the study was done with 5% type 1 error rate (alpha = 0.05), 20% type 2 error rate (beta = 0,20). Confidence interval was 95%. In 95% confidence interval, the deviation (d) was calculated by taking 5%, with 80% power predicted. The minimum sample size was calculated as 306 rotavirus vaccinated children (openepi).
Results
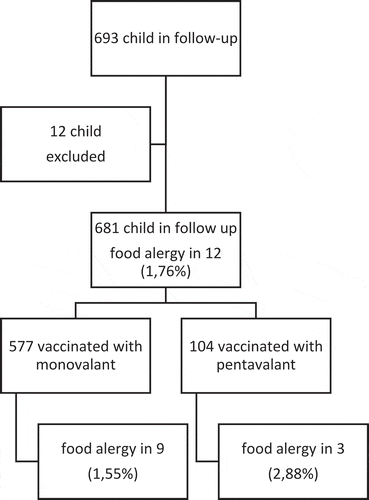
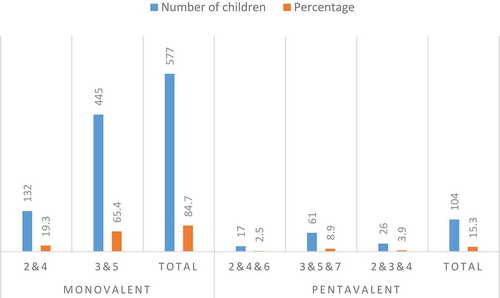
The number of children included in our study was 681. The mean age of these children was 32.7 ± 9.4 months. Sex ratio of study population was equal (M/F: 1.03). As high as 39% of children were born with cesarean. The mean birthweight was 3207 ± 485.29 g and the mean gestational age was 38.7 ± 0.5 weeks (min: 38.1-max: 39.9). The mothers of all children in the study had regular follow-ups during their pregnancy. The mean duration of exclusive breastfeeding was 4.92 ± 0.88 months (min: 4.02-max: 5.82). The mean of the complementary feeding start time was 5.8 ± 0.3 (min: 5.5-max: 6.2) months. Of the children, 577 (84.7%) were vaccinated with monovalent vaccine (rotarix®) and 104 (15.3%) with pentavalent vaccine (rotateq®). The most common vaccination was with the monovalent vaccine (rotarix®) at the 3rd and 5th months (65.4%). The distribution of rotavirus vaccine type and the distribution of months the vaccination was performed at are given in .
Figure 1. The distribution of rotavirus vaccine type and the months the vaccination was performed at.
During the follow-up, the symptoms of food allergy were suspected and the reasons for admission were examined. Suspicion of blood in stool in 21 children, mucus in stool in 28 children, vomiting in 78 children, reluctance to eat and breast refusal in 86 children, and failure to gain weight in 54 children were detected. There was only one child with multiple complaints at the time of admission. When one-year follow-ups of all children were analyzed, it was found that 79 children (11.6%) were diagnosed with two or more bronchiolitis and 96 children (14.1%) were diagnosed with atopic dermatitis.
Of 24 patients (%3,5) diagnosed with food allergy, 12 were diagnosed before the vaccination and thus were excluded from the study. In these 12 children, there were symptoms after being vaccinated against rotavirus. The number of patients diagnosed with food allergy after being vaccinated against rotavirus was 12 (1.76%). The distribution of cases is given in . The characteristics of these children are given in . The mean age of diagnosis was 5.45 (±0.98) months. Three children had a family history of allergy. Of 12 patients who were diagnosed after vaccination, 3 (25%) were vaccinated with pentavalent vaccine and 9 (75%) with monovalent vaccine. In the monovalent vaccination group, food allergy was found in 9 children (1.55%), and in the pentavalent vaccination group, food allergy was found in 3 children (2.88%). The difference between the two vaccination groups in terms of food allergy prevalence was not significant. Monovalent and pentavalent vaccine groups were similar in terms of FA incidence. The time of monovalent vaccination due to food allergy was 2nd and 4th months in 3 children, and 3rd and 5th months in 6 children. The time of pentavalent vaccination due to food allergy was 3rd, 5th, and 7th months in 2 children and 2nd, 4th, and 6th months in one child. Six children were allergic only to milk, 2 children were allergic only to egg, 3 children were allergic to milk and egg, and one child was allergic only to dried nuts. The most common complaint was cutaneous problems (75%) while the second most common complaint was blood in stool (33.3%). Clinical diagnoses were allergic proctocolitis in 4 children, atopic dermatitis in 4 children, urticaria in 2 children, anaphylaxis in one child, and both proctocolitis and atopic dermatitis in one child.
Table 1. The characteristics of these children who had food allergy.
As a result, it was found that the prevalence of FA did not increase compared to the general population.
Discussion
In this retrospective study, it was observed that the prevalence of FA did not increase in children who received rotavirus vaccine compared to the general population. Although it is believed that food allergy, and even cow’s milk protein allergy (CMPA) prevalence increases in infants vaccinated against rotavirus, in this study, increased in food allergy cases could not be related to rotavirus vaccination. Both types of vaccine had similar rates to each other. Most of the food allergies develop in the first or second year of life. In the first year of life, the highest detected prevalence of food allergies is approximately 6–8%. Prevalence tends to decrease until late childhood, and it was reported to become stabilized at approximately 3–4%.Citation9,Citation10 In a cohort study performed in the UK, the prevalence of food allergy in the first year of life confirmed by oral food challenge test was reported as 4%.Citation11 In a Danish study, food allergy prevalence was 3.6% in the first year of life, although it decreased with age.Citation12 The prevalence of food allergy in the first year of life in the US was reported as 5.7%.Citation13 In Asian studies on the prevalence of food allergy in children, it was reported as 12.6% in Japan and 10.9% in South Korea.Citation14,Citation15 The prevalence of CMPA in infancy was reported as 0.1–6% in Europe, 8% in the US and 5,7% in the data from Black Sea region of TurkeyCitation8,Citation15 In the study by Koca and Akcam performed in Turkey, the prevalence of CMPA was found to be 2–3%.Citation16 In our study, particularly after vaccination against rotavirus, the prevalence of food allergy until the 12-month checkup was found to be 1.76%. Only 9 patients (1.32%) had cow’s milk protein allergy. The FA incidence found in our study was found to be similar to FA incidence in the general population, and it can be concluded that FA incidence does not increase after rotavirus vaccine. Although it is believed that food allergy, and even CMPA prevalence increases in infants vaccinated against rotavirus, it was found that there are still no data in the literature on this subject. In this study, no significant increase was observed in the prevalence of food allergy after rotavirus vaccination. Increased in food allergy cases could not be related to rotavirus vaccination. It is also important that there is no significant difference between both types of vaccine. In the literacy, we did not find a study comparing this aspect. However, larger and more prospective studies are needed in this regard.
In their study where orally administered rotavirus vaccine and placebo were compared in terms of side effects, Vesikari et al.Citation17 reported similar prevalence of fever, vomiting, and diarrhea. The most important side effect of rotavirus vaccine is intussusception.Citation18 In 21 infants included in the study, blood in stool was detected and four infants were diagnosed with food allergy. Moreover, only one infant had blood in stool and other infants had the complaint of dermatitis-urticaria. Mucus in stool and hidden blood in stool can be observed after rotavirus vaccination. As this condition resembles allergic proctocolitis complaint, these infants can be misdiagnosed. In the diagnosis of FA, anamnesia, physical examination, personal history, history of immunization, and family history are important. As a result of this study performed with the suspicion of symptoms after rotavirus vaccination leading to the false positivity of FA diagnosis, the importance of food allergy and CMPA diagnosis is clear.
It is observed that clinicians misdiagnose FA even in the presence of any one of these symptoms alone, and because of this, breastfeeding is discontinued or maternal diet for breastfeeding is imposed as the first step of treatment. Besides its numerous positive aspects, the importance of breastfeeding in mother-infant and infant-mother bonding is known.Citation19 Because of misdiagnosis, clinicians may negatively affect this bonding process by asking the mother to stop breastfeeding. Another issue that may create negative influence is anti-vaccine prejudice and vaccine refusal phenomenon. With the perception that the problems may develop after vaccination and concomitant cessation of breastfeeding, families’s trust in vaccination companies and vaccines may decrease.Citation20
The strong point of this study is trying to emphasize the relationship between rotavirus vaccine and FA. It was also to see if there was a difference between both vaccines. However, it should be noted that the study is retrospective. A larger number of cases and a prospective study may be more valuable, especially when comparing both vaccines and stating their association with FA. This relationship should be done in a study with a prospective and with unvaccinated control group.
In conclusion, this is the first study reporting that there is no increase in FA prevalence among infants vaccinated against rotavirus. Studies with a larger population and prospective design are required on this subject. It is important to establish a definitive diagnosis of FA. Symptoms such as presence of blood and mucus in stool, vomiting, colic must be evaluated effectively, and the harms of diets that can affect the mother or harms of cessation of breastfeeding must be considered.
Abbreviation
Author contribution
Conceptualization NMK and OYO, Methodology NMK and OYO, Formal analysis NMK and OYO, Data curation NMK, AS, EA, IA, ZIB, and SC, writing NMK, review NMK, BTK, and OYO. All authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
Ethical approval
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the University Institutional Review and Ethical Board (Project No: KA18/99).
Additional information
Funding
References
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–72. doi:10.3201/eid0905.020562.
- Clark HF, Glass RI, Offitt PA. Rotavirus vaccines. In: Plotkin SA, Orenstein WA editors. Vaccines. 3rd ed. Philadelphia (PA): WBSaunders; 1999. p. 987–1005.
- World Health Organization. Rotavirus vaccines. WHO position paper. 2013 Jan [accessed 2013 Feb 19]. http://www.who.int/wer/2013/wer8805.pdf.
- Chen RT, Zanardi LR. An analysis of rotavirus vaccine reports to the vaccine adverse event reporting system: more than intussusception alone? Pediatr. 2004 Apr;113(4):e353–9.
- Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, Nadeau K, Nowak-Wegrzyn A, Oppenheimer J, Perry TT. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134:1016–25. doi:10.1016/j.jaci.2014.05.013.
- Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, Sigurdadottir ST, Lindner T, Goldhahn K, Dahlstrom J, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–46. doi:10.1016/j.jaci.2007.05.026.
- Hill DJ, Firer MA, Shelton MJ, Hosking CS. Manifestations of milk allergy in infancy: clinical and immunological findings. J Pediatr. 1986;109:270–76. doi:10.1016/S0022-3476(86)80384-5.
- Orhan F, Karakas T, Cakir M, Aksoy A, Baki A, Gedik Y. Prevalence of immunoglobulin E-mediated food allergy in 6–9-year-old urban schoolchildren in the Eastern Black Sea Region of Turkey. Clin Exp Allergy. 2009;39:1027–35. doi:10.1111/cea.2009.39.issue-7.
- Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A; EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014 Aug;69(8):992–1007. doi:10.1111/all.12423. Epub 2014 May 10. Review. PubMed PMID: 24816523.
- Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, Tang MLK, Lowe AJ, Matheson M, Dwyer T, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J Allergy Clin Immunol. 2017;140:145–53. doi:10.1016/j.jaci.2017.02.019.
- Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, Dean T. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006;117:1118–24. doi:10.1016/j.jaci.2005.12.1352.
- Eller E, Kjaer HF, Host A, Andersen KE, Bindslev-Jensen C. Development of atopic dermatitis in the DARC birth cohort. Pediatr Allergy Immunol. 2010;21:307–14. doi:10.1111/pai.2010.21.issue-2p1.
- US Department of Health and Human Services, Centers for Disease Control andPrevention, National Center for Health Statistics. Summary statistics, national health interview survey 2015, table C-2A. 2015.
- Lee SI, Shin MH, Lee HB, Lee JS, Son BK, Koh YY, Kim KE, Ahn YO. Prevalences of symptoms of asthma and other allergic diseases in Korean children: a nationwide questionnaire survey. J Korean Med Sci. 2001;16:155–64. doi:10.3346/jkms.2001.16.2.155.
- Iikura Y, Imai Y, Imai T, Akasawa A, Fujita K, Hoshiyama K, Nakura H, Kohno Y, Koike K, Okudaira H, et al. Frequency of immediate-type food allergy in children in Japan. Int Arch Allergy Immunol. 1999;118:251–52. doi:10.1159/000024089.
- Koca T, Akçam M. Cow’s milk protein allergy. Dicle Med J. 2015;42:268–73.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi:10.1056/NEJMoa052664.
- U.S. Food and Drug Administration. Information on Rotarix–Labeling revision pertaining to intussusception. [accessed 2010 Sept 23]. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm226690.htm.
- Jackson DB. The association between breastfeeding duration and attachment: a genetically informed analysis. Breastfeed Med. 2016;11:297–304. doi:10.1089/bfm.2016.0036.
- Özceylan G, Toprak D, Esen ES. Vaccine rejection and hesitation in Turkey. Hum Vaccin Immunother. 2020;6:1–6. doi:10.1080/21645515.2020.1717182.