ABSTRACT
Introduction: South Africa is yet to introduce rubella-containing vaccines (RCV) into its routine immunization schedule. Selecting the target population when introducing RCV should take into account the ages of susceptible individuals in the population. We aimed to determine the seroprevalence of antibodies to rubella and characterize immunity gaps among individuals of all ages in South Africa.
Methods: We tested for rubella immunoglobulin G (IgG) antibodies with a commercial enzyme-linked immunosorbent assay. We used residual samples collected from 2016 through 2018 as part of the national measles surveillance program. We only tested samples that were negative for measles and rubella immunoglobulin M (IgM) and explored the association between rubella susceptibility (IgG negative) and predictor variables (year of sample collection, age, sex, and province of residence) using logistic regression analysis.
Results: We obtained results for 6057 records. Rubella susceptibility was highest among Individuals aged zero to 11 months (81.9%), followed by children 1 to 5 years old (71.5%), 6 to 10 y old (40.9%) and 11 to 15 y old (31.25) while the smallest proportion of susceptible individuals was among those 16 to 49 y old (19.9%). Females were less likely to be susceptible to rubella compared to males (OR = 0.79 (95%CI: 0.71–0.87), P < .001) in unadjusted analysis but this effect was not observed after adjusting for age and province. In multivariable logistic regression, age (OR = 6.24 (4.52–8.63), P < .001) and province of residence (OR = 0.97 (95%CI: 0.95–0.99), P = .01) were associated with rubella susceptibility.
Conclusion: In the absence of rubella vaccination in the Expanded Program on Immunization in South Africa, the bulk of individuals susceptible to rubella are children under 16 y old. About 20% of individuals 16 to 49 y old are susceptible to rubella. This susceptibility gap must be born in mind during RCV introduction.
Introduction
The Global Measles and Rubella Strategic plan 2012–2020 aimed to eliminate measles and rubella in at least five World Health Organization (WHO) regions by the end of 2020.Citation1 A midterm review suggested that these elimination goals were not likely to be achieved due to several challenges culminating in a shortage of resources for the execution of the plan.Citation2 A number of WHO regions, including Africa, do not have a target for rubella elimination, although many countries have successfully introduced rubella-containing vaccines (RCV) in their Expanded Program on Immunization (EPI) schedules.Citation3 One of the recommendations highlighted in the midterm review involved achieving and maintaining high levels of population immunity to measles and rubella through vaccination.Citation2
Measles vaccination is already part of the EPI schedule in South Africa; however, RCV are only available in the private sector.Citation4 There are several commercially available combinations of RCV, all of which contain the measles vaccine.Citation5 The availability of combination vaccines provides an opportunity to incorporate RCV into already existing measles vaccination activities that entail routine vaccination of infants and supplementary immunization activities (SIAs) targeting older individuals. The WHO recommends introducing RCV when countries achieve at least 80% coverage for measles routine vaccination and/or SIAs.Citation6 Countries that introduced RCV in this manner have experienced considerable reductions in rubella incidence.Citation7
In its guidance document on introduction of RCV, the WHO points out the importance of reviewing the rubella susceptibility profile of the population and targeting a wide age range of individuals during the initial introductory vaccination campaign.Citation8 Identifying age groups of susceptible individuals is therefore important in order to target them during this initial mass campaign. Seroepidemiological studies are used to characterize rubella immunity in populations from results of immunoglobulin G (IgG) testing. The rubella IgG test is unable to distinguish antibodies obtained from passive transfer during pregnancy from antibodies that develop following vaccination or following infection with rubella virus. Furthermore, as an individual acquires rubella antibodies, there is an increase in antibody titers up to a maximum level followed by a decrease in titers. Depending on what time a sample is collected, results might differ in the same individual. Distinguishing between antibodies following vaccination and infection depends on the availability of data on vaccination history in settings where RCV are used. In settings where there is no mass vaccination against rubella, the presence of rubella antibodies can be assumed to be secondary to infection in the majority of cases.
Several serological studies have characterized rubella susceptibility or immunity in different population subgroups in Sub-Saharan Africa.Citation9–11 and in other WHO regions.Citation12–14 When planning RCV introduction, serosurveys provide insight into the population subgroups that should be targeted for vaccination and provide data for modeling rubella transmission dynamicsCitation15–17 including estimation of the burden of congenital rubella syndrome (CRS). Individuals of reproductive age are of particular interest since susceptibility to rubella in this age group has a direct impact on occurrence of CRS, which is the main target of the RCV. Assessing immunity in pregnant women can provide insight into rubella immunity among individuals of reproductive age. Rubella seroprevalence estimates vary in different settings. In Iran, rubella seroprevalence ranged from about 89% among women below 25 y of age to 85% among children under-five, dropping to 81.4% in 11–15 y olds with the highest figures (98.8%) among 21–25 y olds.Citation18 Another Iranian study among pregnant women found that 96% of participants were immune to rubella.Citation14 In Germany, 87.6% of children below 17 yof age were immune to rubellaCitation12 with the age group of 3 to 6 y olds having the highest proportion of immune individuals. A systematic review including several studies in Sub-Saharan AfricaCitation9 reported rubella seroprevalence among individuals of reproductive age ranging from 65% in Sudan to 98% in Nigeria.
An analysis of residual specimens collected from individuals of all age groups in public and private health facilities all over South Africa reported rubella immunity in 93.8% of females aged 12 to 49 y.Citation19 Another study reported rubella immunity in over 95% of pregnant women in the Western Cape province.Citation20 Although these studies report high proportions of immunity to rubella among individuals of reproductive age, it is not certain if rubella infection dynamics remained unchanged over time given that those studies were conducted a decade ago. In order to provide more recent estimates on rubella susceptibility, we aimed to investigate all age groups as the South African government considers introducing RCV.
Methods
Sampling
In this cross-sectional analytic study, we performed rubella immunity testing on residual samples collected in 2016, 2017, and 2018. We included residual samples from measles surveillance that were collected in public facilities and that tested negative for both measles and rubella IgM. Blood samples for measles surveillance are collected from any patient who presents with rash and fever in addition to at least one of the following symptoms; conjunctivitis, coryza, or cough. These samples can be considered to represent the general population since they come from patients in all health districts of the nine provinces in South Africa. Gauteng province has the smallest surface area and the highest population density while the least densely populated is Northern Cape province which has the largest surface area. Johannesburg is the economic hub of the country and is situated in Gauteng province and each province has at least one urban city with rural and semi-rural areas. With the private sector catering for about 15% of the population,Citation21 the public health sector represents about 85% of the South African population and offers free health-care services.
Rubella IgG testing
We tested for the presence of rubella IgG with a test that uses an indirect enzyme-linked immunosorbent assay (ELISA) method (Platelia™, Bio-Rad, Marnes-la-Coquette, France). We determined the presence and concentration of IgG antibodies to rubella by comparing the optical density (OD) of the sample to the concentration in International Units per milliliter (IU/ml) of the calibrators of the standard curve. The Platelia™ Rubella IgG test is standardized to WHO International Standard RUBI 1–94.
We considered a negative (titer < 10 IU/ml) result as indicative of absence of immunity to rubella and a positive result (titer ≥ 15 IU/ml) as indicative of immunity to rubella. We interpreted an equivocal result (titer from 10 IU/ml to ≤15 IU/ml) as inconclusive since we could not obtain a second sample for testing 2 weeks after the first sample as per the manufacturer’s specifications.
Statistical analysis
We summarized categorical data using numbers and percentages and skewed continuous data with medians and ranges. We reported equivocal results when reporting descriptive statistics and subsequently excluded them in all further analyses. The ages of individuals were divided into strata corresponding to individuals below the target for routine immunization (0 to 11 months), individuals who could be targeted for mass vaccination activities (1 to 5 y, 6 to 10 y, and 11 to 15 y), individuals of reproductive age (16 to 49 y), and older individuals (50 and above).
We calculated 99% confidence intervals for proportions of susceptible individuals. We explored the association between rubella susceptibility and predictor variables (age, sex, and province of residence) using univariable and multivariable logistic regression analyses. We applied a stepwise backward automatic method for the multivariable logistic regression and a p-value of 0.05 to select variables that remained in the final model. We cleaned and analyzed the data using STATA (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Ethical considerations
The Human Research Ethics Committee at Stellenbosch University approved the study (Reference number: S18/08/177(PhD)). All participant data were available only to the study team and stored on password-protected computers.
Results
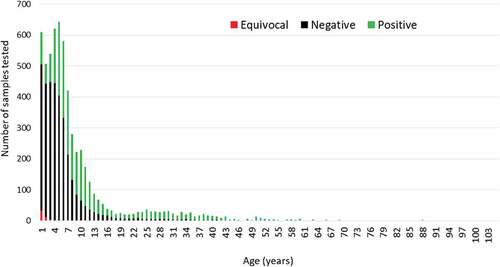
We retrieved 6216 eligible samples and obtained 6057 rubella IgG results. The sample volume was insufficient for 75 records while 82 had equivocal results. Gauteng province had the highest number of samples tested while Free State province had the fewest. Participant ages ranged from 1 month to 104 y with a median age of 5 y (). Overall 43% of individuals were immune (IgG positive) while 57% were susceptible (IgG negative)
Table 1. Demographic characteristics of samples included from 2016 through 2018 (N = 6216)
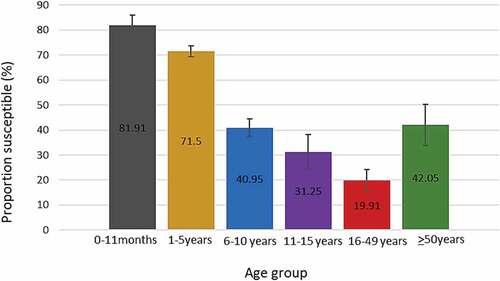
Individuals between 1 and 5 y of age represented the majority of participants and about 67% (55/82) of equivocal results were in this age group (). Rubella susceptibility was highest among Individuals aged zero to 11 months (81.9%), followed by children 1 to 5 y old (71.5%), 6 to 10 y old (40.9%) and 11 to 15 y old (31.25) while the smallest proportion of susceptible individuals was among those 16 to 49 y old (19.9%).
The proportion of susceptible individuals was higher amongst males (59.13%, 99%CI: 56.84–61.39) compared to females (53.31%, 99%CI: 50.86–55.75) (). Most susceptible individuals were 1 to 5 y old among males (62.11%) and females (58.36%), while the smallest proportion of susceptible individuals was the 16 to 49 y old age group for males (2.71%) and those 50 y and above (2.49%) for females (). Women of reproductive age (16–49 y) represented 5.39% of susceptible females.
Figure 2. Proportion of rubella susceptible (IgG negative) individuals among males and females. Error bars represent 99% confidence intervals
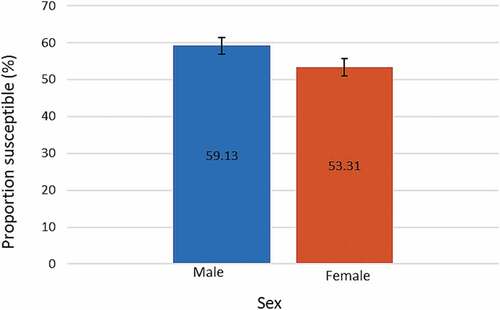
Figure 3. Proportion of rubella susceptible (IgG negative) individuals in each age group among males and females
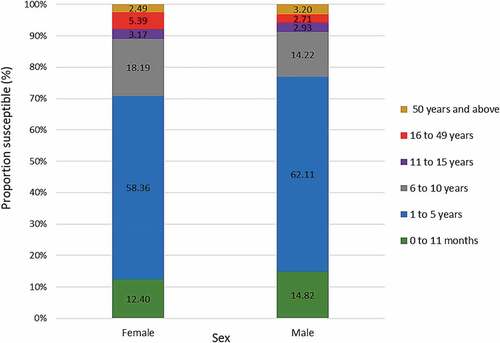
shows the proportion of susceptible individuals in each province in descending order. Susceptibility ranged from 48.07%, in North West province to 61.05% in Western Cape province. The proportion of susceptible individuals decreased with increasing age group except for individuals 50 y and above (). The highest proportion of susceptible individuals were children aged 0 to 11 months (81.91%) while individuals 16 to 49 y old had the lowest (about 19.91%).
Figure 4. Proportion of rubella susceptible (IgG negative) individuals for each province. Error bars represent 99% confidence intervals
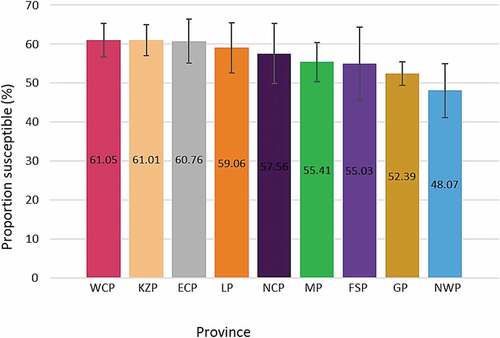
Figure 5. Proportion of rubella susceptible (IgG negative) individuals by age group. Error bars represent 99% confidence intervals
Risk factors associated with rubella susceptibility
shows the results of unadjusted (univariable) and adjusted (multivariable) logistic regression analyses. In unadjusted analysis female individuals were less likely to be susceptible compared to men (OR = 0.79, 95%CI: 0.71–0.87) but after adjusting for age group and province of residence, this association was no more observed (OR = 0.91, 95%CI: 0.81–1.01). Province of residence and age group were associated with rubella susceptibility in both unadjusted and adjusted analyses.
Table 2. Unadjusted and adjusted logistic regression analyses
Discussion
We present a cross-sectional snapshot of current immunity levels in South Africa, showing that about 57% of individuals are susceptible to rubella. We found a decrease in the proportion of susceptible individuals with increasing age. Age group, sex, and province were associated with rubella susceptibility in unadjusted analyses but only province of residence and age group remained associated with rubella susceptibility in multivariable analysis.
Several studies in Sub-Saharan Africa have described the predominance of rubella infections in childrenCitation22–25 and a recent analysis of rash-based surveillance data in South Africa revealed similar results.Citation26 Rubella infections occurring in childhood result in most individuals being immune by the time they are adolescents or adults. This translates to decrease in susceptibility with increasing age. This natural process of immunization leaves out a number of individuals who age into the reproductive age group while being susceptible. Introducing RCV should address this immunity gap, conditional on achieving coverage figures that are high enough.
Rubella susceptibility among individuals of reproductive age is an indication of the risk of CRS. Our estimates of rubella susceptibility are lower than those reported in individuals of reproductive ageCitation9,Citation10 and among pregnant womenCitation11,Citation27 in other Sub-Saharan countries prior to RCV introduction. This could be due to lower virus circulating in South Africa as a result of rubella vaccination in the private sector,Citation4 or due to differences in study design, especially the community-based sampling framework used in several of these studies. The unexpectedly high susceptibility among individuals aged 50 y and older could be explained by the small number of samples obtained from individuals in this age group, leading to biased estimates.
We observed an association between age and rubella susceptibility, which is a finding that is similar to several studies.Citation10–13,Citation27 This could be due to the nature of contacts between younger individuals that favor transmission of infections such as rubella when compared to contacts between older individuals.Citation28 Increased susceptibility to rubella among younger individuals coupled with evidence of increased rubella incidence in this age groupCitation22–24,Citation26 justifies targeting children below 15 y oldCitation29 for mass vaccination during RCV introduction rather than just infants in routine immunization activities. Following rubella vaccine introduction into the routine EPI schedule, the average age of infection is likely to increase and a similar study will be required in future to assess population immunity and adapt vaccination strategies to minimize the risk of CRS.
Although men were more likely to be susceptible to rubella in unadjusted analysis, we found no association between gender and susceptibility to rubella in adjusted analysis. Although females of reproductive age could be vaccinated as part of specific CRS control measures,Citation6 omitting males from vaccination activities could lead to a persistence of viral circulation which will eventually pose a risk to susceptible pregnant women.
The association between susceptibility to rubella and province of residence has limited impact on vaccine introduction since countries do not selectively introduce RCV in certain provinces leaving out others. The provinces with the highest proportion of susceptible individuals are among the most populated in South Africa.Citation30 However, Gauteng province, which has the highest number of individuals and is the smallest province in terms of surface area has the second-lowest proportion of susceptible individuals. This suggests that there are different drivers of rubella transmission in various provinces.
We did not match the age structure of our sample to that of the general population of South Africa since most samples for measles surveillance are collected from children. Most of the individuals in our sample were children under 10 y of age and it could be argued that this predominance of children influenced our estimates. Blaizot et al.Citation31 compared sampling structures and sample sizes for estimating epidemiological parameters from serological data for a number of infectious diseases, including rubella. They found that using our sampling approach which predominantly includes children would provide similar estimates compared to a sampling approach that represented the country’s population age structure or a sample with similar numbers of individuals from all age groups.
Our study has two limitations. Firstly, we used residual sera from individuals at public health facilities. This institution-based sampling could have influenced our results since it reflects health-seeking behavior of individuals included in the study. However, the facilities from which our samples were obtained include peripheral clinics and hospitals. Another limitation relates to the fact that samples from private health facilities were not included in the analysis. Given that RCV are available in the private sector, it is unclear to what extent the susceptibility profile of individuals using private health care differs from those using public health facilities.
The main strength of our study is the national representativeness of our sample. Given that we included samples from all provinces, our estimates are a reliable reflection of the situation in the general population. Another factor that contributes to the robustness of our results is the large sample size including residual sera from three consecutive years. This enabled us to report rubella seroprevalence with 99% confidence intervals thereby increasing the precision of our estimates.
Conclusion and recommendations
In the absence of rubella vaccination in the Expanded Program on Immunization in South Africa, the bulk of individuals susceptible to rubella are children under 16 y old. About 20% of individuals 16 to 49 y old are susceptible to rubella. This has an impact on the risk of congenital rubella syndrome since this group comprises most females of reproductive age. Age group and province of residence are associated with susceptibility to rubella. Although vaccine introduction is not likely to be a selective process with respect to provinces, any rollout strategy should be cognizant of the age-specific susceptibility profile.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (143 KB)Acknowledgments
The authors would like to acknowledge Emmanuel Phalane at the Centre for Vaccines and Immunology for his assistance during testing of samples.
Supplementary material
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2020.1738834..
Additional information
Funding
References
- World Health Organization. Global measles and rubella strategic plan: 2012–2020. [Internet]. 2012 [accessed 2017 Jan 23]. http://apps.who.int/iris/bitstream/10665/44855/1/9789241503396%5Feng.pdf
- Orenstein WA, Hinman A, Nkowane B, Olive JM, Reingold A. Measles and Rubella Global Strategic Plan 2012–2020 midterm review. Vaccine. 2018 Jan;36:A1–34. doi:10.1016/j.vaccine.2017.09.026.
- Endean C (ed.). Gavi Progress Report 2015. The Global Alliance for Vaccines and Immunization; 2015. Report No.: 2016.
- Motaze NV, Suchard M, Cohen C, Baker L, Blumberg L (eds), Vaccine information for parents & caregivers [Internet]. Johannesburg: Ideas Wise and Wonderful for National Institute for Communicable Diseases; 2016 [accessed 2018 Feb 15]. http://www.nicd.ac.za/assets/files/NICD_Vaccine_Booklet_D132_FINAL.pdf
- Organization WH, others. Immunological basis for immunization: rubella. 2008 [accessed 2017 Jan 22]. http://apps.who.int/iris/handle/10665/43922
- World Health Organization. Rubella vaccines: WHO position paper. Wkly Epidemiol Rec. 2011;29(86):301–16.
- Luce R, Masresha B, Katsande R, Fall A, Shibeshi M. The impact of recent rubella vaccine introduction in 5 countries in the African region. J Immunol Sci. 2018 Sep 1;2(SI1):108–12. doi:10.29245/2578-3009/2018/si.1116.
- World Health Organization, Expanded Programme on Immunization, World Health Organization, Department of Immunization V and B. Introducing rubella vaccine into national immunization programmes: a step-by-step guide [Internet]. 2015 [accessed 2017 May 18]. http://apps.who.int/iris/bitstream/10665/184174/1/9789241549370_eng.pdf
- Mirambo MM, Majigo M, Aboud S, Groß U, Mshana SE. Serological makers of rubella infection in Africa in the pre vaccination era: a systematic review. BMC Res Notes. 2015 Dec;8(1):716. doi:10.1186/s13104-015-1711-x.
- Hayford K, Mutembo S, Carcelen A, Matakala HK, Munachoonga P, Winter A, Wanyiri JW, Searle K, Mwansa FD, Mwiche A, et al. Measles and rubella serosurvey identifies rubella immunity gap in young adults of childbearing age in Zambia: the added value of nesting a serological survey within a post-campaign coverage evaluation survey. Vaccine. 2019 Apr;37(17):2387–93. doi:10.1016/j.vaccine.2019.02.037.
- Barreto J, Sacramento I, Robertson SE, Langa J, de Gourville E, Wolfson L, Schoub BD. Antenatal rubella serosurvey in Maputo, Mozambique: antenatal rubella serosurvey in maputo, mozambique. Trop Med Int Health. 2006 Apr;11(4):559–64. doi:10.1111/tmi.2006.11.issue-4.
- Poethko-Müller C, Mankertz A. Seroprevalence of measles-, mumps- and rubella-specific IgG antibodies in german children and adolescents and predictors for seronegativity. Borrow R, editor. PLoS ONE. 2012 Aug 6;7(8):e42867. doi:10.1371/journal.pone.0042867.
- Lebo EJ, Kruszon-Moran DM, Marin M, Bellini WJ, Schmid S, Bialek SR, Wallace GS, McLean HQ. Seroprevalence of measles, mumps, rubella and varicella antibodies in the united states population, 2009–2010. Open Forum Infect Dis. 2015 Feb 19;2(1):ofv006–ofv006. doi:10.1093/ofid/ofv006.
- Honarvar B, Moghadami M, Moattari A, Emami A, Odoomi N, Lankarani KB. Seroprevalence of anti-rubella and anti-measles IgG antibodies in pregnant women in Shiraz, Southern Iran: outcomes of a nationwide measles-rubella mass vaccination campaign. PLoS One. 2013;8(1):e55043. doi:10.1371/journal.pone.0055043.
- Vynnycky E, Adams EJ, Cutts FT, Reef SE, Navar AM, Simons E, Yoshida L-M, Brown DWJ, Jackson C, Strebel PM, et al. Using seroprevalence and immunisation coverage data to estimate the global burden of congenital rubella syndrome, 1996–2010: a systematic review. PLoS One. 2016;11(3):e0149160. doi:10.1371/journal.pone.0149160.
- Grenfell BT, Anderson RM. The estimation of age-related rates of infection from case notifications and serological data. J Hyg (Lond). 1985;95(2):419–36. doi:10.1017/S0022172400062859.
- Kinoshita R, Nishiura H. Assessing herd immunity against rubella in Japan: a retrospective seroepidemiological analysis of age-dependent transmission dynamics. BMJ Open. 2016;6(1):e009928. doi:10.1136/bmjopen-2015-009928.
- Ghafourian M, Shakunia A, SM A, Kooti W, Shakerinejad G, Serajian A, Chinipardaz Z. Seroepidemiology of rubella in women under 25 years old attending medical centers in Ahvaz, Iran in 2013. Jundishapur J Microbiol [Internet]. 2015 Dec 26;8(12). [accessed 2016 Apr 11]. http://www.jjmicrobiol.com/?page=article&article_id=27896
- Schoub BD, Harris BN, McAnerney J, Blumberg L. Rubella in South Africa: an impending Greek tragedy? SAMJ South Afr Med J. 2009;99:515–19.
- Corcoran C, Hardie D. Seroprevalence of rubella antibodies among antenatal patients in the Western Cape. S Afr Med J. 2005;95:688–90.
- Council for Medical Schemes Annual Report 2018/19 [Internet]. Council for Medical Schemes; 2019 [accessed 2019 Dec 10]. http://www.medicalschemes.com/files/Annual%20Reports/CMSAR2018_19.pdf
- Chimhuya S, Manangazira P, Mukaratirwa A, Nziramasanga P, Berejena C, Shonhai A, Kamupota M, Gerede R, Munyoro M, Mangwanya D, et al. Trends of rubella incidence during a 5-year period of case based surveillance in Zimbabwe. BMC Public Health. 2015 Dec;15(1):294. doi:10.1186/s12889-015-1642-4.
- Mirambo MM, Aboud S, Mushi MF, Seugendo M, Majigo M, Groß U, Mshana SE. Serological evidence of acute rubella infection among under-fives in Mwanza: a threat to increasing rates of congenital rubella syndrome in Tanzania. Ital J Pediatr. 2016 Dec;42(1):54. doi:10.1186/s13052-016-0264-5.
- Nimpa Mengouo M, Ndze VN, Baonga F, Kobela M, Wiysonge CS. Epidemiology of rubella infection in Cameroon: a 7-year experience of measles and rubella case-based surveillance, 2008–2014. BMJ Open. 2017 Apr;7(4):e012959. doi:10.1136/bmjopen-2016-012959.
- Njeru I, Onyango D, Ajack Y, Kiptoo E. Rubella outbreak in a Rural Kenyan District, 2014: documenting the need for routine rubella immunization in Kenya. BMC Infect Dis [Internet]. 2015 Dec;15(1). [accessed 2016 Mar 18]. http://www.biomedcentral.com/1471-2334/15/245
- Hong H, Makhathini L, Mashele M, Malfeld S, Motsamai T, Sikhosana L, Manamela J, Ntshoe G, Motaze NV, Smit S, et al. Annual measles and rubella surveillance review, South Africa. Commun Dis Surveill Bull. 2017;16(2):14.
- Tamirat B, Hussen S, Shimelis T. Rubella virus infection and associated factors among pregnant women attending the antenatal care clinics of public hospitals in Hawassa City, Southern Ethiopia: a cross-sectional study. BMJ Open. 2017 Oct;7(10):e016824. doi:10.1136/bmjopen-2017-016824.
- Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, Massari M, Salmaso S, Tomba GS, Wallinga J, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3):e74. doi:10.1371/journal.pmed.0050074.
- Global vaccine action plan. Vaccine. World Health Organization. 2013 Apr;31:B5–31. doi:10.1016/j.vaccine.2013.02.015.
- Statistics South Africa. Mid-year population estimates 2016 [Internet]. 2017 [accessed 2017 Mar 14]. https://www.statssa.gov.za/publications/P0302/P03022016.pdf
- Blaizot S, Herzog SA, Abrams S, Theeten H, Litzroth A, Hens N. Sample size calculation for estimating key epidemiological parameters using serological data and mathematical modelling. BMC Med Res Methodol. 2019 Dec;19(1):51. doi:10.1186/s12874-019-0692-1.