ABSTRACT
Knowledge, attitudes and beliefs (KABs) toward influenza vaccination (IV) play a key role in HCWs’ decisions to receive vaccination and can strongly influence patients’ IV uptake. We examined the knowledge, attitudes and beliefs of GPs toward IV, exploring their opinion on IV in the elderly, mandatory HCW vaccination and the desirability of an IV trial in the elderly with hospitalization/mortality as effect measure. From November 2018 to March 2019, surveys were emailed to GPs and GP-practices (n = 1676) in three regions of the Netherlands. We assessed the self-reported IV in GPs, reasons for (not) advising IV to personnel, (not) supporting mandatory IV for personnel and (not) desiring a trial on IV in the elderly on hospitalization/mortality. Multivariable logistic regression models were used to determine predictors for GP IV. A total of 552 surveys were completed and 71.9% of the GPs reported receiving IV. Determinants for IV in GPs were male sex (aOR 1.62, 95%CI 1.06–2.49, p = .03) and age ≥60 y (aOR 5.25, 95%CI 1.51–18.32, p = .01). Seventy-nine percent of the GPs recommend IV for their practice personnel. Mandatory IV for personnel was supported by 41.2% of the GPs with GP self-reporting IV being the only determinant (aOR 10.03 (95%CI 5.69–17.70 p = .00)). An IV trial on hospitalization and/or mortality was desired by 60.5% of the GPs. We concluded that the majority of Dutch GPs receives IV and recommends IV to their personnel. These high rates along with the hesitancy of GPs toward mandatory HCW IV should be considered when policymakers decide on a mandate for IV in HCW in general.
Introduction
During an epidemic, influenza can affect all in society and elderly especially. Suddenly increased elderly care needs and absenteeism among caregivers and healthcare workers (HCWs) can disrupt the healthcare system.Citation1, Citation2 Besides vaccinating high-risk patients against influenza, also HCW vaccination is proposed to reduce its impact by preventing absenteeism and transmission to elderly and their caregivers.Citation3 However, many HCWs choose to stay unvaccinated leaving coverage rates far below WHO-recommended levels.Citation4
Knowledge, attitudes and beliefs (KABs) toward influenza vaccination (IV) play a key role in HCWs’ decisions to receive vaccination and can strongly influence patients’ IV uptake.Citation5–7 Determinants of vaccine refusal in HCWs have been studied extensively.Citation8,Citation9 However, most studies have been conducted in hospitals or elderly homes, whereas in many high-income countries like the Netherlands, general practitioners (GPs) are key actors in implementing IV policy. Since previous findings cannot be translated to primary care,Citation10 little remains known about the KAB of GPs toward IV, especially in the largest target group; the elderly. New insights in KAB of GPs toward IV could help developing interventions aimed at increasing vaccination uptake in the elderly and in primary healthcare personnel.
In the Netherlands, GPs have a long history as main implementers of the National Prevention Influenza Programme according to which high-risk groups are vaccinated against influenza free of charge. Given this background, the KAB of Dutch GPs toward IV in HCWs and elderly is relevant. Moreover, their attitude toward currently debated topics regarding IV – such as the desirability of (mandatory) HCW influenza vaccinationCitation11 and of a trial designed to evaluate the effect of IV in the elderly on hospitalization and/or mortality,Citation12 – is important to consider for both policymakers and the scientific community when deciding on mandatory IV or setting the agenda for new IV-related research.
Therefore, we examined (1) the KAB of Dutch GPs toward IV, (2) evaluated demographic predictors for KAB, and (3) explored GPs’ opinions on IV in the elderly, mandatory HCW influenza vaccination and the desirability of an IV trial on mortality, both before and during the influenza-epidemic of 2018/2019.
Materials and methods
Work-up
From November 2018 to March 2019, invitations to complete a survey, hosted on FormDesk®, were emailed to GPs and GP-practices in three regions (North, West and South) of the Netherlands. E-mail addresses were retrieved from databases provided by contact persons of the collaborating academic departments of Family Medicine in these regions.
To evaluate the effect of seasonality on survey results, GP-contacts in the Southern-group were randomly divided into two groups, receiving requests to participate either before (n = 350) or during (n = 337) the influenza epidemic. To increase survey response, by the end of November 2018 electronic newsletters with a link to our survey were posted on GP-platforms used in the Southern region. End of January, during the epidemic, e-mail requests were sent out to all other GP contacts; i.e. GPs in the Northern (n = 642) and Western region (n = 160) and the remaining GP-contacts in the Southern region (n = 337). By chance, the academic department of Family Medicine in the Western region only held a small database of contacts. Therefore, in this region, we provided permission for participating GPs to distribute the survey to their regional GP colleagues.
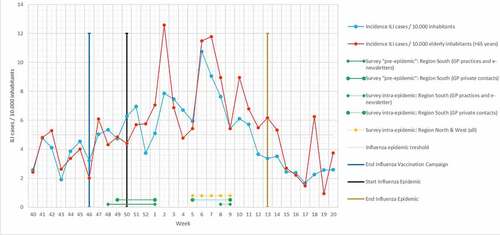
Reminders were sent 1 and 2 weeks after sending the first invitation. The surveys were closed on January 2 and March 1. illustrates the start and end of the surveys in relation to the influenza-like-illness (ILI) incidence.
Figure 1. Timescale illustrating the start and end of the surveys in relation to the influenza-like-illness (ILI) incidence.Citation13
Questionnaire
The survey consisted of 14 closed-ended questions mostly using 5-point Likert scales (Appendix A). We adopted some of the questions of a survey of the influenza vaccination rate among Dutch GPs in 2008, so that we could compare reported IV rates among Dutch GPs over time.Citation14 When applicable, the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) statement was followed.Citation15
Draft questionnaires were previously reviewed by 10 university staff members specialized in primary care research and adjusted accordingly. The medical ethics committee of the Maastricht University Medical Center confirmed that the Medical Research Involving Human Subjects Act did not apply since no patients were included. To avoid any effect on the GPs’ vaccination behavior, surveys were sent out only 2 weeks after the IV campaign had ended ().
Outcome measures
Outcome measures were the self-reported IV coverage in GPs and the assessment of determinants and reasons for (not) being vaccinated against influenza, (not) advising (mandatory) HCW vaccination, (not) supporting the current IV policy and (absence of) desirability of a trial on IV in the elderly evaluating hospitalization and/or mortality as an endpoint.
Analysis
Only fully completed forms were used for analyses. After testing for multicollinearity, all demographic variables (by all means: age, gender, working experience, part-time/full-time work, practice form) were entered in an ordinal logistic regression model to calculate the adjusted odds ratio (aOR). If assumptions regarding goodness of fit and/or proportional odds assumption were violated, dependent variables with Likert-scale (five parameters) were recoded into three parameter variables and assumptions were retested. If assumptions were still not met, outcome variables were reduced to two levels to perform binary logistic regression. We used the chi-square test to test for any demographic differences between the GPs per region and per time frame (pre or intra-epidemically). When comparing continuous data, the Shapiro–Wilk test was used to test for normality. If not normally distributed, non-parametric tests (Mann–Whitney Test) were used.
Results
Demographics
A total of 1676 GPs or GP practices were directly asked to participate. Since the survey was also accessible by electronic newsletter, an unknown number of potential participants was indirectly reached. Eventually, 601 GPs participated in completing 552 surveys. Since the denominator was not known exactly, the total response rate is estimated as being ≤32.9% (552/1676). Accurate response rates were calculated from the survey groups including GPs who were only addressed personally, varying between 29.1% and 39.5% (mean 33.6%). The basic characteristics of responders and national figures are presented in . Demographics of those participating in the Southern region before and during the epidemic did not differ significantly (results not shown).
Table 1. Basic characteristics of respondents compared with national figures
Survey results
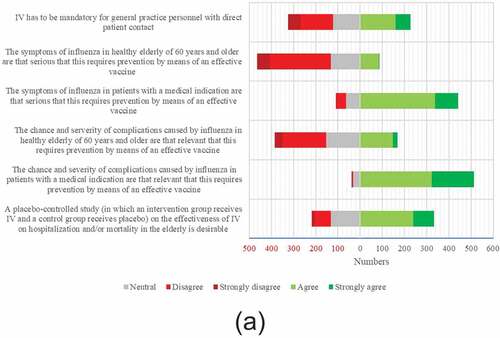
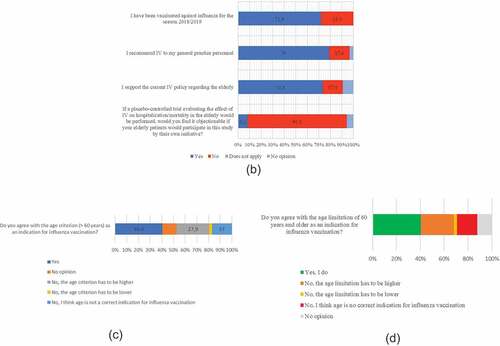
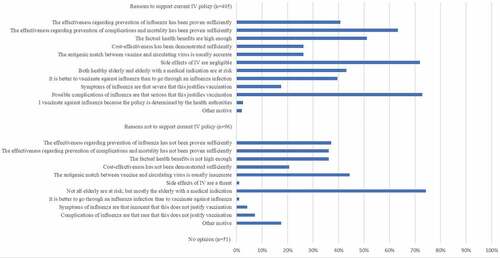
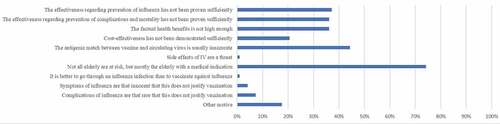
Survey results are shown in . In the 2018/2019 season, 397 (71.9%) of the GPs reported receiving IV. Most important reasons were reducing the risk of getting influenza (61.2%) and transmitting influenza to patients (87.7%). Most important reasons for refusing IV were not belonging to a risk group (51.0%) and the conviction that one was already protected against influenza (25.2%) (). Among “other motives” uncertainty regarding the usefulness of the vaccine was most frequently mentioned (13/41). Regression analysis showed two determinants for self-reported IV among GPs: male sex (aOR 1.62, 95%CI 1.06–2.49, p = .03) and age ≥60 y (aOR 5.25, 95% CI 1.51–18.32, p = .01). Reasons for supporting the IV policy (or not) can be found in .
Mandatory IV in practice personnel
Of all responding GPs, 436 (79.0%) recommend IV for their practice personnel (). Mandatory IV of personnel is supported by 41.2% and rejected by 36.9% of the GPs (). Reported IV in GPs was a predictor for supporting mandatory IV in practice personnel; aOR 10.03 (95%CI 5.69–17.70 p = .00).
Estimated vaccine efficacy and vaccination rate
Median estimated efficacy of IV for preventing influenza in the elderly was 60% (range 0-100%) and significantly higher in those who received IV (60% (range 1-95%)) than those not receiving IV (50% (range 0-100%), p = .00). The median estimated vaccination rate of risk groups within the GP’s practice was 60% (range 0-95%) and did not differ between vaccinated and unvaccinated GPs (median 60% (range 0-95%) vs. 60% (range 3-90%), p = .44).
Desirability of a placebo-controlled IV trial on hospitalization/mortality
Of all GPs, 60.5% desires a trial evaluating the effect of IV in the elderly on hospitalization and/or mortality. GPs not receiving IV desired such a trial more frequently than those receiving IV (68.4% vs. 57.4%, p = .02).
Seasonal and regional differences
No significant differences in survey results were demonstrated between the three regions (results not shown), except for the estimated vaccination rate which was significantly lower in the Western-group compared to the Southern and Northern regions; 50% (range 20-75%) vs. 60% (range 0-95%), p = .00.
Pre-epidemically 36.6% of the GPs supported mandatory IV in HCWs, increasing to 48.0% intra-epidemically (overall 41.2%). The unadjusted OR of the intra-epidemic season vs. the pre-epidemic season on support of an IV mandate (agree vs. neutral or disagree) was 1.60 (95%CI 1.03–2.48), p = .04). After correcting for demographic variables we found an aOR of 1.49 (95%CI 0.94–2.38, p = .09).
Discussion
We examined the knowledge, attitudes and beliefs of general practitioners toward influenza vaccination in different regions in the Netherlands before and during the 2018/2019 influenza epidemic. Our study showed that the majority of GPs receives IV (71.9%), recommends IV for their practice personnel (79.0%) and desires for a trial evaluating the effect of IV on hospitalization and/or mortality in the elderly (60.5%). Male sex (aOR 1.62) and age ≥60 y (aOR 5.25) were significant determinants for self-reported IV among GPs. Reported IV in GPs was a strong predictor for supporting mandatory IV in practice personnel (aOR 10.03).
Strengths of our study are the generalizability since we conducted our study in different regions. In the Netherlands, GPs have always been key actors in implementing IV. Given their experience and expertise, the attitude of Dutch GPs toward this topic is particularly interesting. Our methodological approach also allowed us to compare survey responses before and during the influenza season which has not been done before. Also – by adopting some questions of a previous studyCitation14 – we could compare GP vaccination rates over time. Finally, this study evaluates currently debated topics regarding IV (i.e. mandatory HCW IV and desirability of an IV trial on mortality) that have not been evaluated in such large populations of GPs before.
Some limitations of this study should be discussed. Although the response rate of approximately 30% is fair in this field,Citation16 and attempts to increase questionnaire response by using online platforms were made, selective response cannot be excluded. Also, since the participating GPs were affiliated to an academic department of Family Medicine, this could negatively affect the representativeness of our respondents. However, the basic characteristics of our respondents were roughly comparable to national statistics. Also, participating GPs did not seem to have a preoccupation toward IV since their median estimated vaccination coverage rates and estimated vaccine efficacy rates correspond well to the national statistics (49.9% in risk groups)Citation17 and literature (vaccine efficacy 50-60%)Citation18 respectively. For these reasons and given their affiliation to different departments in different regions in the Netherlands, we consider our study population as representative. Finally, post-hoc sample size calculation (not presented) showed that at least 369 participants needed to be included in order to have an acceptable margin of error of 0.05 from the 95%CI based on a proportion of 0.6 of GPs being vaccinated. Thus, responses (n = 552) can be considered high enough to draw conclusions based on these findings.
Because IV in GPs was self-reported, social desirability bias cannot be excluded. However, research shows that questionnaire data overestimates vaccination rates by less than 10%,Citation19 limiting the potential effect of such bias. Finally, for methodological reasons previously explained, we were still enrolling participants in our “pre-epidemic” group when the epidemic had already started. But by that time ILI-incidence was still low, and the incidence of ILI in the elderly – which has our special interest – started to increase later, coinciding with our second (intra-epidemic) survey rounds (). Therefore, we think our surveys still discriminate between the “pre-epidemic” and intra-epidemic season.
Coverage rate, motives and predictors of IV in GPs
IV coverage among European HCWs is generally below 30%,Citation20 and has been showing declining trends.Citation21 Vaccination rates among GPs vary widely; ranging from 12% in SloveniaCitation22 to over 75% in some Italian regions.Citation23 Most recent statistics on Dutch GP IV date back from the pandemic 2009/2010 season, reporting a (seasonal) IV coverage rate of 63%.Citation24 Especially when compared to the latest non-pandemic rates of 36% for seasonal IV in 2007/2008,Citation14 the coverage rate of 71.9% found in our study suggests a relevant increase. For reasons previously explained, this increase is not likely to be explained by selection bias. A potential explanation for the increase could be the gradual implementation of the recommendations for HCW to be vaccinated against influenza that were introduced in 2007.Citation25
Besides age >60 y (which is an indication for IV), male sex was a predictor for vaccination uptake in GPs, which is consistent with previous studies among GPs.Citation14,Citation24In our study, these differences in vaccination coverage between sex were consistent over all age groups, whereas in the general (non-GP) population females tend to be vaccinated more often.Citation26 This is suggested to be related to the greater propensity of women for seeking health care.Citation27 Although this effect is likely to be smaller in healthcare professionals, our study did not yield supportive data to explain for the inverse relation that we found.
In our study, most frequently reported motives in GPs for receiving IV were personal protection against influenza and lowering of the risk of transmitting influenza to patients, whereas having no medical indication for IV and the conviction that one is already protected against influenza were the most common reasons for not being vaccinated. These motives do not seem to have changed over the past decadeCitation14 and apply internationally.Citation9 Similar to our study, concerns about side effects, forgetfulness, and doubts about the vaccine’s efficacy are less frequently reported motives (ranging from 2.5% to 28.6%) for non-vaccination in GPs.Citation9 Besides all quantitative data, qualitative data showed that also the belief that influenza is not a serious illness is a potential barrier for HCW vaccination.Citation8 Our study clarifies that – unlike in patients with a medical indication – the majority of GPs do not agree that the seriousness of symptoms and the severity and chance of complications of influenza in healthy elderly are that relevant that this requires prevention by means of an effective vaccine.
Attitude toward mandatory IV in HCW and toward a new trial
To our best knowledge, this is the first study to examine the attitudes of GPs toward mandatory IV in practice personnel. Whereas only 41.2% of the GPs in our study supports mandatory vaccination of practice personnel with direct patient contact, Desante et al. showed that in the United States, 84% of the physicians working in internal medicine or emergency medicine supported mandatory IV among HCW.Citation28 Differences in the severity of complications caused by influenza in patients presenting in this setting compared to primary care may partly explain for these differences.
The efficacy of IV in the elderly has long been a topic of debate, mainly due to the lack of direct RCT-based evidence on effects of IV on severe morbidity and mortality in the elderly. This study is the first now to report an uninformed opinion of a majority (60.5%) of GPs desiring such a trial.
Regional and seasonal influence on the attitude of GPs toward IV in the elderly
Regional variations in GPs’ perceptions and practices regarding vaccination have been observed in France.Citation29 However, this study did not focus on IV explicitly. Besides the estimated coverage rates of IV varying up to 12% per region, we did not find regional differences in attitudes of GPs toward IV. This could be explained by close adherence to Dutch medical guidelines.
Up to our knowledge, no studies quantified the effect of seasonality on attitudes of GPs on IV before. A qualitative Australian study did suggest that the severity of the previous influenza season could affect the attitude of stakeholders toward mandatory IV in HCW 1 y later.Citation30 Although we examined this effect within the same season, we could not demonstrate a significant effect of the epidemic occurrence of influenza on the GPs' attitude toward mandatory IV in HCW. It should be noted that the 2018/2019 influenza season in the Netherlands was relatively mild, potentially limiting the near significant (p = .09) effect.
Implications and novelty of our study
Our study found a high IV coverage rate in GPs (71.9%), indicating a relevant increase over the past decade.Citation14 In the absence of surveillance systems monitoring IV coverage rates in primary care on any (national or European) level and considering the present-day debate on introducing mandatory IV for HCWs, our up-to-date information on GP coverage rates and their attitudes toward IV is relevant for both policymakers and researchers. In this study, we did not evaluate vaccination rates in HCWs other than GPs. Previous studies learned that coverage rates in other HCWs are generally lower than in physicians (ranging between 20% and 40%).Citation31 We do know that physician’s encouragement to get vaccinated is an important factor in HCW vaccine uptake.Citation32,Citation33 Considering that the majority of GPs is vaccinated and recommends IV to their practice personnel, we expect that this translates into high vaccination rates in practice personnel as well, although other HCWs than GPs were not included in this study. The high vaccination coverage in GPs as such, the hesitancy of GPs to mandate IV in HCWs, the fact that non-institutionalized elderly are easily exposed to many potential vectors of influenza other than HCWs, but also the fact that GPs also regularly visit patients that may be very ill and institutionalized, should all be taken in to account when policymakers decide on mandatory IV for HCWs in general and – more explicitly – in primary care.
This is the first study to objectify the effect of seasonality on attitudes of GPs on IV. Our finding that GPs’ attitudes toward mandatory IV in HCW does not significantly change during the influenza season should be interpreted with care, as explained previously. In case stakeholders or, by referendum, HCWs are involved in deciding on such a mandate, policymakers should be aware that, especially during a severe influenza epidemic, a potential seasonal effect on this opinion, cannot be ruled out.
A novel finding is that 60.5% of the GPs' desires a placebo-controlled trial on IV in the elderly evaluating mortality as an endpoint and that 86.1% would have no objections if their elderly patients would participate in such a trial, exposing them to the harmful risks of influenza. This (uninformed) opinion has no direct implications for the justification and feasibility of such a trial. However, it should be seen as an important sign for the scientific community to clearly communicate on the state of evidence on the effects of IV on morbidity and mortalityCitation34 in the elderly and the substantial ethical, methodological and practical barriers to be addressed,Citation12 before a placebo-controlled trial with severe complications and mortality as outcomes would be justified.
Finally, if new research in the field is conducted, this study provides new insights in knowledge gaps or outstanding questions that could be addressed. For instance, the observed uncertainty among GPs in our study on the need for IV in “healthy” elderly.
Conclusion
Whereas the positive behavior toward IV is reflected by the majority of GPs receiving IV and recommending IV to their practice personnel, the GPs’ attitude toward mandatory IV and the beneficial effects of IV in the healthy elderly on severe morbidity and mortality should considered hesitant. Given the majority of GPs that likes to see a trial being conducted on hospitalization/mortality as an endpoint, and the identified barriers for conducting such a trial, it is important to explicitly discuss the current state of evidence on the effects of IV and these barriers with the GP community. Our findings should be taken into consideration when policymakers decide on a mandate for IV and when researchers set up the agenda for influenza-related research or communicate on their findings.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Wim Opstelten and Dr Ted van Essen for allowing us to use parts of their questionnaire about vaccination behavior of general practitioners. We thank Dr Gé Donker (Nivel, Netherlands Institute for Health Services Research) for sharing data and Dr Bjorn Winkens (statistician, Maastricht University) for his feedback on the statistical analysis. We also thank B. Doorn (project manager in Maastricht University (northern region)), Dr Pauline Slottje (Amsterdam University Medical Centers, Vrije Universiteit Amsterdam (western region)) and Nynke Schouwenaars (medical scientist, University of Groningen (northern region)) for distributing the surveys among GPs affiliated to their universities.
Additional information
Funding
References
- European Centre for Disease Prevention And Control. Factsheet about seasonal influenza. [ accessed 2019 October 3]. https://ecdc.europa.eu/en/seasonal-influenza/facts/factsheet
- European Commission. Importance of HCW vaccination. [accessed 2019 October 3]. https://ec.europa.eu/health/vaccination/influenza_en
- Maltezou HC, Poland GA. Vaccination policies for healthcare workers in Europe. Vaccine. 2014;32(38):4876–80. doi:10.1016/j.vaccine.2013.10.046.
- Wicker S, Marckmann G. Vaccination of health care workers against influenza: is it time to think about a mandatory policy in Europe? Vaccine. 2014;32(38):4844–48. doi:10.1016/j.vaccine.2013.09.062.
- Martinez-Baz I, Aguilar I, Moran J, Albeniz E, Aldaz P, Castilla J. Factors associated with continued adherence to influenza vaccination in the elderly. Prev Med. 2012;55(3):246–50. doi:10.1016/j.ypmed.2012.06.020.
- Bovier PA, Chamot E, Bouvier Gallacchi M, Loutan L. Importance of patients’ perceptions and general practitioners’ recommendations in understanding missed opportunities for immunisations in Swiss adults. Vaccine. 2001;19(32):4760–67. doi:10.1016/S0264-410X(01)00223-7.
- Chiatti C, Barbadoro P, Lamura G, Pennacchietti L, Di Stanislao F, D’Errico MM, Prospero E. Influenza vaccine uptake among community-dwelling Italian elderly: results from a large cross-sectional study. BMC Public Health. 2011;11(1):207. doi:10.1186/1471-2458-11-207.
- Lorenc T, Marshall D, Wright K, Sutcliffe K, Sowden A. Seasonal influenza vaccination of healthcare workers: systematic review of qualitative evidence. BMC Health Serv Res. 2017;17(1):732. doi:10.1186/s12913-017-2703-4.
- Collange F, Verger P, Launay O, Pulcini C. Knowledge, attitudes, beliefs and behaviors of general practitioners/family physicians toward their own vaccination: a systematic review. Hum Vaccin Immunother. 2016;12(5):1282–92. doi:10.1080/21645515.2015.1138024.
- Ridda I, Lindley IR, Gao Z, McIntyre P, Macintyre CR. Differences in attitudes, beliefs and knowledge of hospital health care workers and community doctors to vaccination of older people. Vaccine. 2008;26(44):5633–40. doi:10.1016/j.vaccine.2008.07.070.
- Ministry of health, welfare and sport. Letter of State Secretary Paul Blokhuis on evaluation of mandatory influenza vaccination in HCW [Kamerbrief Blokhuis over Maatregelen Griep]. [accessed 2019 October 3]. https://www.rijksoverheid.nl/ministeries/ministerie-van-volksgezondheid-welzijn-en-sport/documenten/kamerstukken/2018/10/10/kamerbrief-over-maatregelen-griep
- Verhees RAF, Dondorp W, Thijs C, Dinant GJ, Knottnerus JA. Influenza vaccination in the elderly: is a trial on mortality ethically acceptable? Vaccine. 2018;36(21):2991–97. doi:10.1016/j.vaccine.2018.04.027.
- Nivel. Incidence of influenza like illness during the 2018/2019 influenza season. [accessed 2019 October 3]. https://www.nivel.nl/nl/zorgregistraties-eerste-lijn/surveillance
- Opstelten W, van Essen GA, Ballieux MJ, Goudswaard AN. Influenza immunization of Dutch general practitioners: vaccination rate and attitudes towards vaccination. Vaccine. 2008;26(47):5918–21. doi:10.1016/j.vaccine.2008.08.049.
- Eysenbach G. Improving the quality of Web surveys: the checklist for reporting results of internet E-Surveys (CHERRIES). J Med Internet Res. 2004;6(3):e34. doi:10.2196/jmir.6.3.e34.
- Morton SM, Bandara DK, Robinson EM, Carr PE. In the 21st Century, what is an acceptable response rate? Aust N Z J Public Health. 2012;36(2):106–08. doi:10.1111/j.1753-6405.2012.00854.x.
- Nivel. Report on vaccination coverage rates in the Netherlands. [accessed 2019 October 3]. https://www.nivel.nl/nl/publicatie/monitor-vaccinatiegraad-nationaal-programma-grieppreventie-2017
- Govaert TM. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. Jama. 1994;272(21):1661–65. doi:10.1001/jama.1994.03520210045030.
- Brien S, Kwong JC, Buckeridge DL. The determinants of 2009 pandemic A/H1N1 influenza vaccination: a systematic review. Vaccine. 2012;30(7):1255–64. doi:10.1016/j.vaccine.2011.12.089.
- Mereckiene J, Cotter S, Nicoll A, Lopalco P, Noori T, Weber J, D'Ancona F, Levy-Bruhl D, Dematte L, Giambi C, et al. Seasonal influenza immunisation in Europe. Overview of recommendations and vaccination coverage for three seasons: pre-pandemic (2008/09), pandemic (2009/10) and post-pandemic (2010/11). Euro Surveill. 2014 Apr 24;19(16):20780. https://www.ncbi.nlm.nih.gov/pubmed/24786262
- Dini G, Toletone A, Sticchi L, Orsi A, Bragazzi NL, Durando P. Influenza vaccination in healthcare workers: a comprehensive critical appraisal of the literature. Hum Vaccin Immunother. 2018;14(3):772–89. doi:10.1080/21645515.2017.1348442.
- Petek D, Kamnik-Jug K. Motivators and barriers to vaccination of health professionals against seasonal influenza in primary healthcare. BMC Health Serv Res. 2018;18(1):853. doi:10.1186/s12913-018-3659-8.
- Desiante F, Caputi G, Cipriani R, Nanula C, Aprile I, Pesare A, Conversano M. Assessment of coverage and analysis of the determinants of adherence to influenza vaccination in the general practitioners of Taranto. Annali di igiene: medicina preventiva e di comunita. 2017;29(4):256–63. doi:10.7416/ai.2017.2157.
- Opstelten W, van Essen GA, Heijnen M-L, Ballieux MJ, Goudswaard AN. High vaccination rates for seasonal and pandemic (A/H1N1) influenza among healthcare workers in Dutch general practice. Vaccine. 2010;28(38):6164–68. doi:10.1016/j.vaccine.2010.07.031.
- Health council of the Netherlands. Influenza vaccination: revision of the indication. [accessed 2020 January8]. https://www.healthcouncil.nl/documents/advisory-reports/2007/03/08/influenza-vaccination-revision-of-the-indication
- Centers for disease control and prevention. Influenza vaccination coverage rates by sex. [ accessed 2020 January 8]. https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm#by-sex
- Chambers C, Skowronski DM, Rose C, Serres G, Winter A-L, Dickinson JA, Jassem A, Gubbay JB, Fonseca K, Drews SJ, et al. Should sex be considered an effect modifier in the evaluation of influenza vaccine effectiveness? Open Forum Infectious Diseases. 2018;5(9):ofy211. doi:10.1093/ofid/ofy211.
- deSante JE, Caplan A, Shofer F, Behrman AJ. Physician attitudes towards influenza immunization and vaccine mandates. Vaccine. 2010;28(13):2517–21. doi:10.1016/j.vaccine.2010.01.042.
- Collange F, Zaytseva A, Pulcini C, Bocquier A, Verger P. Unexplained variations in general practitioners’ perceptions and practices regarding vaccination in France. Eur J Public Health. 2019;29(1):2–8. doi:10.1093/eurpub/cky146.
- Moran A, Agaliotis M, Seale H. The views of key stakeholders around mandatory influenza vaccination of hospital and aged care staff: examining the current climate in Australia. Vaccine. 2019;37(5):705–10. doi:10.1016/j.vaccine.2018.12.029.
- Abramson ZH, Levi O. Influenza vaccination among primary healthcare workers. Vaccine. 2008;26(20):2482–89. doi:10.1016/j.vaccine.2008.03.011.
- Corace K, Prematunge C, McCarthy A, Nair RC, Roth V, Hayes T, Suh KN, Balfour L, Garber G. Predicting influenza vaccination uptake among health care workers: what are the key motivators? Am J Infect Control. 2013;41(8):679–84. doi:10.1016/j.ajic.2013.01.014.
- Boey L, Bral C, Roelants M, De Schryver A, Godderis L, Hoppenbrouwers K, Vandermeulen C. Attitudes, believes, determinants and organisational barriers behind the low seasonal influenza vaccination uptake in healthcare workers - A cross-sectional survey. Vaccine. 2018;36(23):3351–58. doi:10.1016/j.vaccine.2018.04.044.
- Armstrong BG, Mangtani P, Fletcher A, Kovats S, McMichael A, Pattenden S, Wilkinson P. Effect of influenza vaccination on excess deaths occurring during periods of high circulation of influenza: cohort study in elderly people. BMJ (Clinical Research Ed). 2004;329(7467):660. doi:10.1136/bmj.38198.594109.AE.
Appendix A. Survey questions
1 I have been vaccinated against influenza for the season 2018/2019.
No (continue to question 2b)
2a I have been vaccinated for the following reason(s): (Multiple answers possible)
I belong to one of the influenza risk groups (by age criterion and/or by medical indication)
Vaccination will reduce the risk of getting influenza
Vaccination will reduce the risk of transmitting influenza to high-risk patients
There were some vaccines left
Because of reporting in scientific journals and in the media
Other motive …
2b I have not been vaccinated for the following reason(s): (Multiple answers possible)
I do not belong to one of the influenza risk groups (neither by age criterion nor by medical indication)
I am protected against influenza by frequent professional exposure to the virus
I question whether in my case vaccination will be effective
I forgot to get vaccinated
I fear side effects of IV
Because of reporting in scientific journals and in the media
Other motive …
3 I recommend influenza vaccination to my general practice personnel.
Yes
No
Does not apply; I do not have any personnel
4 Influenza vaccination should become mandatory for general practice personnel with direct patient contact.
-Strongly disagree-Disagree-Neutral-Agree-Strongly agree
5 Do you agree with age criterion (≥ 60 years) as an indication for influenza vaccination?
Yes, I do
No, the age criterion has to be higher
No, the age criterion has to be lower
No, I think age is not a correct indication for influenza vaccination
No opinion
6 How would you estimate the effectiveness of influenza vaccination in the elderly on preventing influenza in the elderly, in case of good antigenic match between the vaccine and the circulating influenza viruses?
…… %
7 How would you estimate the vaccination rate of your risk patients in the season 2018/2019?
… %
8 The symptoms of influenza in healthy elderly of 60 years and older are that serious that this requires prevention by means of an effective vaccine.
-Strongly disagree-Disagree-Neutral-Agree-Strongly agree
9 The symptoms of influenza in patients with a medical indication are that serious that this requires prevention by means of an effective vaccine.
-Strongly disagree-Disagree-Neutral-Agree-Strongly agree
10 The chance and severity of complications caused by influenza in healthy elderly of 60 years and older are that relevant that this requires prevention by means of an effective vaccine.
-Strongly disagree-Disagree-Neutral-Agree-Strongly agree
11 The chance and severity of complications caused by influenza in patients with a medical indication are that relevant that this requires prevention by means of an effective vaccine.
-Strongly disagree-Disagree-Neutral-Agree-Strongly agree
12 I support the current influenza vaccination policy regarding the elderly.
Yes
No
No opinion
Why DO you support the current policy regarding the elderly? (Multiple answers possible)
The effectiveness regarding prevention of influenza has been proven sufficiently
The effectiveness regarding prevention of complications and mortality has been proven sufficiently
The health benefits are high enough
Cost-effectiveness has been demonstrated sufficiently
The antigenic match between vaccine and circulating virus is usually accurate
Side effects of IV are negligible
Both healthy elderly and elderly with a medical indication are at risk
It is better to vaccinate against influenza than to go through an influenza infection
Symptoms of influenza are that severe that this justifies vaccination
Possible complications of influenza are that serious that this justifies vaccination
I vaccinate against influenza because the policy is determined by the health authorities, but personally I do not support this policy
Other motive …
Why DO you NOT support the current policy regarding the elderly? (Multiple answers possible)
The effectiveness regarding prevention of influenza has not been proven sufficiently
The effectiveness regarding prevention of complications and mortality has not been proven sufficiently
The factual health benefits are not high enough
Cost-effectiveness has not been demonstrated sufficiently
The antigenic match between vaccine and circulating virus is usually inaccurate
Side effects of influenza vaccination are a threat
Not all elderly are at risk, but mostly the elderly with a medical indication
It is better to go through an influenza infection than to vaccinate against influenza
Symptoms of influenza are that innocent that this does not justify vaccination
Complications of influenza are that rare that this does not justify vaccination
Other motive …
13 A placebo-controlled study (in which the intervention group receives the influenza vaccine and the control group receives placebo) on the effectiveness of influenza vaccination on hospitalization and/or mortality in the elderly is desirable.
-Strongly disagree-Disagree-Neutral-Agree-Strongly agree
14 The desirability of a placebo-controlled trial on influenza vaccination in the elderly is currently debated. If such a trial would be performed, would you find it objectionable if your elderly patients would participate in this study by their own initiative?
Yes
No
No opinion
Gender
Male
Female
Age
… … years old
For how long have you been working as a practicing GP?
… … years
How many days per week do you work as a practicing GP?
Three or less days per week
Four or more days per week
Practice form
Solo
Non-solo (duo/group)
I work as a locum
Remarks