ABSTRACT
Infectious diseases can impact chronic medical conditions. However, it is currently not clear how pertussis correlates with preexisting or underlying disorders. We reviewed literature from the last 25 years to describe the burden and impact of pertussis infection in specific risk groups in individuals aged ≥11 years.
Our literature search returned 543 hits, of which 18 were eligible for this review. Adolescents and adults with underlying conditions, such as asthma, chronic obstructive pulmonary disease (COPD), or obesity are potentially at increased risk of pertussis infection. Immunodeficiency and smoking have also been associated with worsened pertussis symptoms and an increased pertussis-related hospitalization rate. In patients with pertussis and preexisting asthma or COPD, symptoms were worsened, and health-care costs were consequently increased.
Further efforts are needed to close the knowledge gap and to understand the burden of pertussis in at-risk adolescent and adult populations to help inform vaccination strategies and recommendations.
PLAIN LANGUAGE SUMMARY
What is the context?
Pertussis, or whooping cough, is an infectious disease of the respiratory tract. It can range from a mild cough to a potentially fatal illness.
Most severe and fatal cases occur in infants. However, older children and adults—especially those who suffer from other medical conditions—can also develop a serious pertussis infection.
What is new?
We reviewed the literature from the last 25 years to evaluate the burden of pertussis infection in individuals of at least 11 years of age.
We also aimed to identify those at increased risk of the disease.
We found that:
Adolescents and adults with asthma, chronic obstructive pulmonary disease (COPD) or obesity are at increased risk of pertussis infection.
People with asthma, COPD, an impaired immune system, or those who smoke are more severely affected by pertussis and are hospitalized more often for pertussis-related complications.
What is the impact?
The current knowledge on pertussis in at-risk populations is limited. Further studies are needed to more accurately evaluate the true burden of disease and to help guide prevention, control and treatment strategies.
Background
Chronic diseases, as well as general medical conditions, can be exacerbated by different infectious diseases.Citation1–5 Since life expectancy in developed countries is rapidly increasing, predominantly due to a lower mortality rate among older individuals,Citation6,Citation7 the prevalence of age-related chronic diseases is predicted to increase, along with healthcare-associated costs.Citation8 In addition, multi-morbidity is known to increase with age.Citation8 For instance, a cross-sectional study assessing the prevalence of 40 morbidities in 1.7 million individuals from Scotland, UK, showed that at least two chronic medical conditions were present in 30.4% of adults aged 45–64 years, in 64.9% of those aged 65–84 years, and in >80% of >85 year-olds.Citation9 Prevention of infectious diseases in all age groups, especially older adults, could, therefore, contribute to maintaining or improving quality-of-life by limiting exacerbations of underlying medical conditions.
Pertussis, commonly known as “whooping cough,” is an acute infectious and highly contagious disease that affects people of all ages, and is caused by the bacterium Bordetella pertussis.Citation10 Since the introduction of effective vaccines in the 1940s, pertussis incidence has, for example, steadily decreased in the United States (US) by more than 80% among all age groups.Citation11 Neither natural immunity due to previous infection nor vaccination provides life-long protection; thus, despite the high infant pertussis vaccination coverage rates, there has been a global resurgence of the disease in developed regions in adolescents and adults.Citation12–14 Besides waning of immunity and other previously published contributing factors,Citation13–17 absent or inconsistent recommendations for booster vaccinations in adults, and thus low vaccine coverage in specific populations or regions, may also significantly add to the recent resurgence. Consequently, pertussis booster vaccination throughout life is important to help prevent pertussis infection and its transmission in those at risk of infection.
With respect to pertussis vaccination, in the US it is recommended that children should receive a diphtheria-tetanus-acellular pertussis (DTaP) containing vaccine at 2, 4, 6 months, between 15 and 18 months, and at 4–6 years. A booster dose of a reduced antigen content tetanus-diphtheria-pertussis (Tdap) vaccine is recommended for those 11 or 12 years of age and for any adult 19 years of age or older who has never received a dose of Tdap.Citation18 The Advisory Committee on Immunization Practices recently updated their recommendation for adults to receive one dose of either a Td or Tdap vaccine every 10 years. In addition, pregnant women should receive one dose of Tdap during each pregnancy. Despite these recommendations in the US, uptake of pertussis-containing vaccines in adults is still well below target.Citation19 In addition, adult recommendations vary globally, for example, the UK does not have an adult pertussis vaccination recommendation except for vaccination during pregnancy.Citation20
B. pertussis infection has a clinical presentation ranging from a relatively mild cough illness to a severe and potentially fatal illness with pneumonia, seizures, encephalopathy, and respiratory failure.Citation12 While most severe and fatal pertussis cases occur in infants, pertussis infection can also have serious manifestations in older children and adults.Citation21,Citation22 Similarly, most pertussis-related hospitalizations occur in infants,Citation23,Citation24 although up to 12% of adults with pertussis may also require hospitalization.Citation25 In fact, the true burden of pertussis disease in adults is unknown, with cases in adults being frequently missed or misdiagnosed.Citation13,Citation26,Citation27 A high proportion of adults hospitalized for pertussis have underlying medical conditions,Citation24,Citation28 suggesting that these may be a contributing factor to pertussis disease severity. Between 1990 and 2004, the US. Centers for Disease Control and Prevention received 5 reports of pertussis-associated deaths in adults (aged 49–82 years), all of whom suffered from comorbid conditions, such as diabetes, multiple sclerosis with asthma, multiple myeloma (requiring immunosuppressive therapy), myelofibrosis, and chronic obstructive pulmonary disease (COPD).Citation12 Thus, it is important to ascertain which patients are at increased risk of pertussis disease (and related complications) and whether they could benefit from targeted prevention strategies.
Few data exist on the current burden and characteristics of severe pertussis disease in adolescents and adults.Citation24 A recent review paper provided a detailed overview of the disease burden and the role of vaccination in older adults.Citation26 Here we have reviewed the literature from the last 25 years to describe the current knowledge on the burden and impact of pertussis infection in specific risk groups in individuals aged ≥11 years, an age beyond which pertussis vaccination recommendations are variable and uptake is below the rates seen for pediatric pertussis-containing vaccines.
Methods
Search strategy
A search was performed in PubMed on July 17, 2019 to identify papers related to pertussis infections in different risk groups as well as the burden of disease associated with underlying conditions in these groups. The string used in our search is provided in the supplementary material. The studies related to children <11 years, diagnostic, molecular and immunology, outcome of infection, mechanisms of infection, or reviews were excluded.
Results
Study selection
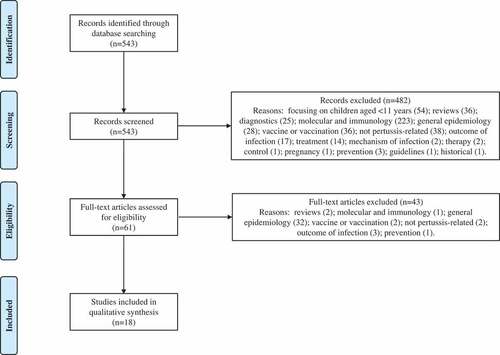
Our search returned 543 results. Based on the screening of titles, abstracts, or main texts, 482 were excluded. Sixty-one full-text articles were further screened, of which 18 were included in this review. Articles were excluded for the following reasons: focusing on children aged <11 years (54), reviews (38), diagnostics (25), molecular and immunology (224), general epidemiology (60), vaccine or vaccination (38), not pertussis-related (40), outcome of infection (20), treatment (14), or based on other reasons (12). A flow diagram showing the inclusion/exclusion of studies is presented in . Eight case studies appeared in our search, and whilst they are not representative of the general population, they do provide further support of severe or atypical pertussis diagnosis in patients with preexisting or underlying conditions.
Underlying conditions associated with an increased risk of severe pertussis in adults
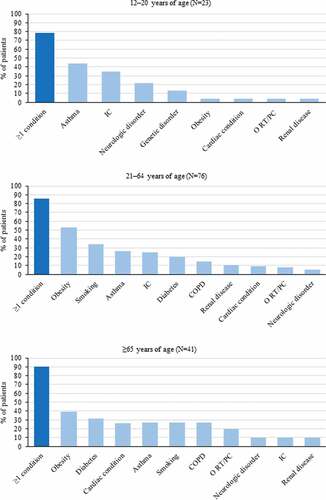
Enhanced Pertussis Surveillance and inpatient medical record data collected via the US. Emerging Infections Program Network between 2011 and 2015 showed that among 15,942 reported pertussis cases (mean annual incidence: 20.3 per 100,000 persons), 3.2% required hospitalization. Of these, 29.6% of cases and 4.5% of hospitalizations occurred in 12–20-year-olds, 18% of cases and 14.8% of hospitalizations in 21–64-year-olds, and 2.1% of cases and 8.3% of hospitalizations in >65-year-olds. Of the hospitalized patients, 32.6% had underlying conditions, with the highest proportion (87.2%) observed among adults aged ≥21 years. The most frequent conditions in 12–20–year-olds were asthma, immunocompromising conditions, and the use of immunocompromising medication. Obesity and smoking (current or former) were most frequent in 21–64-year-olds, and asthma and cardiac conditions in ≥65-year-olds (). Of the three hospitalized patients who died, one was an infant aged 42 days and two were adults with underlying medical conditions: one 48-year-old adult who had a history of human immunodeficiency virus and one 76-year-old adult who had a previous history of asthma and COPD.Citation24
Figure 2. The most frequent underlying medical conditions in hospitalized pertussis patients by different age groups (Mbayei, Clin Infect Dis 2018)Citation24 IC, immunocompromising condition; N, number of hospitalized pertussis patients; O RT/PC, other respiratory tract/pulmonary condition; COPD, chronic obstructive pulmonary disease
Asthma
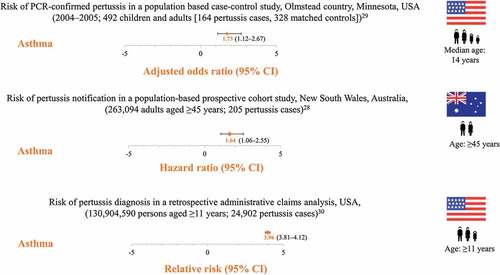
Three studies have shown that individuals with underlying asthma are at increased risk of pertussis diagnosis compared to individuals without, as further illustrated by two case studies ().Citation28–32 A retrospective, population-based case–control study compared the history of asthma in children and adults who had pertussis (confirmed by polymerase chain reaction, PCR) with age- and sex-matched controls during a pertussis outbreak between 2004 and 2005 in California, USA. Of the 164 cases, 38% had asthma before the pertussis diagnosis compared to 26% of the 328 controls, resulting in an adjusted odds ratio of 1.73 (95% confidence interval [CI]: 1.12–2.67, P = .013). The population-attributable risk of pertussis in asthma patients was 17%.Citation29 In a study between 2006 and 2008, 263,094 adults aged ≥45 years (mean 62.8 years) were recruited in New South Wales, Australia, in a prospective, population-based cohort study and followed-up for laboratory-confirmed pertussis (PCR or culture) occurrence. Among the 205 cases, a history of preexisting asthma was a significant predictor of pertussis incidence, with a hazard ratio of 1.64 (95%CI: 1.06–2.55) for pertussis in individuals with asthma compared to those without.Citation28 A retrospective analysis using administrative claims data in the United States evaluated the incidence of pertussis among 1,041 adolescents and adults aged >11 years with preexisting asthma and a matched cohort consisting of 1,041 pertussis patients without preexisting asthma. The incidence of pertussis among patients with preexisting asthma was 0.27 per 1000 person-years (95%CI: 0.27–0.29), resulting in a relative risk of pertussis of 3.96 (95%CI: 3.81–4.12) when compared to controls. The risk was highest among 19–64-year-olds with preexisting asthma (relative risk 4.06 [95%CI: 3.86–4.27]).Citation30
Figure 3. Increased risk of pertussis in patients with asthma PCR, polymerase chain reaction; CI, confidence interval
During the 1998 pertussis outbreak in Quebec, Canada, positive cases (either culture-positive or meeting the Canadian surveillance case definition for pertussis and having no other apparent cause) were evaluated in 280 patients aged 12–17 years and in 384 patients aged ≥18 years. Respectively, 24% and 13% had asthma. The authors observed higher asthma prevalence in pertussis patients compared to the general adolescent population of Canada, which was estimated to be ~12%. In pertussis patients with asthma, the mean duration of paroxysmal cough (5 vs. 4 weeks, P = .004), risk of sinusitis (16% vs. 12%, P = .4) and mean number of nights disturbed by pertussis (26 vs. 20 nights, P = .03) were also increased compared to pertussis patients who did not have asthma.Citation33
Among individuals hospitalized for pertussis, approximately 44% of 12–20-year-olds and approximately 26% of ≥21-year-olds had asthma, proportions that are considerably higher than the prevalence of asthma in the general US. population, estimated at 10% of the adolescent and 8% of the adult population.Citation24 Asthma (and/or COPD) exacerbations were among the most frequent diagnoses leading to pertussis-related hospitalizations in both adolescents and adults.Citation24 An increased all-cause and pertussis-related hospital admission rate was also observed for pertussis patients with asthma compared to controls at 45 days, 3 months, or 6-months post-pertussis diagnosis.Citation30
In a case–control study in Finland in 1999, involving 103 adults with stable asthma (53 mild, 50 moderate) and 30 healthy controls, B. pertussis was detected by PCR in the sputum of 28.3% of mild asthmatics, in 20.0% of moderate asthmatics, and in 16.7% of controls. Asthma symptom scores were above the median in 66% of B. pertussis-positive asthmatics compared to 47% in B. pertussis-negative asthmatics. In addition, as shown by the forced expiration volume in 1 s/forced vital capacity, lung function was lower in asthmatics with B. pertussis compared to those without (77.1% vs. 80.7%).Citation34
In pertussis patients with or without asthma, health-care costs adjusted to 2014 US. dollars using the medical care component of the US. Consumer Price Index were also assessed.Citation35 Within 45 days of pertussis diagnosis, pertussis patients with asthma accrued 2,007 USD in all-cause adjusted health-care costs (medical and pharmacy), whereas matched pertussis patients without asthma accrued 814 USD resulting in 1,193 USD more in all-cause adjusted costs. This difference increased to 1,301 USD in the 3 month post-pertussis diagnosis and to 1,639 USD in the 6 month post-diagnosis. Within 45 days of pertussis diagnosis, the greatest difference ((2,510 USD)) was seen in ≥65-year-olds while the smallest (746 USD) in 11–18-year-olds.Citation30 The difference in pertussis-related adjusted costs between pertussis patients with or without asthma was 230 USD within 45 days of pertussis diagnosis, 241 USD within 3 months, and 251 USD within 6 months, and was similar between all age groups.Citation30
COPD
Two studies have shown that individuals with COPD are potentially at increased risk of pertussis diagnosis or pertussis-related hospitalizations compared to adults without COPD.Citation24,Citation30 Emerging Infections Program Network data collected between 2011 and 2015 showed that COPD was more frequent among hospitalized adult pertussis patients (19%) compared to the proportion of adults in the United States with a history of COPD (6.4%).Citation24 COPD exacerbations were among the most frequent diagnoses leading to pertussis-related hospitalizations in adults.Citation24 In a retrospective administrative claims analysis, the relative risk of pertussis in individuals aged ≥11 years with preexisting COPD was over two times higher than in matched controls (relative risk 2.53 [95%CI: 2.40–2.68]), with the highest relative risk (3.59 [95%CI: 3.35–3.84]) observed among patients aged 19–64 years.Citation30 Within 45 days, 3 months, and 6 month post-pertussis diagnosis, all-cause hospitalization rates were higher in pertussis patients with COPD than in those without compared to the pre-diagnosis date. COPD patients were almost two times more likely to be hospitalized due to pertussis across all three periods evaluated.Citation30 Health-care costs were also assessed in pertussis patients with or without COPD. The accrued adjusted health-care cost (medical and pharmacy) per pertussis patient with COPD was 4,751 USD and 1,057 USD per matched pertussis patient without COPD, resulting in 3,694 USD difference in all-cause adjusted costs within 45 days of pertussis diagnosis. This difference increased to 6,154 USD in the 6 month post-pertussis diagnosis for the patients with pertussis and COPD.Citation30
In a case–control study comparing seroprevalence of B. pertussis in 90 patients with COPD and 90 age- and sex-matched controls with other lung diseases who attended a tertiary care hospital in Hamedan, Iran, a statistically significant association was found between COPD and anti-pertussis toxin (PT) immunoglobulin (Ig) G seropositivity, noting that the presence of IgG antibodies in study participants is indicative of past infection(s). However, an association between B. pertussis seroprevalence and severity of COPD was not shown.Citation36 These results should be interpreted taking into account that the threshold for seropositivity was selected arbitrarily and that there is no international threshold indicative for previous pertussis infection. Some inconsistencies can also be noted in the assessments made by COPD severity. Overall, the authors hypothesized that the colonization by B. pertussis could be a potential risk factor for the development of COPD.Citation36
Chronic bronchitis
In an observational study between 2000 and 2002 in Basel, Switzerland, the occurrence of B. pertussis and B. parapertussis infection was evaluated in 26 patients (age: median 71, range 34–86) diagnosed with acute exacerbations of chronic bronchitis. Nasopharyngeal swabs from all 26 participants were negative by both culture and PCR. However, serology, using an arbitrary seropositivity threshold, indicated B. pertussis infection in 19%, and dubious B. parapertussis or pertussis infection in 12% of cases.Citation37
Smoking
Enhanced Pertussis Surveillance and inpatient medical record data collected between 2011 and 2015 showed that among ≥21-year-olds hospitalized with pertussis, 31.6% were current/former smokers.Citation24 (In perspective, 15.7% of the US. adults ≥18 years of age were current cigarette smokers in 2016Citation38).
Two other studies also support the hypothesis that smoking could contribute to the worsening of pertussis symptoms.Citation33,Citation39 During the 1998 pertussis outbreak in Quebec, Canada, the mean duration of paroxysmal cough (5 vs. 4 weeks, P = .004), risk of sinusitis (19% vs. 11%, P = .008), and mean number of nights disturbed by pertussis (25 vs. 20 nights, P = .4) were increased in smokers compared to nonsmokers aged ≥12 years.Citation33 A population-based prospective cohort study in Australian adults aged ≥45 years found no association between smoking and the occurrence of pertussis.Citation28 Nevertheless, a nested case–control study suggests that smoking increases the risk for severe pertussis disease, as shown by the increased rate of pertussis-related hospitalizations in smokers compared to nonsmokers aged ≥45 years (odds ratio 2.37 [95%CI: 1.11–5.06]).Citation39
Obesity
Data from the US. Emerging Infections Program Network collected between 2011 and 2015 show that 47.9% of ≥21-year-old pertussis patients were obese.Citation24 (In perspective, 38% of the general US. population are obeseCitation38). In a population-based prospective cohort study in Australia, a high body mass index (BMI) was also associated with an increased pertussis risk in ≥45-year-olds (relative risk 1.52 [95%CI: 1.06–2.19] for a BMI of 30+ kg/m2 vs a BMI of <25 kg/m2).Citation28 However, in the population-based nested case–control study evaluating hospitalized pertussis cases, the association between obesity and pertussis-related hospitalization was not statistically significant, although the point estimate for the adjusted odds ratio (2.26 [95%CI: 0.89–5.74]) suggests that individuals with a BMI ≥30 may be at increased risk of being hospitalized due to pertussis infection.Citation39
Immunocompromising conditions
Among adult pertussis patients aged ≥21 years included in the US. Emerging Infections Program Network, a higher prevalence of immunocompromising conditions was observed compared to the general adult population in the United States: 19.7% vs. approximately 3%.Citation24,Citation40 Even though the prevalence of immunocompromising conditions was relatively low (6.8%) when considering all ages (<2 months to ≥65 years), the prevalence was 34.8% in 12–20-year-olds. Taken together with the frequent underlying neurologic or genetic disorders in 12–20-year-olds participating in the study, these data suggest that many of the few adolescents hospitalized for pertussis have complex medical histories, which might explain the higher rate of pertussis-related complications in this age group, such as new-onset seizures and encephalopathy.Citation24
Six case studies reported mild to severe pertussis disease in adults aged 31–82 years with underlying conditions as Diabetes Mellitus Type II, Wegener’s granulomatosis, multiple myeloma, renal transplantation, and acquired immune deficiency syndrome.Citation41–46
Mannose-binding lectin deficiency (MBL)
One study evaluated the risk of pertussis in patients with MBL. Among study participants of all ages (median age of 15 years, age range: 1–71), severe MBL deficiency was significantly more frequent among the 125 pertussis patients than among the 430 controls (11.2% vs. 5.8%, odds ratio 2.0 [95%CI: 1.0–4.1]). Severe MBL deficiency was detected in 20.4% and 8.6% of adults (>18 years of age) with and without pertussis, respectively (odds ratio 2.7 [95%CI: 1.1–6.5]).Citation47
Discussion
Several studies have shown that underlying diseases may increase the risk of pertussis diagnosis. In particular, our literature search identified studies focusing on asthma, COPD, and obesity. Smoking is a risk factor for illnesses like COPD or lung cancer and it also appears to increase the risk of pertussis diagnosis.
Asthma is the most frequent chronic medical condition during childhood and is also a major cause of morbidity in adults. Its prevalence increased in the United States between 2001 and 2010 and appeared to start plateauing in the mid-2010s.Citation48,Citation49 In 2017, according to the World Health Organization, 235 million people were suffering from asthma worldwide.Citation50 Bacterial organisms, such as Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, have been demonstrated to be clinically relevant contributors to asthma exacerbations.Citation51 In addition, studies have demonstrated an increased risk of invasive or serious pneumococcal diseases among adults with asthma and subsequently updated recommendations by the Advisory Committee on Immunization Practices indicate that adults with asthma should receive a single dose of 23-valent pneumococcal polysaccharide vaccine to prevent serious pneumococcal infections.Citation52 Thus, bacterial organisms are an important consideration in the clinical management of patients with asthma. However, to-date, there is no consensus as to whether individuals with preexisting asthma have an increased risk of pertussis infection.
Studies included in this review, covering >130 million individuals aged ≥11 years and >25,000 pertussis cases indicate that adolescents and adults with asthma are at an increased risk of pertussis and pertussis-related hospitalizations compared to individuals without asthma.Citation28–30 Asthma may also be worsened in individuals with pertussis, as reflected by a reduction in lung function,Citation34 worsening of symptoms,Citation33 and an increase in pertussis-related hospitalizations.Citation24,Citation30 In addition, one study demonstrated that all-cause and even pertussis-related health-care expenses are higher in pertussis patients with asthma than in those without.Citation30
COPD is a chronic inflammatory disease and usually progressive conditions are associated with an irreversible decline of pulmonary function, a substantial deterioration of quality-of-life, and an increase in the use of health-care resources. It is also anticipated that COPD will become the third leading cause of deaths worldwide by 2030.Citation53 The complexity of COPD is reflected by the transient and apparently stochastic nature of acute exacerbations, when additional medical treatment and even hospitalization are usually required.Citation54 However, the most significant issue in patients with COPD is respiratory system dysbiosis which increases susceptibility to bacterial and viral infections that could trigger acute COPD exacerbations.Citation55,Citation56
The association between COPD and pertussis in clinical settings has not been investigated extensively, and as such the overall burden of pertussis in COPD patients is unclear. Since we conducted our search in Pubmed, an additional study was published describing the occurrence of respiratory pathogens in nasopharyngeal aspirate samples from adults admitted with acute exacerbation of COPD (AECOPD) or asthma exacerbation. Interestingly, B. pertussis was detected in patients with AECOPD, although the sample size was limited and did not allow for a comparison.Citation57 Two studies included in this review have also shown, albeit in a non-definitive manner, an increase in the risk of pertussis diagnosis and pertussis-related hospitalizations in patients with COPD, as compared to patients without COPD.Citation24,Citation30 In addition, another study found a correlation between IgG antibodies against B. pertussis and COPD, which could indicate a persistent subclinical infection.Citation36 All-cause costs and pertussis-related health-care expenses were also higher in pertussis patients with COPD than in those without,Citation30 suggesting an additional cost to the healthcare system of managing a patient with both COPD and pertussis. In contrast, non-typeable Haemophilus influenzae (NTHi) and Moraxella catarrhalis were the most frequently identified bacteria in sputum collected from 127 COPD patients aged 40–85 years collected at acute exacerbations.Citation58 Among the subset of patients with bronchiectasis, Haemophilus was the dominant genus observed both in stable state and exacerbation events. The same study also failed to show an association between COPD and B. pertussis infection.Citation58 We note however several limitations that may have potentially impacted the detection of B. pertussis in COPD patients in this study. Most importantly, assessments were only performed on sputum samples, which might not be optimal for the detection of B. pertussis infection, especially if collected after initiation of antibiotic or oral corticosteroid treatment for an exacerbation.Citation58,Citation59 As such, further investigation is required to understand the possible impact of pertussis infection in patients with COPD.
Smoking is accepted to be the highest risk factor for the development of COPD, while chronic bronchitis can affect between 14% and 74% of all patients with COPD.Citation60 Targeted and early disease prevention strategies may, therefore, benefit patients with COPD. Studies identified by our literature search show that a relatively high proportion of individuals hospitalized with pertussis were smokersCitation24,Citation39 and that smoking was associated with the worsening of pertussis symptoms.Citation33 However, no association between smoking and occurrence of pertussis was found in the study by Liu et al.Citation28 A high B pertussis seroprevalence was demonstrated among 26 chronic bronchitis patients,Citation37 although the small sample size and locational homogeneity of study participants, arbitrary selection of seropositivity thresholds and classification of cases, and lack of PCR or culture confirmation of any seropositive cases may hamper the generalizability of these findings.
Pertussis-related fatalities were reported for adults with a history of immunodeficiency, asthma, and COPD, and in other immunocompromised patients.Citation24,Citation41,Citation44 This is consistent with reports received by the US. Centers for Disease Control and Prevention between 1990 and 2004, when five adults aged 49–82 years with serious underlying medical conditions (e.g. severe diabetes, severe multiple sclerosis with asthma, multiple myeloma on immunosuppressive therapy, myelofibrosis, and COPD) died due to pertussis.Citation41
Obesity was also identified as a potential risk factor for severe pertussis disease.Citation24,Citation61 Although the association between obesity and pertussis-related hospitalization was found not to be significant, a point estimate for the odds ratio suggests that hospitalization risk may be increased.Citation39
Our search also identified one article showing a relation between pertussis and MBL deficiency,Citation47 which is a relatively common condition and which affects the immune system in 5–30% of people. This condition is characterized by low levels of MBL in the blood, which increases the risk of recurrent infections.Citation62
Many of the articles reviewed herein face similar limitations. Whether the observed associations between underlying conditions and B. pertussis infection are a result of increased awareness and a higher likelihood of these individuals to seek medical attention or a predisposition for a more severe disease is not clear. In addition, the increased pertussis-related hospitalization rates in individuals with underlying medical conditions might be confounded by the perceived risk of disease, which is higher in such populations. Although outside the direct scope of this review, it is also important to note that the exact mechanism by which underlying diseases influence the systemic response to an infection is not fully understood.Citation63 It is assumed that when underlying conditions are present, the organism reacts with a weak local and systemic inflammatory response to pathogens,Citation64 which might explain the increased risk of infection and exacerbation of underlying diseases.Citation55,Citation56 Future observational studies are therefore needed to unravel the mechanism by which underlying disorders interfere with infections. However, trials involving underlying conditions are difficult to set up since the heterogeneity of acute and/or chronic conditions affect the reproducibility of results.
Conclusion
Adolescents and adults with underlying conditions, such as asthma, COPD, or obesity are potentially at increased risk of pertussis infection or exacerbation. In addition to these, immunodeficiency and smoking have also been associated with worsened pertussis symptoms and an increased pertussis-related hospitalization rate. In patients with pertussis who had preexisting asthma or COPD, symptoms were worsened and health-care costs were consequently increased. An improved understanding of pertussis infections and clinical pathway for those at risk of complications from pertussis will help guide prevention, control, and treatment strategies. Thus, further studies are needed to establish the true burden of pertussis in adolescent and adult populations and to formulate a conclusive vaccination strategy on an international level to help protect those at risk.
A plain language summary contextualizing the results and potential clinical research relevance and impact is presented in .
Authors’ contributions
All authors were involved in the conception of this publication. Victoria A Jenkins performed the literature search, the initial screening of the results, and wrote the initial draft. All authors contributed to the interpretation of the results, writing (review and editing) of the manuscript, and read and approved the submitted publication.
Disclosure of potential conflicts of interest
Victoria A Jenkins, Miloje Savic, and Walid Kandeil were employees of the GSK group of companies at the time the development of this manuscript was initiated. Walid Kandeil is currently an employee of Takeda Pharmaceuticals International AG. Victoria A Jenkins, Miloje Savic, and Walid Kandeil hold shares in the GSK group of companies as part of their remuneration.
Supplemental Material
Download MS Word (27.8 KB)Acknowledgments
The authors would like to thank Martin Ryser for reviewing the manuscript and for sharing his expertise on COPD. The authors are also grateful to the Modis platform C/O GSK for editorial assistance and manuscript coordination. Medical writing services were provided by Maria C. Maior and Alpár Pöllnitz, and editorial assistance and publication coordination were provided by Sander Hulsmans.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2020.1738168.
Additional information
Funding
References
- Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283(4):499–505. doi:10.1001/jama.283.4.499.
- Rohde G, Wiethege A, Borg I, Kauth M, Bauer TT, Gillissen A, Bufe A, Schultze-Werninghaus G. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi:10.1136/thorax.58.1.37.
- Sethi S. New developments in the pathogenesis of acute exacerbations of chronic obstructive pulmonary disease. Curr Opin Infect Dis. 2004;17(2):113–19. doi:10.1097/00001432-200404000-00008.
- Sethi S. Molecular diagnosis of respiratory tract infection in acute exacerbations of chronic obstructive pulmonary disease. Clin Infect Dis. 2011;52(suppl_4):S290–S5. doi:10.1093/cid/cir044.
- Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–71. doi:10.1056/NEJMoa012561.
- United Nations. World population prospects 2019; [ accessed 2019 Jul 16] https://population.un.org/wpp/.
- World Health Organization. Life expectancy; [ accessed 2019 Jul 16]. https://www.who.int/gho/mortality_burden_disease/life_tables/situation_trends_text/en/.
- Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44(4):1055–68. doi:10.1183/09031936.00059814.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi:10.1016/s0140-6736(12)60240-2.
- Nieves DJ, Heininger U. Bordetella pertussis. Microbiol Spectr. 2016;4(3). doi:10.1128/microbiolspec.EI10-0008-2015.
- Centers of Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. The pink book: course textbook -. 13th ed. 2015.
- Kilgore PE, Salim AM, Zervos MJ, Schmitt H-J. Pertussis: microbiology, disease, treatment, and prevention. Clin Microbiol Rev. 2016;29(3):449–86. doi:10.1128/CMR.00083-15.
- Tan T, Dalby T, Forsyth K, Halperin SA, Heininger U, Hozbor D, Plotkin S, Ulloa-Gutierrez R, Wirsing von Konig CH.Pertussis across the globe: recent epidemiologic trends from 2000 to 2013. Pediatr Infect Dis J. 2015;34(9):e222–32. doi:10.1097/inf.0000000000000795.
- World Health Organization. Pertussis reported cases; [ accessed 2019 Jul 16]. http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencepertussis.html.
- Domenech de Celles M, Magpantay FM, King AA, Rohani P. The pertussis enigma: reconciling epidemiology, immunology and evolution. Proc Biol Sci. 2016;283(1822):20152309. doi:10.1098/rspb.2015.2309.
- Mooi FR, Van Der Maas NA, De Melker HE. Pertussis resurgence: waning immunity and pathogen adaptation - two sides of the same coin. Epidemiol Infect. 2014;142(4):685–94. doi:10.1017/s0950268813000071.
- Mooi FR, van Loo IH, King AJ. Adaptation of Bordetella pertussis to vaccination: a cause for its reemergence? Emerg Infect Dis. 2001;7(3Suppl):526–28. doi:10.3201/eid0707.017708.
- Centers for Disease Control and Prevention. Pertussis: summary of vaccine recommendations. For healthcare professionals. Summary of DTaP and Tdap vaccine recommendations across the lifespan; 2020 [accessed 2020 Feb 2]. https://www.cdc.gov/vaccines/vpd/pertussis/recs-summary.html.
- Centers for Disease Control and Prevention. Vaccination coverage among adults in the United States, National Health Interview Survey, 2016;2020 [accessed 2020 Feb 2]. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html.
- Public Health England. The routine immunisation schedule from January 2020; 2020 [accessed 2020 Feb 2]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/849184/PHE_complete_immunisation_schedule_Jan2020.pdf.
- von Konig CH, Halperin S, Riffelmann M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis. 2002;2(12):744–50. doi:10.1016/S1473-3099(02)00452-8.
- Riffelmann M, Littmann M, Hulsse C, Hellenbrand W, Wirsing von Konig CH.Pertussis: not only a disease of childhood. Dtsch Arztebl Int. 2008;105(37):623–28. doi:10.3238/arztebl.2008.0623.
- Clarke MF, Rasiah K, Copland J, Watson M, Koehler AP, Dowling K, Marshall HS. The pertussis epidemic: informing strategies for prevention of severe disease. Epidemiol Infect. 2013;141(3):463–71. doi:10.1017/s095026881200091x.
- Mbayei SA, Faulkner A, Miner C, Edge K, Cruz V, Peña SA, Kudish K, Coleman J, Pradhan E, Thomas S, et al. Severe pertussis infections in the United States, 2011–2015. Clin Infect Dis. 2019;69(2):218–26. doi:10.1093/cid/ciy889.
- Kretsinger K, Broder KR, Cortese MM, Joyce MP, Ortega-Sanchez I, Lee GM, Tiwari T, Cohn AC, Slade BA, Iskander JK, et al. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep. 2006;55(RR–17):1–37.
- Kandeil W, Atanasov P, Avramioti D, Fu J, Demarteau N, Li X. The burden of pertussis in older adults: what is the role of vaccination? A systematic literature review. Expert Rev Vaccines. 2019;18(5):439–55. doi:10.1080/14760584.2019.1588727.
- Syed MA, Bana NF. Pertussis. A reemerging and an underreported infectious disease. Saudi Med J. 2014;35:1181–87.
- Liu BC, McIntyre P, Kaldor JM, Quinn HE, Ridda I, Banks E. Pertussis in older adults: prospective study of risk factors and morbidity. Clin Infect Dis. 2012;55(11):1450–56. doi:10.1093/cid/cis627.
- Capili CR, Hettinger A, Rigelman-Hedberg N, Fink L, Boyce T, Lahr B, Juhn YJ. Increased risk of pertussis in patients with asthma. J Allergy Clin Immunol. 2012;129(4):957–63. doi:10.1016/j.jaci.2011.11.020.
- Buck PO, Meyers JL, Gordon LD, Parikh R, Kurosky SK, Davis KL. Economic burden of diagnosed pertussis among individuals with asthma or chronic obstructive pulmonary disease in the USA: an analysis of administrative claims. Epidemiol Infect. 2017;145(10):2109–21. doi:10.1017/s0950268817000887.
- Goebbels K, Gieseler U, Schoffl V, Kupper T. Cough and dyspnoea of an asthmatic patient at Mt. Kilimanjaro: a difficult differential diagnosis. Travel Med Infect Dis. 2010;8(1):22–28. doi:10.1016/j.tmaid.2009.11.001.
- Spector J, Rathlev NK. Pertussis in an elderly Asian-American man: a case report. J Emerg Med. 2010;38(4):434–38. doi:10.1016/j.jemermed.2007.07.024.
- De Serres G, Shadmani R, Duval B, Boulianne N, Dery P, Douville Fradet M, Rochette L, Halperin SA. Morbidity of pertussis in adolescents and adults. J Infect Dis. 2000;182(1):174–79. doi:10.1086/315648.
- Harju TH, Leinonen M, Nokso-Koivisto J, Korhonen T, Raty R, He Q, Hovi T, Mertsola J, Bloigu A, Rytila P, et al. Pathogenic bacteria and viruses in induced sputum or pharyngeal secretions of adults with stable asthma. Thorax. 2006;61(7):579–84. doi:10.1136/thx.2005.056291.
- Bureau of Labor Statistics. United States department of labor medical care component of consumer price index – all urban consumers (current series ID: CUUR0000SAM), not seasonally adjusted; [ accessed 2019 Sep 26]. http://data.bls.gov/timeseries/cuur0000SAM.
- Hashemi SH, Nadi E, Hajilooi M, Seif-Rabiei MA, Samaei A. High seroprevalence of Bordetella pertussis in patients with chronic obstructive pulmonary disease: a case-control study. Tanaffos. 2015;14:172–76.
- Bonhoeffer J, Bar G, Riffelmann M, Soler M, Heininger U. The role of Bordetella infections in patients with acute exacerbation of chronic bronchitis. Infection. 2005;33(1):13–17. doi:10.1007/s15010-005-4004-9.
- Centers for Disease Control and Prevention. National Center for Health Statistics. Health, United States, 2017: with special feature on mortality; 2018 [accessed 2019 Dec 4]. https://www.cdc.gov/nchs/data/hus/hus17.pdf.
- Karki S, McIntyre P, Newall AT, MacIntyre CR, Banks E, Liu B. Risk factors for pertussis hospitalizations in Australians aged 45 years and over: a population based nested case-control study. Vaccine. 2015;33(42):5647–53. doi:10.1016/j.vaccine.2015.08.068.
- Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA. 2016;316(23):2547–48. doi:10.1001/jama.2016.16477.
- Kenyon C, Miller C, Ehresmann K, Boxrud D, Cassiday P, Sanden GN, Bisgard KM, Kiang K. Fatal case of unsuspected pertussis diagnosed from a blood culture–Minnesota, 2003. MMWR Morb Mortal Wkly Rep. 2004;53(6):131–32. doi.
- Colebunders R, Vael C, Blot K, Van Meerbeeck J, Van den Ende J, Ieven M. Bordetella pertussis as a cause of chronic respiratory infection in an AIDS patient. Eur J Clin Microbiol Infect Dis. 1994;13(4):313–15. doi:10.1007/bf01974608.
- Garbiras M, Shabaka A, Calvo N, Martin L, Moreno MA. Lopez de la Manzanara V, Sanchez-Fructuoso AI Whooping cough in a renal transplant recipient. Transpl Infect Dis. 2016;18(2):280–83. doi:10.1111/tid.12503.
- Janda WM, Santos E, Stevens J, Celig D, Terrile L, Schreckenberger PC. Unexpected isolation of Bordetella pertussis from a blood culture. J Clin Microbiol. 1994;32(11):2851–53. doi:10.1128/JCM.32.11.2851-2853.1994.
- Koufakis T, Paschala A, Siapardanis D. Pertussis reinfection in an adult: a cause of persistent cough not to be ignored. Case Rep Infect Dis. 2017;2017:4786141. doi:10.1155/2017/4786141.
- Troseid M, Jonassen TO, Steinbakk M. Isolation of Bordetella pertussis in blood culture from a patient with multiple myeloma. J Infect. 2006;52(1):e11–3. doi:10.1016/j.jinf.2005.04.014.
- Gröndahl-Yli-Hannuksela K, Viander M, Mertsola J, He Q. Increased risk of pertussis in adult patients with mannose-binding lectin deficiency. APMIS. 2013;121(4):311–15. doi:10.1111/apm.12000.
- Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, Liu X. National surveillance of asthma: United States, 2001–2010. Vital Health Stat. 2012;3:1–58.
- Lundback B, Backman H, Lotvall J, Ronmark E. Is asthma prevalence still increasing? Expert Rev Respir Med. 2016;10(1):39–51. doi:10.1586/17476348.2016.1114417.
- Organization WH. Asthma fact sheet; [ accessed 2019 Jul 16]. https://www.who.int/news-room/fact-sheets/detail/asthma.
- Kraft M. The role of bacterial infections in asthma. Clin Chest Med. 2000;21(2):301–13. doi:10.1016/S0272-5231(05)70268-9.
- Nuorti JP, Whitney CG, MD for the ACIP Pneumococcal Vaccines Working Group. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). Morb Mortal Wkly Rep. 2010;59(34):1102–06.
- World Health Organization. Chronic Obstructive Pulmonary Disease (COPD); [ accessed 2019 July 16]. https://www.who.int/respiratory/copd/en/.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–65. doi:10.1056/NEJMra0800353.
- Sethi S. Infection as a comorbidity of COPD. Eur Respir J. 2010;35(6):1209–15. doi:10.1183/09031936.00081409.
- Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:95–109. doi:10.2147/COPD.S54473.
- Ko FW-S, Chan PK-S, Chan RWY, Chan K-P, Ip A, Kwok A, Ngai JC-L, Ng -S-S, On CT, Hui DS-C. Molecular detection of respiratory pathogens and typing of human rhinovirus of adults hospitalized for exacerbation of asthma and chronic obstructive pulmonary disease. Respir Res. 2019;20(1):210. doi:10.1186/s12931-019-1181-0.
- Wilkinson TMA, Aris E, Bourne S, Clarke SC, Peeters M, Pascal TG, Schoonbroodt S, Tuck AC, Kim V, Ostridge K, et al. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax. 2017;72(10):919–27. doi:10.1136/thoraxjnl-2016-209023.
- Lopez Caro JC, Santibanez M, Garcia Rivero JL, Villanueva M, Sainz J, Gonzalez Astorqui P, Hierro M, Rodriguez Porres M, Paras Bravo P, Mira A, et al. Sputum microbiome dynamics in chronic obstructive pulmonary disease patients during an exacerbation event and post-stabilization. Respiration. 2019:1–8. doi:10.1159/000501988.
- Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(3):228–37. doi:10.1164/rccm.201210-1843CI.
- Health, United States, 2016: with chartbook on long-term trends in health. Hyattsville (MD): National Center for Health Statistics (US); 2017.
- Primary Immunodeficiency UK. Mannose-binding lectin (MBL) deficiency; [ accessed 2019 Jul 16]. http://www.piduk.org/static/media/up/MBLdeficiency.pdf.
- Dhainaut J-F, Claessens Y-E, Janes J, Nelson DR. Underlying disorders and their impact on the host response to infection. Clinical Infectious Diseases. 2005;41(Supplement_7):S481–S9. doi:10.1086/432001.
- Dhainaut JF, Laterre PF, LaRosa SP, Levy H, Garber GE, Heiselman D, Kinasewitz GT, Light RB, Morris P, Schein R, et al. The clinical evaluation committee in a large multicenter phase 3 trial of drotrecogin alfa (activated) in patients with severe sepsis (PROWESS): role, methodology, and results. Crit Care Med. 2003;31(9):2291–301. doi:10.1097/01.ccm.0000085089.88077.af.