ABSTRACT
Background: Compared with trivalent influenza vaccines, quadrivalent influenza vaccines are expected to provide wider protection against influenza B virus infections. We developed a novel quadrivalent subunit influenza vaccine which was distinct from the influenza vaccines available on the market in production process. In this research, we evaluated the safety and immunogenicity of the quadrivalent subunit influenza vaccine in animal models.
Methods: In toxicity assessment, 40 SD rats were randomly assigned to be intramuscularly injected with 1.0 ml of the tested vaccine (33 μg/ml) or 0.9% sodium chloride solution. In irritation assessment, eight rabbits were randomly assigned to receive 0.5 ml of tested vaccine or phosphate buffer solution intramuscularly. Thirty-two guinea pigs were randomly assigned to be intramuscularly injected with high-dose tested vaccine (0.5 ml), low-dose tested vaccine (0.05 ml), ovalbumin, or 0.9% sodium chloride solution, respectively, for sensitization assessment. In immunogenicity assessment, 50 BALB/c mice were equally randomized to receive one dose of tested vaccine, two doses of tested vaccine with an interval of 14 days, 0.5 ml of trivalent subunit influenza vaccine, 0.5 ml of monovalent subunit influenza vaccine, or 0.5 ml of phosphate buffer solution. Orbital blood was collected before and 28 and 42 days after administration of the injections for detecting influenza antibody titers.
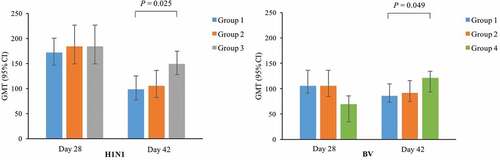
Results: No abnormal toxicity and irritation in rats and rabbits showed in the gross autopsy and histopathological examinations. The results of sensitization in guinea pigs indicated that no obvious allergic symptoms observed in the high-dose and low-dose vaccine groups within 30 min after twice provocations, and the result of sensitization evaluation was negative. Vaccine induced significant immune responses in mice with 100% seroconversion rates at 28 and 42 days after the first dose. The geometric mean titers (GMTs) of hemagglutination inhibition (HI) antibodies at day 28 in one-dose quadri–vaccine and two-dose quadri–vaccine groups were comparable to those in the tri–vaccine or mono-vaccine groups for shared influenza strains. However, the GMTs of HI antibodies against H1N1 (P = 0.025) and BV (P = 0.049) at day 42 in one-dose quadri–vaccine group were significantly lower than those in the tri–vaccine or mono-vaccine groups. The GMTs of HI antibodies against H1N1, H3N1, BY, and BV at day 28 and day 42 were comparable between one-dose quadri–vaccine and two-dose quadri–vaccine groups.
Conclusions: The quadrivalent subunit influenza vaccine was safe and immunogenic in animal models. One dose of the vaccine could elicit a satisfactory antibody response in mice.
Introduction
Influenza remains a major public health concern worldwide. The World Health Organization (WHO) estimates that seasonal influenza may result in 290,000–650,000 deaths each year due to respiratory diseases alone.Citation1 The best way to prevent seasonal influenza is to get vaccination annually. Due to the constant evolving of influenza viruses, the WHO Global Influenza Surveillance and Response System continuously monitors the influenza viruses circulating in humans and updates the composition of influenza vaccines twice a year.Citation2
At present, licensed seasonal influenza vaccines include recombinant influenza vaccines, live attenuated influenza vaccines, and widely used inactivated influenza vaccines throughout the world.Citation3 There are three types of inactivated influenza vaccines available on the market: whole virus vaccines, split virus vaccines, and subunit vaccines. Whole virus vaccines have become less common because of the relatively high rates of adverse reactions associated with them,Citation4,Citation5 which have been replaced by split virus vaccines and subunit vaccines in most countries.Citation6 For split virus vaccines, the influenza viruses are detergent-disrupted while retaining the immunogenic potential of the viral proteins.Citation7 Subunit vaccines are further purified to reduce the amount of non-hemagglutinin (HA) proteins, such as structural proteins.Citation8 Since 1982, an inactivated subunit influenza vaccine has been approved for the market. Then, it has been a trivalent vaccine containing 15 μg HA per strain since 1992.Citation9 Wide clinical experience since 1982 justified the use of inactivated subunit influenza vaccines as a routine measure for the control of influenza.Citation10
Currently, only one domestic trivalent subunit influenza vaccine is available in China, which is in short supply. Besides, on account of the trivalent subunit influenza vaccine contains only one influenza B strain (either Victoria or Yamagata lineage), the effectiveness of the vaccine would be compromised when two distinct lineages of influenza B with limited cross-reactivity cocirculate, especially when the dominant one is not included in the vaccine.Citation11 Compared with trivalent influenza vaccines, quadrivalent influenza vaccines are expected to provide wider protection against influenza B virus infections.Citation12–15 Therefore, we developed a novel quadrivalent subunit influenza vaccine which was different from the influenza vaccines available on the market in production process and evaluated the safety and immunogenicity of it in animal models.
Materials and methods
Vaccine
The tested quadrivalent subunit influenza vaccine was developed by Jiangsu Ab&b Biotechnology Co., Ltd, containing H1N1 (California/7/2009), H3N2 (Switzerland/9715293/2013), B Yamagata (Phuket/3073/2013) (BY), and B Victoria (Brisbane/60/2008) (BV) influenza strains. The influenza strains of the vaccine were cultured in eggs, and the HA concentration of each was 33 μg/ml. The vaccine was 0.5 ml per dose, with no thiomersal preservative. In the preparation of the quadrivalent subunit influenza vaccine, Triton X-100 was selected as the lysis buffer, and the effect of it was mild. In terms of purification, the lysis and density gradient centrifugation were carried out simultaneously, which had little damage to the integrity of HA and neuraminidase (NA), and could remove other impurities of the influenza virus strains such as M protein and nucleoprotein. The trivalent subunit influenza vaccine (H1N1, H3N2, and BY) and monovalent subunit influenza vaccine (BV) tested in mice were both self-made, and the manufacturing process was consistent with that of the tested quadrivalent subunit influenza vaccine. In addition, some precautions were taken to prepare the vaccines, and the preparation process was carried out in accordance with GMP. For example, the influenza virus seed bank was established in a biosafety level 2 laboratory, the process steps before the inactivation of the product were conducted in a negative-pressure workshop, and the preparers were administered with the influenza vaccine in advance.
Toxicity in rats
Forty SD rats with male and female in equal were randomly assigned (1:1) according to gender and body weight to receive an intramuscular injection of quadrivalent subunit influenza vaccine or 0.9% sodium chloride solution. The total dosing volume for each rat was 1.0 ml (0.2 ml per forelimb, and 0.3 ml per hindlimb). All kinds of abnormal manifestations in rats were observed and recorded, including appearance, signs, behavioral activities, glandular secretion, respiration, feces, and death. The observation time points are before, immediately after, and 10 min, 30 min, 1 h, 2 h, 4 h, and 6 h after injection, as well as two times per day (at morning and afternoon, respectively) for the next 14 days. Body weights were measured before and 3, 7, and 14 days after the administration of the injections, and food intakes were measured once a week. At the end of the 14-day observation, all rats were subjected to gross necropsy, and then, all the diseased organs were examined histopathologically.
Irritation in rabbits
Eight rabbits composed of male and female in equal were randomly assigned by gender and body weight in a ratio of 1:1 into vaccine group or vehicle control group. Rabbits were sheared with electric hair clippers, with the left and right quadriceps muscles exposed. Then, intramuscular injections of 0.5 ml of 0.01 mol/L phosphate buffer solution (PBS) or quadrivalent subunit influenza vaccine and 0.5 ml of 0.9% sodium chloride solution were administered on the right and left depilation sites of each rabbit by sterile manipulation. Four rabbits were sacrificed at 48 h and 14 days after administration, half male and half female, respectively. Quadriceps muscles were dissected and cut longitudinally, and the irritation reactions of muscle tissues at the injection sites were visually observed and scored. The tissue sections were then fixed with 10% neutral formalin for histopathological examination.
Sensitization in guinea pigs
A total of 32 guinea pigs with equal number of males and females were randomized and equally assigned into low-dose vaccine, high-dose vaccine, positive control, or negative control groups, stratified by gender and body weight. In the sensitization phase, guinea pigs in four groups were intramuscularly injected with 0.05 ml of quadrivalent subunit influenza vaccine, 0.5 ml of quadrivalent subunit influenza vaccine, 0.5 ml of 1% ovalbumin, or 0.5 ml of 0.9% sodium chloride solution, respectively, every other day for three times. Then, 14 and 21 days after the last sensitization, half of the guinea pigs in each group (equal number of male and female) were injected into plantar metatarsal veins with twice volumes of sensitizing concentration solution for provocation. The symptoms of anaphylaxis were observed and recorded within 30 min after the provocation. The presence and intensity of anaphylaxis were evaluated according to systemic sensitization evaluation criteriaCitation16 released by the Center for Drug Evaluation of China (Appendix 1). All guinea pigs were observed for clinical symptoms, once per day on non-administration day, before and 30 min after administration on sensitization day, and before administration on provocation day. Any death, near-death, activity, trauma, feces, appearance, and coat were recorded. Body weights were measured before grouping, the last sensitization, the first provocation, and the second provocation, respectively.
Immunogenicity in mice
Fifty BALB/c mice weighed 20–25 g were randomly assigned equally to receive one dose of quadrivalent subunit influenza vaccine (one-dose quadri–vaccine group), two doses of quadrivalent subunit influenza vaccine with an interval of 14 days (two-dose quadri–vaccine group), one dose of trivalent subunit influenza vaccine (H1N1, H3N2, and BY, HA concentration 33 μg/ml) (tri–vaccine group), one dose of monovalent subunit influenza vaccine (BV, HA concentration 33 μg/ml) (mono-vaccine group), or one dose of PBS (PBS group). The volume of each dose of vaccine or PBS was 0.1 ml which was calculated according to the weight of mice and humans and was injected in the tibialis anterior muscle of the hind leg. Orbital blood was collected before and 28 and 42 days after the first dose. Serum influenza antibody titers were assayed by the hemagglutination inhibition (HI) method.Citation17 Seroconversion is defined as pre-vaccination titer less than 1:8 and post-vaccination titer 1:40 or more or pre-vaccination titer 1:8 or more and at least fourfold increase post-vaccination.
Statistical analysis
Statistical analyses were performed using SPSS Statistics standard 23.0. Body weights and food intakes were compared between groups using Student’s t-test, one-way ANOVA, or Wilcoxon rank-sum tests. Samples with antibody titers below the detection limit (1:10) were given an arbitrary value of 1:5 for calculations. Antibody titers were log-transformed in order to calculate the geometric mean titers (GMTs). We used one-way ANOVA or Wilcoxon rank-sum tests to compare the GMTs of HI antibodies among groups and did multiple comparisons based on a Bonferroni-adjusted α when a significant difference was found.
Results
Safety in rats
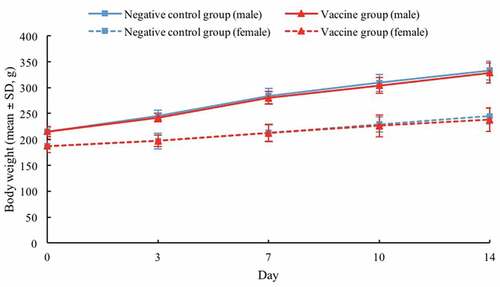
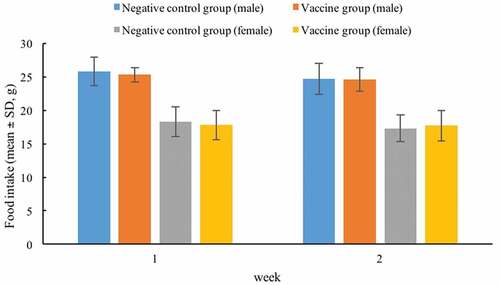
SD rats injected with quadrivalent subunit influenza vaccine or 0.9% sodium chloride solution were generally in good condition, no obvious clinical abnormal symptoms were observed, and no death during the observation period. The body weights in male negative control and vaccine groups increased by 54.88% and 52.56% compared with the initial weights, while those in female negative control and vaccine groups increased by 31.02% and 27.27%, at the end of the observation period, respectively (). The food intakes of male negative control and vaccine groups during the first week were 25.8 and 25.3, respectively, and those during the second week were 24.7 and 24.6, respectively. The food intakes of female negative control and vaccine groups during the first week were 18.3 and 17.8, respectively, and those during the second week were 17.3 and 17.7, respectively (). There were no significant differences in body weights and food intakes noted between the vaccine and negative control groups throughout the study period. Furthermore, no abnormal changes were found in the gross autopsy results of all rats.
Safety in rabbits
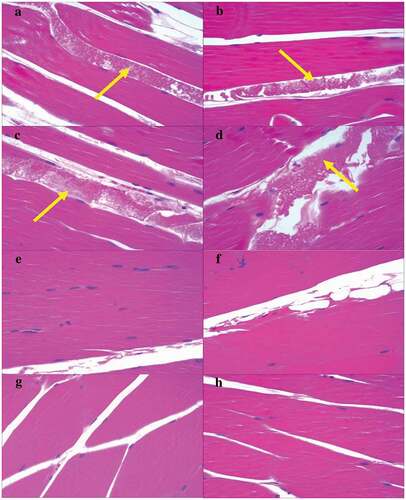
All the rabbits were generally in good condition and survived after the administration of the injections. Forty-eight hours and 14 days after administration, no local reactions such as redness, swelling, hyperemia, exudation, degeneration, or necrosis were observed in the necropsy results of both injection sides of all rabbits. No obvious histopathological changes of irritation were observed 48 h and 14 days after administration, with an exception of protein fluid exudation observed at injection sites in the rabbits of both groups at 48 h after administration ().
Figure 3. Histopathological results of quadriceps muscles of rabbits 48 h (A–D) and 14 days (E–H) after administration. Magnification: 200x. Yellow arrow: protein fluid exudation. A and B (vehicle control group), C and D (vaccine group), E and F (vehicle control group), and G and H (vaccine group) are the left and right quadriceps muscles, respectively, of the same rabbit
Safety in guinea pigs
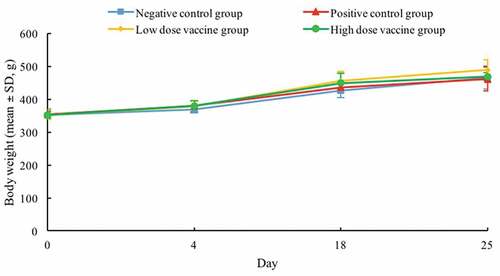
No obvious abnormality was observed in the guinea pigs after the injections during the study. Compared with the initial body weights, those at the end of the 25-day observation period in negative control, positive control, low-dose vaccine, and high-dose vaccine groups increased by 32.39%, 30.23%, 38.53%, and 32.95%, respectively (). There were no statistically significant differences in the body weights found across the groups. In addition, no obvious allergic symptoms were observed in negative control, low-dose vaccine, and high-dose vaccine groups within 30 min after twice provocations, and the result of sensitization evaluation was negative. However, the positive control group showed obvious allergic reaction symptoms after twice provocations, and the result of sensitization evaluation was extremely strong positive.
Immunogenicity
Before administration, antibody titers of H1N1, H3N2, BY, and BV in all mice were below the detection limit. At 28 and 42 days after the first dose, the seroconversion rates of H1N1, H3N2, and BY were all 100% in one-dose quadri–vaccine, two-dose quadri–vaccine, and tri–vaccine groups, and those of BV were all 100% in one-dose quadri–vaccine, two-dose quadri–vaccine, and mono-vaccine groups. GMTs of HI antibodies against vaccine contained influenza strains increased significantly at day 28 and decreased slightly at day 42 in most groups, whereas those against BY in one-dose quadri–vaccine and tri–vaccine groups and BV in mono-vaccine group had a slight increasing or remained unchanged at day 42 (). GMTs of HI antibodies against vaccine contained strains at day 28 and day 42 were comparable among different groups, except for those against H1N1 at day 42 (P = 0.018) and BV at day 28 (P = 0.022) and day 42 (P = 0.040). Multiple comparisons of GMTs of HI antibodies against H1N1 among one-dose quadri–vaccine, two-dose quadri–vaccine, and tri–vaccine groups and those against BV among one-dose quadri–vaccine, two-dose quadri–vaccine, and mono-vaccine groups were showed in Appendix 2. The GMTs of HI antibodies at day 28 in one-dose quadri–vaccine and two-dose quadri–vaccine groups were comparable to those in tri–vaccine or mono-vaccine groups for shared influenza strains. However, the GMTs of antibodies against H1N1 (P = 0.025) and BV (P = 0.049) at day 42 in one-dose quadri–vaccine group were significantly lower than those in tri–vaccine or mono-vaccine groups. Furthermore, the GMTs of antibodies against H1N1, H3N1, BY, and BV at day 28 and day 42 were comparable between one-dose quadri–vaccine and two-dose quadri–vaccine groups.
Table 1. GMTs of HI antibodies against influenza strains among different groups of BALB/c mice
Discussion
We developed a novel quadrivalent subunit influenza vaccine which was distinct from the influenza vaccines available on the market in production process. The selection of Triton X-100 as the lysis buffer and the purification method of performing the lysis and density gradient centrifugation concurrently ensured the high purity of HA and NA. The novelty of the production process of our quadrivalent subunit influenza vaccine contributed to the field of influenza vaccine development to some extent.
Before a novel vaccine is approved for human clinical trials, the safety information of it must be provided to support the clinical development and licensure of the product.Citation18 In our study, comprehensive preclinical safety assessments of a quadrivalent subunit influenza vaccine including toxicity, irritation, and sensitization were conducted in rats, rabbits, and guinea pigs, respectively. The findings showed that the quadrivalent subunit influenza vaccine had good safety profiles in rats, rabbits, and guinea pigs. Although the results of irritation test in rabbits showed that protein fluid exudation was observed in muscle fiber interstitium at injection sites on the left and right quadriceps muscles in the rabbits of both groups at 48 h after administration, no significant difference was observed between both sides. In addition, no obvious histopathological changes were observed at both injection sites of all remaining rabbits 14 days after administration, suggesting that the histopathological changes could be recovered.
As can be seen from the immunogenicity results of mice, the GMTs of HI antibodies against H1N1 and BV at day 42 in one-dose quadri–vaccine group were significantly lower than those in tri–vaccine or mono-vaccine groups. However, GMTs of HI antibodies against H1N1 and BV at day 42 in one-dose quadri–vaccine group were still at high levels, which were much higher than the protective antibody level of influenza (1:40). Besides, the GMTs of HI antibodies in one-dose quadri–vaccine and two-dose quadri–vaccine groups were comparable to those in tri–vaccine or mono-vaccine groups for shared influenza strains at day 28, and those against H3N2 and BY at day 42 remained comparable in one-dose quadri–vaccine, two-dose quadri–vaccine, and tri–vaccine groups. Overall, the additional B-strain in quadrivalent subunit influenza vaccine did not compromise the immunogenicity compared to trivalent subunit influenza vaccine for shared influenza strains, and the immunogenicity against the additional B-strain was comparable to the monovalent subunit influenza vaccine. Previously, a phase III randomized clinical trial of a similar quadrivalent subunit influenza vaccine also demonstrated that the immunogenicity of the influenza strains in quadrivalent vaccine was non-inferior to the shared strains and superior to the alternate-lineage B-strains in trivalent vaccine.Citation19 Compared with one dose of quadrivalent subunit influenza vaccine, two-dose regimen did not improve the immunogenicity, which indicated that one-dose regimen might be adopted in subsequent human clinical trials. One-dose regimen will help reduce the number of vaccination and is in line with current immunization schedule of influenza vaccines recommended by the Advisory Committee on Immunization Practices.Citation20,Citation21 However, the observation period for immunogenicity evaluation in mice in our study was set to 42 days after the first-dose vaccination, and the difference of antibody persistence between one-dose and two-dose regimens was unobtainable. Whether two doses of the quadrivalent subunit influenza vaccine could induce higher immunogenicity may be needed to be observed in the long term, and we will take this as a potential focus of our future work.
In our study, the limitation did exist in the selection of control groups in immunogenicity assessment in mice. Both the trivalent and monovalent subunit influenza vaccines tested were self-made, and the manufacturing process was consistent with that of the quadrivalent subunit influenza vaccine. Because neither domestic licensed nor imported trivalent subunit influenza vaccines were available when we conducted this study. In the absence of licensed similar vaccines, this approach of using self-made vaccines as control groups was also adopted in another phase III clinical trial of a quadrivalent subunit influenza vaccine in children.Citation22 Furthermore, 42 days after the first-dose vaccination, no histopathological examination was performed on mice after execution, and no inflammatory response such as eosinophil infiltration was observed. This issue will be taken into account in subsequent clinical trials.
In conclusion, the quadrivalent subunit influenza vaccine was safe in rats, rabbits, and guinea pigs and immunogenic in mice. The immunogenicity of two doses of quadrivalent subunit influenza vaccine in mice was equivalent to that of one dose. The results of this study provide evidence for this quadrivalent subunit influenza vaccine candidate to be further evaluated in subsequent clinical trials in humans.
Author contributions
Fengcai Zhu designed the study and contributed to the revision of the manuscript. Huayue Ye and Siyue Jia contributed to drafting of the manuscript and data interpretation. Yuhui Zhang performed laboratory tests. Jingxin Li contributed to statistical analysis. Each author listed on the manuscript has seen and approved the submission of this version of the manuscript.
Disclosure of potential conflicts of interest
Yuhui Zhang is an employee of Jiangsu Ab&b Biotechnology Co., Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
We thank all participants who contributed to this work.
References
- World Health Organization. Estimate of influenza deaths due to respiratory disease; [accessed 2020 Jan 2]. https://www.who.int/influenza/surveillance_monitoring/bod/en/.
- World Health Organization. Influenza (seasonal); [accessed 2020 Jan 2]. https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal).
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices — United States, 2019–20 influenza season; [accessed 2020 Jan 3]. https://www.cdc.gov/mmwr/volumes/68/rr/rr6803a1.htm?s_cid=rr6803a1_w.
- Wright PF, Cherry JD, Foy HM, Glezen WP, Hall CB, McIntosh K, Monto AS, Parrott RH, Portnoy B, Taber LH, et al. Antigenicity and reactogenicity of influenza A/USSR/77 virus vaccine in children – a multicentered evaluation of dosage and safety. Rev Infect Dis. 1983;5(4):758–64. PMID: 6353530. doi:10.1093/clinids/5.4.758.
- Beyer WEP, Palache AM, Osterhaus ADME. Comparison of serology and reactogenicity between influenza subunit vaccines and whole virus or split vaccines. Clin Drug Investig. 1998;15(1):1–12. PMID: 18370460. doi:10.2165/00044011-199815010-00001.
- World Health Organization. Vaccines against influenza WHO position paper – November 2012 [accessed 2020 Jan 3]. https://www.who.int/wer/2012/wer8747.pdf.
- Verma R, Khanna P, Chawla S. Influenza vaccine: an effective preventive vaccine for developing countries. Hum Vaccin. 2012;8(5):675–78. PMID: 22634439. doi:10.4161/hv.19516.
- Kawaoka Y, Neumann G. Influenza virus surveillance, vaccine strain selection, and manufacture. Methods Mol Biol (Clifton, NJ). 2012;865:147–62. PMID: 22528158. doi:10.1007/978-1-61779-621-0_9.
- Van de witte SV, Nauta J, Giezeman-Smits KM, De Voogd JM. Trivalent inactivated subunit influenza vaccine Influvac®: 30-year experience of safety and immunogenicity. Trials Vaccinology. 2012;1:42–48. doi:10.1016/j.trivac.2012.10.001.
- Gian Vincenzo Z, Valentina F. Influvac, a trivalent inactivated subunit influenza vaccine. Expert Opin Biol Ther. 2011;11(1):89–98. PMID: 21110767. doi:10.1517/14712598.2011.541436.
- Mckeage K. Inactivated quadrivalent split-virus seasonal influenza vaccine (Fluarix® quadrivalent): a review of its use in the prevention of disease caused by influenza A and B. Drugs. 2013;73(14):1587–94. PMID: 24022123. doi:10.1007/s40265-013-0114-3.
- Domachowske JB, Pankowculot H, Bautista M, Feng Y, Claeys C, Peeters M, Innis BL, Jain V. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3–17 years. J Infect Dis. 2013;207(12):1878–87. PMID: 23470848. doi:10.1093/infdis/jit091.
- Beran J, Peeters M, Dewé W, Raupachová J, Hobzová L, Devaster JM. Immunogenicity and safety of quadrivalent versus trivalent inactivated influenza vaccine: a randomized, controlled trial in adults. BMC Infect Dis. 2013;13(1):224–33. PMID: 23688546. doi:10.1186/1471-2334-13-224.
- Rodriguez Weber MA, Claeys C, Aranza DC, Feng Y, Innis BL, Jain VK, Peeters M. Immunogenicity and safety of inactivated quadrivalent and trivalent influenza vaccines in children 18-47 months of age. Pediatr Infect Dis J. 2014;33(12):1262–69. PMID: 25386965. doi:10.1097/INF.0000000000000463.
- Bart S, Cannon K, Herrington D, Mills R, Forleo-Neto E, Lindert K, Abdul Mateen A. Immunogenicity and safety of a cell culture-based quadrivalent influenza vaccine in adults: a phase III, double-blind, multicenter, randomized, non-inferiority study. Hum Vaccin. 2016;12(9):2278–88. PMID: 27322354. doi:10.1080/21645515.2016.1182270.
- Center for Drug Evaluation, NMPA. Technical guidelines of drug irritation, sensitization and hemolytic study; [accessed 2020 Dec 10]. http://www.cde.org.cn/zdyz.do?method=largePage&id=188.
- Palmer DF, Dowdle WR, Coleman MT, Schild GC. Advanced laboratory techniques for influenza diagnosis. Part 2. Hemagglutination inhibition test. Atlanta: US Department of Health Education and Welfare, PHS; 1975. p. 25–62.
- Forster R. Study designs for the nonclinical safety testing of new vaccine products ☆. J Pharmacol Toxicoll Methods. 2012;66(1):1–7. PMID: 22561062. doi:10.1016/j.vascn.2012.04.003.
- Witte SVD, Nauta J, Montomoli E, Weckx J. A phase III randomised trial of the immunogenicity and safety of quadrivalent versus trivalent inactivated subunit influenza vaccine in adult and elderly subjects, assessing both anti-haemagglutinin and virus neutralisation antibody responses. Vaccine. 2018;36(40):6030–38. PMID: 29709447. doi:10.1016/j.vaccine.2018.04.043.
- Advisory Committee on Immunization Practices. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2019 [accessed 2019 Mar 6]. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html.
- Advisory Committee on Immunization Practices. Recommended adult immunization schedule for ages 19 years or older, United States, 2019 [accessed 2019 Mar 6]. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
- Hartvickson R, Cruz M, Ervin J, Brandon D, Forleo-Neto E, Dagnew AF, Chandra R, Lindert K, Mateen AA. Non-inferiority of mammalian cell-derived quadrivalent subunit influenza virus vaccines compared to trivalent subunit influenza virus vaccines in healthy children: a phase III randomized, multicenter, double-blind clinical trial. Int J Infect Dis. 2015;41:65–72. PMID: 26585940. doi:10.1016/j.ijid.2015.11.004.
Appendix 1.
Systemic sensitization evaluation criteria
Appendix 2.
Appendix 2. Multiple comparisons of GMTs of HI antibodies against H1N1 among one-dose quadri–vaccine, two-dose quadri–vaccine, and tri–vaccine groups and those against BV among one-dose quadri–vaccine, two-dose quadri–vaccine, and mono-vaccine groups