?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective: The seroepidemiologic study aimed to delineate the immune status against tetanus in Hangzhou and its influential factors to provide evidence on strategies for tetanus prevention.
Methods: We used a multi-stage stratified random sampling method to recruit participants from several Community Health Centers in Hangzhou during 2009–2018. Demographic data and vaccination histories of all subjects born during the period from 2004 to 2018 were collected from Hangzhou Immunization Information System (HZIIS), while the data of all subjects born before 2003 were acquired through case investigation. We used Enzyme Linked Immunosorbent Assay (ELISA) to detect Immunoglobulin G (IgG) antibodies against tetanus in serum samples and univariate and multivariate analysis to detect association between level of tetanus IgG and related factors.
Results: A total of 3695 subjects were included, of which were 1904 males and 1791 females. About 59.84% of people had a clear immunization history of tetanus-toxoid-containing vaccines (TTCVs). The percentage of people whose tetanus IgG antibody titers were above the minimum protective level (0.1 IU/ml) was 76.02% (95% CI: 74.6–77.4%) and the geometric mean concentration (GMC) for tetanus IgG was 1.70 IU/ml. Antibody levels of people were higher in rural areas than in urban areas, in males than in females, in subjects who had inoculation vaccine history than those without inoculation of vaccine or with unknown history, and lower in groups aged >15 y compared to <15 y.
Conclusions: The positive rate and GMC of tetanus IgG were significantly influenced by gender, age and immunization history of TTCVs. We should seek to achieve early and timely infant vaccination and maintain high coverage of the complete 3-dose primary series plus 3-dose booster series prior to adolescence. Besides basic immunization, we should stress the importance of using booster doses of tetanus vaccine in adolescents and adults; it is necessary to adopt corresponding immunization measures for women at childbearing age.
KEYWORDS:
1. Introduction
Tetanus is an acute bacterial infection with a high fatality rate caused by toxigenic strains of the bacterium Clostridium tetani (C. tetani), but monitoring its incidence is difficult. Tetanus may occur at any age but mainly occurs in newborns and maternals with unclean childbirth and poor postpartum health conditions.Citation1 World Health Organization (WHO) estimates that approximately 34,000 neonates died of neonatal tetanus in 2015 which represents a 96% reduction since 1988.Citation2 However, the tetanus surveillance systems that focus on detection of neonatal tetanus cases in health facilities may be not well established in many countries. In 2014, the total reported tetanus incidence in the European Union (EU) was 0.01 per 100,000 population, with 65% of cases aged ≥65 y.Citation3 The incidence of tetanus was not accurate, for many cases occurred outside the reach of the health system and were not reported. In 2015, a total of 10301 tetanus cases including 3551 neonatal cases were reported through the WHO and United Nations International Children’s Emergency Fund (UNICEF) Joint Reporting Form, reflecting the low reporting sensitivity for tetanus cases and uncertainty about the true disease incidence.Citation4
TTCVs may not always work. The immunity against tetanus can only be acquired through active or passive immunization, but not after recovery from tetanus.Citation1 Vaccination is the most effective and reliable strategy for preventing the morbidity of tetanus. TT vaccine was first produced in 1924 and used extensively for the first time among soldiers during World War II. Since then, immunization programs using TTCVs have been highly successful in preventing Maternal and Neonatal Tetanus (MNT) as well as injury-associated tetanus. TT is valid as a single-antigen vaccine and also as a combination vaccine to protect against other vaccine-preventable diseases. Many different TTCVs were licensed worldwide.Citation5 According to the Hangzhou’s Vaccination Mandate record, children had been vaccinated by the TT vaccine since 1959 in Hangzhou, but it was not recommended for pregnant women. Afterward, the main vaccines used for the prevention of tetanus were DTP, diphtheria–tetanus (DT), DTaP-Haemophilus influenzae type b (Hib) and DTaP-inactivated poliovirus vaccine (IPV)/Hib. The main components of DTP were diphtheria toxoid and tetanus toxoid combined with either whole-cell pertussis (DTwP) or acellular pertussis (DTaP). DTwP was introduced in 1978 in China, while in 1983 in Hangzhou. DTaP was introduced in 1999 in Hangzhou, replaced DTwP since 2008 and completely replaced it in 2011. In Hangzhou, primary vaccination of infants and young children consists of three doses of DTP in 3, 4 and 5 months old followed by a fourth dose at 18–24 months old, with DT booster doses being recommended at aged 6 y. The vaccination rates achieved with four doses of the DTP vaccination in childhood have been more than 99% since 2011.Citation6 In the developing countries, where extensive efforts have been made to provide TTCVs in routine immunization programs, in particular targeting children and pregnant women, the tetanus incidence has decreased in recent years.Citation7,Citation8 However, as cases of tetanus have been documented in individuals with antibody concentrations above thresholds, a normally protective antibody concentration may not be a guarantee of immunity in every circumstance. The aim should be to sustain high antibody concentrations throughout life.Citation9,Citation10 The immune response to TTCVs tends to decrease with age increasing. Comparative studies suggested that children tend to develop higher antibody levels than adults.
IgG antibodies against tetanus are one of the indicators for monitoring the effectiveness of TTCVs in the vaccinated population. Sero-surveillance is an essential tool to monitor vaccine-preventable diseases, as it can detect subgroups at risk and therefore deliver information that is often lacking in routine surveillance and vaccination coverage studies.Citation11 It is suggested that Sero-surveillance is conducive to the improvement of the methods to prevent tetanus occurred. In this study, we have measured the levels of IgG antibodies against tetanus. We aim to delineate the immune status against tetanus among the ‘healthy’ population in Hangzhou City and to provide evidence on strategies for tetanus prevention in China.
2. Methods
2.1. Subjects and data collection
The present sero-surveillance of healthy population was performed in Hangzhou during 2009–2018, which was carried out once a year. A multi-stage stratified random sampling method was employed to select the participant. A sample size of at least 940 subjects was included for cross-sectional investigation based on the formula: (p-tetanus = 72.35%, δ = 3%, α = 0.05, p: the rates of tetanus IgG seropositivity).Citation12 The seroepidemiology of tetanus IgG was compared among 11 various age groups: <1, 1, 2, 3–4, 5–6, 7–9, 10–14, 15–19, 20–29, 30–39, ≥40 y. There are at least 30 samples from each age group per year.
The samples used were residual specimens via regular physical examination for subjects from kindergarten, primary school and secondary school students and in the Community Health Centers in Hangzhou. Basic socio-demographic information and vaccination history of all subjects born from 2004 to 2018 derived from HZIIS, while all subjects who were born before 2003 were acquired by case investigations. HZIIS included all residents and new immigrants who had been vaccinated in Hangzhou since 2004. Subjects were linked to the HZIIS via matching both the names and the dates of birth. Meanwhile, blood samples were collected and serums were isolated for antibody detection.
2.2. Experimental approach and results calculation
ELISA was used to detect the tetanus IgG antibody quantitatively by commercial ELISA kits (IBL: RE56901 for tetanus, Serion ELISA classic, Institut Virion/Serion GmbH, Würzburg, Germany) according to the manufacturer’s instructions. Two levels of immunity to tetanus were defined: tetanus antibody levels of <0.1 IU/ml = ‘sero-negativity’, ≥0.1 IU/ml = ‘sero-positivity’. Tetanus IgG antibody positive means successful vaccination or immunoglobulin recipient.
2.3. Statistical analysis
Quantitative variables with normal distributions were expressed as means± standard deviations (SD), and those with non-normal distributed variables were expressed as medians (interquartile range). Statistical significance between groups was examined by two independent variable t-test or one-way ANOVA test for the GMC for tetanus IgG levels. The prevalence rates of tetanus IgG were expressed as frequencies (percentages), and Pearson’s chi-square test or Fisher’s exact test was used to determine group differences. Multivariate analysis including gender and age groups was used: Logistic regression was used to analyze the associations between changes in the rate of tetanus IgG seropositivity and related factors. Linear regression was used to analyze the associations between changes in the GMC levels and related factors. The 95% confidence intervals (CIs) were calculated. All statistical analyses were performed and graphs were created using SPSS statistical software for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA). A value of P< .05 (2-sided) was considered statistically significant.
2.4. Ethical considerations
All of the participants were given a brief oral description of the aims of the present study, and verbal consent was obtained from the participants. This program was approved by the ethics committee of the Hangzhou Center for Disease Control and Prevention (HZCDC).
3. Results
3.1. Socio-demographic information and vaccination
In all, 3695 subjects with available information were included in the current study, with 100% response rate. There were 1904 males and 1791 females. Samples were collected during 2009–2018,11 various age groups were included and the average age was 25.70 y old. There were 1494 subjects in urban while 2201 ones in rural.
Out of 3695 people, 2211 (59.84%) had a clear immunization history of TTCVs, 5.17% (191/3695) of subjects had no immunization history, while the remaining subjects’ vaccination histories were unknown. The vaccination rates of TTCVs among 11 different age groups were statistically significant (χ2 = 1489.76, P for trend < 0.001), which had a declining trend in older age groups. The vaccination rate of TTCVs was significantly higher in males (65.86%) than in females (53.43%), higher in rural (62.06%) than in urban (56.55%). Among the subjects with clear vaccination history, 0.58% (13/2211), 0.86% (19/2211), 24.06% (532/2211), 73.50% (1625/2211), 1.00% (22/2211) of the subjects had 1 dose, 2 doses, 3 doses, 4 doses and 5 doses, respectively. Among the subjects with unknown vaccination history, 41.92% (542/1293) were males and 58.08% (751/1293) were females, 44.78% (579/1293) lived in urban zones while 55.22% (714/1293) in rural and 75.10% (971/1293) were older than 15 y old.
3.2. Seroepidemiology of tetanus IgG among various groups via univariate analysis
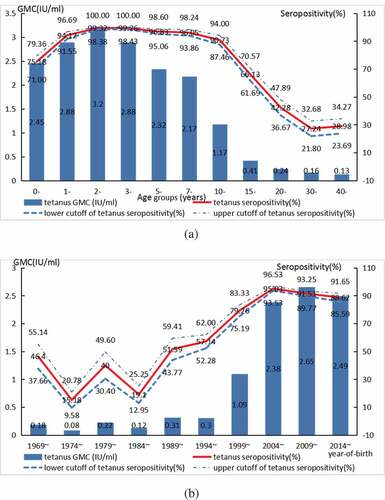
In total, the rate of tetanus IgG seropositivity was 76.02% (95% CI: 74.64–77.40%) and the GMC for tetanus IgG was 1.70 IU/ml. In univariate analysis, overall, significantly higher seropositivity and GMC for tetanus IgG were found in males compared with females, in rural compared with in urban, in subjects had inoculation TTCVs history when comparing those without inoculation or unknown history of TTCVs () (all P-values <0.05). In addition, in the present study, it is found that the tetanus IgG antibody was lowest in the age group ≥15 y (66.36%), and was the highest in the age group of 1–2 y (99.32%). Significantly lower seropositivity and GMC for tetanus IgG were found in age groups ≥15 y when compared to <15 yage groups (χ2 = 1347.84, P< .001; F = 221.64, P< .001) (, ). Seropositivity and GMC for tetanus IgG in the population increased significantly by year-of-birth (χ2 = 1189.72, P for trend<0.001, F = 188.65, P< .001) ().
Table 1. Seroepidemiology of Tetanus IgG in population among various groups via univariate analysis
Among the subjects with clear immunization history of TTCVs, the rate of tetanus IgG seropositivity was 90.68% (95% CI: 89.47–91.89%) and the GMC for tetanus IgG was 2.31 IU/ml. Significantly higher seropositivity and GMC for tetanus IgG were found in males compared with females, in rural compared with in urban, in age groups <15 y compared with that in the age group ≥15 y (all P-values <0.05).
3.3. Associations of seroepidemiology of tetanus IgG and related variables via multivariate analysis
In multivariate analysis, overall, significantly lower seropositivity and GMC for tetanus IgG were found in females compared with males (OR = 0.798, 95% CI: 0.656–0.970; OR = 0.896, 95% CI: 0.813–0.988, all P-values < 0.05); higher seropositivity tetanus IgG were found in rural compared with urban (OR = 1.916, 95% CI: 1.578–2.326; P-value < 0.05) and in subjects that had inoculation TTCVs history (OR = 1.644, 95% CI: 1.096–2.467; OR = 1.657, 95% CI: 1.322–2.076, all P-values<0.05) ().
Table 2. Association of Seroepidemiology of tetanus IgG and related variables via multivariate analysis
In addition, age is one of the important factors affecting the seroepidemiology of tetanus. Taking the <1-y-old group as a reference, the seropositivity and GMC for tetanus IgG in subjects aged 1–14 y were significantly higher and those in subjects aged ≥15 y were significantly lower (all P-values <0.05) ().
In short, both the rates of tetanus IgG seropositivity and the GMC for tetanus IgG were significantly influenced by gender, age and the immunization history of TTCVs.
4. Discussion
We found that the percentage of people who had tetanus IgG antibody titers above the minimum protective level was 76.02% and the GMC for tetanus IgG was 1.70 IU/ml. People in rural areas, who were males, younger than 15 y and who had inoculation vaccine history tended to have higher antibody levels. In European and American countries, the positive rate of healthy adults against tetanus IgG is as high as 95.0%,Citation13 which is 69.6–84.6% in Africa.Citation14 In studies carried out in China, the positive rate of anti-tetanus IgG in healthy people varied significantly, ranging from 55.7% to 98.3%, probably because of the difference in survey methods and laboratory tests.Citation15–17 The positive rate of anti-tetanus IgG in healthy children who have completed immunization between ages of 1 and 10 can be as high as 99.0%.Citation18 Studies conducted in China on the level of anti-tetanus IgG in population only used univariate analysis. In our study, multivariate logistic regression analysis was used to explore the real factors influencing the positive rate of anti-tetanus IgG in population. And results show that the rate of tetanus IgG seropositivity was 76.02% and the GMC for tetanus IgG was 1.70 IU/ml. Antibody concentrations of at least 0.1–0.2 IU/ml are defined as protective when using standard ELISA.Citation9,Citation10 The “Regulations on the Management of Planned Immunization Technology” issued by the Ministry of Public Health proposed that the proportion of tetanus IgG to reach the level of protection needs to be more than 85%. Results show that the tetanus IgG among the ‘healthy’ population in Hangzhou was below the protection level, lower than other studies in the country,Citation18 which was close to Guangzhou.Citation19 Considering that it may be due to the differences among various kits, it is not excluded that the floating population in Hangzhou is large and the TTCVs vaccination situation of the floating population is not ideal. Factors that affect the level and duration of tetanus IgG antibodies including the age, the vaccination, the type of TTCVs, the doses of inoculations, the interval between inoculations and so on.
Age is one of the important factors affecting the level of tetanus IgG. Studies at home and abroad have found that the positive rate of anti-tetanus IgG decreased with age.Citation15,Citation16 In the present study, the protective rate of tetanus IgG antibody in the population under 15 y of age reached above 90% and the GMC maintained at a high level, and a small peak occurred after immunization enhancement at the age of 1.5 to 2 y; but the antibody level decreased significantly with the increase of age, especially in people over the age of 15 y, which was consistent with some other scholars’ findings.Citation16 The level of tetanus antibody and GMC in the population under 15 y of age maintained at a high level showed that the combination of early immunity, 18 months of age and 6 y of age had achieved good results. However, the anti-tetanus IgG positive rate and GMC value of people <10 y old, 10–14 y old and ≥15 y old showed a decreasing trend, which was similar to the results of other studies at home and abroad.Citation14,Citation20 The positive rate of anti-tetanus IgG in people aged ≥15 y was 27.24–66.13%, similar to the situation in South Korea.Citation21 According to the immunization procedure, the population aged ≥15 y had been more than 10 y after inoculation, and the positive rate of anti-tetanus IgG in the population decreased from 90.73% in the group aged 10–14 y to 66.13%, which was consistent with the half-life of anti-tetanus IgG of about 10 y.Citation13 According to the CDC, the proportions of adults who received TTCVs within the last 10 y were 64% for 19–64 y and 53% for people over 65 y, in 2010,Citation22 and the proportion of people between 19 and 64 y of age who had received TTCVs in the past 5 y were only 8.2%. Immunity gaps may exist in the elderly due to waning immunity or non-vaccination.Citation23 The immunity of adolescents and adults should be taken seriously, and further research is required to fully assess the duration of protection against tetanus in the elderly. Seropositivity and GMC for tetanus IgG in population increased significantly by year-of-birth. It reached the first growth point in 1999 and the second peak in 2009, and indicated key events in tetanus vaccination. DTaP was introduced in 1999 in Hangzhou, replaced DTwP since 2008 and completely replaced it in 2011.
Vaccination is the most effective and reliable method to prevent tetanus, though they remain in the population. With the rise in vaccination rate of TTCVs, the incidence, mortality and health care costs associated with tetanus declined dramatically. In the present study, there were 59.84% subjects having immunization history of TTCVs. Compared with those without inoculation TTCVs history, both the rate of tetanus IgG seropositivity and GMC of subjects with inoculation TTCVs history were significantly higher, which was consistent with relevant literature reports.Citation19,Citation24 Additionally, the level of tetanus IgG has a great relationship with the number of inoculation needles. TTCVs’ manufacturers recommended various booster doses, depending on the product. In children, a 3-dose primary series of DTP induced an antibody titer above the minimum protective threshold, with a mean level above 0.2 IU/ml as reported in several studies.Citation10 Although tetanus antibody levels are high after 3 primary TTCV doses in infancy, they decline over time, and the percentage of unprotected vaccinators increases to 20% 1 y after vaccination and the average titer drops to the protective threshold. A booster dose at 18–24 months old can rapidly increase antibody levels. A 5-dose will provide protection into adolescence, and the 6-dose during adolescence will induce immunity that lasts through much of adulthood, thus protecting women through their childbearing years.Citation10 In the Netherlands, a study demonstrated that tetanus immunity persisted for at least 20 y after the 6-dose, with a GMC of 0.44 IU/ml in individuals aged 30–34 y.Citation25 Serosurvey data illustrating the decline of sero-protection with increasing age in the absence of booster doses demonstrate the need for booster doses for both sexes in order to provide lifelong protection. In the present study, 0.58%, 0.86%, 24.06%, 73.50%, 1.00% subjects had 1dose, 2doses, 3doses, 4doses and 5doses, respectively. The vaccination rate for over 5-doses was low in Hangzhou. At present, the majority of children in China adopt DTaP for basic immunization and enhanced immunization, and DT booster doses being recommended at aged 6 y, but there is no vaccination recommendation for pregnant women/women of child-bearing age, which still lags behind the majority of developed countries and even some developing countries.Citation26 Since there are no TT vaccination recommendations for pregnant women and women of child-bearing age, in order to prevent tetanus incidence, opportunities should be taken to provide or complete the full TTCVs series for those who were not vaccinated, or incompletely vaccinated. Additionally, we should stress the importance of employment of booster dose of tetanus vaccine in adolescents and adults. Immunization programs should ensure that 3 TTCVs booster doses are provided, i.e. a total of 6 doses, preferably administered in childhood and completed by adolescence, in order to provide protection throughout adolescence and adulthood. Considering the results of the present study that the anti-tetanus IgG positive rate and GMC value of people <10 y old, 10–14 y old and ≥15 y old showed a decreasing trend, it is recommended that an appropriate Td booster vaccine coverage after the age of 10, which can effectively protect the susceptible population, reduce disease occurrence.
This study also found that the level of tetanus IgG was significantly influenced by gender. It suggested that it may be meaningful to analyze the difference between the gender, such as the different occupations, exposure opportunities, immunity between males and females. Some men were vaccinated during military service and women had relatively weak immunity.Citation23 In the present study, the positive rate and GMC value of anti-tetanus IgG in males were significantly higher than those in females. The results of this study suggest that women in Hangzhou are at greater risk of contracting tetanus, and that infants can be protected by maternally transmitted anti-tetanus antibodies passed from placenta to fetus.Citation1 Improving tetanus immunity in women is also an important measure to prevent neonatal tetanus. The serum level of tetanus IgG at birth tends to be higher or the same as that of the mother. Therefore, the level of tetanus immunity in women of childbearing age should be monitored and immunized. In a study in pregnant women with no previous TTCVs vaccination, 78% of those who received 2 doses during pregnancy had tetanus-specific antibody levels above the protective threshold at 3 y later. Infants whose mothers had suboptimal levels of tetanus antibodies may be at risk of tetanus.Citation10 Two recent systematic reviews evaluated the use of alum-adsorbed TTCVs administered to pregnant women, and got similar results.Citation27,Citation28 One of these reviews concluded through a meta-analysis that the vaccine effectiveness of ≥2 properly timed doses of TT given to pregnant women against neonatal tetanus mortality was 94% (95% CI: 80–98%).Citation29 Thus, it is important for women of childbearing age to strengthen their level of tetanus immunity. The maternal immunization strategy with Tdap is already included in some countries. In Australian, immunization changed from being permissive to maternal vaccination with Tdap to actively recommending a pregnant woman be vaccinated in the third trimester of each pregnancy.Citation30 In the United States, it is recommended that 1 dose of Tdap should be given at the age of 11–12 too, and 1 dose of Tdap should be given to adults aged 19, and 1 dose of Tdap should be given to women of childbearing age every time.Citation31 However, Tdap vaccine that would make vaccination of adolescents and adults much easier is not licensed in China. Licensing and using Tdap vaccine in pregnancy would be an effective solution to the challenges. Not only will women and their infants be protected from tetanus but also will they be protected from pertussis.
The strength of our study was that we used HZIIS inquiry combined with case investigation to collect data, which ensured the immunization information as complete as possible. Limitations in this study included that the impact of differences in vaccine types had not been thoroughly analyzed in this study; the differences in vaccine types could be analyzed in subsequent studies to further improve the development and the use of vaccines.
In conclusion, the positive rate and GMC value of anti-tetanus IgG were significantly influenced by gender, age and the immunization history of TTCVs. In order to prevent tetanus transmission, all children worldwide should be immunized against tetanus. We should seek to achieve early and timely infant vaccination and maintain high coverage of the complete 3-dose primary series plus 3-dose booster series prior to adolescence. Firstly, while doing basic immunization, we should stress the importance of application of booster doses of tetanus vaccine in adolescents and adults; secondly, the results in the present study reflect an appropriate Td booster vaccine coverage at 10 y of age; thirdly, it is necessary to adopt corresponding immunization measures in population with low antibody level in Hangzhou, such as the women of childbearing age. It is important to strengthen their level of tetanus immunity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- World Health Organization. Tetanus vaccines: WHO position paper-February 2017. Wkly Epidemiol Rec. 2017;92(6):53–76.
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388(10063):3027–35. doi:10.1016/S0140-6736(16)31593-8.
- European Center for Disease Control. Surveillance atlas of infectious diseases. 2018. http://atlas.ecdc.europa.eu/public/index.aspx.
- WHO vaccine-preventable diseases: monitoring system 2016 global summary. 2019. http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencettetanus.html.
- WHO Prequalified Vaccines. 2016. http://www.who.int/immunization_standards/vaccine_quality/PQ_vaccine_list_en/en/
- Zhang Q, Zheng H, Liu M, Han K, Shu J, Wu C, Xu N, He Q, Luo H. The seroepidemiology of immunoglobulin G antibodies against pertussis toxin in China: a cross sectional study. BMC Infect Dis. 2012;12:138. doi:10.1186/1471-2334-12-138.
- Chatchatee P, Chatproedprai S, Warinsathien P, Tharmaphornpilas P, Yoocharoen P, Warintrawat S, Theamboonlers A, Chongsrisawat V, Poovorawan Y. Seroprevalence of tetanus antibody in the Thai population: a national survey. Asian Pac J Allergy Immunol. 2007;25:219–23.
- Gouveia PA da C, Silva CEF, Miranda Filho D de B, Bernardino SN, Escarião AG, Ximenes RA de A. Mortality trend due to accidental tetanus from 1981 to 2004 in Pernambuco and analysis of the impact on intensive care unit attendance. Rev Soc Bras Med Trop. 2009;42(1):54–57. doi:10.1590/s0037-86822009000100011.
- Roper MH, Wassilak SGF, Tiwari TSP, Orenstein WA. Tetanus toxoid. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. 6th. Philadelphia: Sauders; 2013. p. 447–92.
- Borrow R, Balmer P, Roper MH. The immunologic basis for immunization: module 3: tetanus. Geneva: World Health Organization; 2007. http://apps.who.int/iris/bitstream/10665/43687/1/9789241595551_eng.pdf
- Theeten H, Hutse V, Hens N, Hens N, Yavuz Y, Hoppenbrouwers K, Beutels P, Vranckx R, VAN Damme P. Are we hitting immunity targets? The 2006 age-specific seroprevalence of measles, mumps, rubella, diphtheria and tetanus in Belgium. Epidemiol Infect. 2011;139:494–504. doi:10.1017/S0950268810001536.
- Li-ming H, Er-ping X, Luo-xian Y. Observation of immunity level of pertussis-diphtheria-tetanus in healthy people in Hangzhou City during 1995–2006. Chin J Vaccines Immunization. 2009;15(1):68–71. (In Chinese).
- Borella-Venturini M, Frasson C, Paluan F, DE Nuzzo D, Di Masi G, Giraldo M, Chiara F, Trevisan A. Tetanus vaccination, antibody persistence and decennial booster: a serosurvey of university students and at- risk workers. Epidemiol Infect. 2017;145(9):1757–62. doi:10.1017/S0950268817000516.
- Scobie HM, Patel M, Martin D, Mkocha H, Njenga SM, Odiere MR, Pelletreau S, Priest JW, Thompson R, Won KY, et al. Tetanus immunity gaps in children 5–14 years and men ≥15 years of age revealed by integrated disease serosurveillance in Kenya, Tanzania, and Mozambique. Am J Trop Med Hyg. 2017;96(2):415–20. doi:10.4269/ajtmh.16-0452.
- Wang SL, Wang HJ. Monitoring analysis of national immunization program vaccine antibody levels in healthy people of Wuhai city Inner Mongolia. J Med Pest Control. 2017;33(4):415–17. In Chinese
- Liu XL, Li GP, Wang YJ. Serological surveillance of antibody levels to pertussis, diphtheria and tetanus among healthy people in Tongchuan,Shanxi. Dis Surveillance. 2012;27(7):516–19. (In Chinese).
- Wu ZJ, Hu H, Li Y, Gao Y. Surveillance and analysis on serum immune antibody levels of 8 vaccine-preventable diseases among migrant children aged 0–7 years in Yinchuan. Hainan Med J. 2016;27(10):1701–03. (In Chinese).
- Li JX, Liu S, Tan XF. The serological surveillance of epidemic cerebrospinal meningitis groups A and C,pertussis, diphtheria,and tetanus among 1–10 years old children in Ganyu district Liangyungang city. Mod Preventive Med. 2016;43(18):3332–34. (In Chinese).
- Chun-huan Z, Ye-jian W, Qing H. The serological surveillance of tetanus antibodies among Guangzhou people in 2016. J Trop Med. 2018;18(9):1242–44. (In Chinese)
- Qm X, Su N, Wu DP. Surveillance on immunity levels of measles,rubella,tetanus,varicella among healthy populations in Panyu district,Guangzhou. J Med Pest Control. 2016;32(7):723–725,728. (In Chinese)
- Sung H, Jang MJ, Bae EY, Han SB, Kim J-H, Kang JH, Park Y-J, Ma SH. Seroepidemiology of tetanus in Korean adults and adolescents in 2012. J Infect Chemother. 2014;20(7):397–400. doi:10.1016/j.jiac.2014.03.008.
- Centers for Disease Control and Prevention (CDC). Adult vaccination coverage-United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:66–72.
- Bourée P. Immunity and immunization in elderly. Path Biol. 2003;51(10):581–85. doi:10.1016/j.patbio.2003.09.004.
- Wang Q, Sun M, Shi S. Analysis of diphtheria antibody level in healthy population in Xicheng district of Beijing. Capital J Public Health. 2013;7(5):226–28. In Chinese
- de Melker HE, van den Hof S, Berbers GAM, Nagelkerke NJD, Rümke HC, Conyn-van Spaendonck MAE. A population-based study on tetanus antitoxin levels in the Netherlands. Vaccine. 1999;18(1–2):100–08. doi:10.1016/S0264-410X(99)00186-3.
- Jing-shan Z, Lei C, Shi-cheng G. Immunization coverage of the national immunization program vaccines at the township level, based on a survey conducted by provincial CDCs in China, 2013. Chin J Vaccines Immunization. 2014;20(6):492–98. In Chinese.
- Demicheli V, Barale A, Rivetti A. Vaccines for women for preventing neonatal tetanus. In: Cochrane database of systematic reviews [Internet]. John Wiley & Sons, Ltd; 2015 7: CD002959. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002959.pub4/pdf/abstract
- Blencowe H, Lawn J, Vandelaer J, Roper M, Cousens S. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int J Epidemiol. 39(Suppl 1):i102–9. DOI: 10.1093/ije/dyq027.
- GRADE table 4. 2017. http://www.who.int/immunization/policy/position_papers/tetanus/en/.
- Australian Technical Advisory Group on Immunisation (ATAGI). The Australian immunisation Handbook. 10th ed. (2015 update) ed. Canberra (Australian): Government Department of Health; 2015.
- Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367(11):1012–19. doi:10.1056/NEJMoa1200850.