ABSTRACT
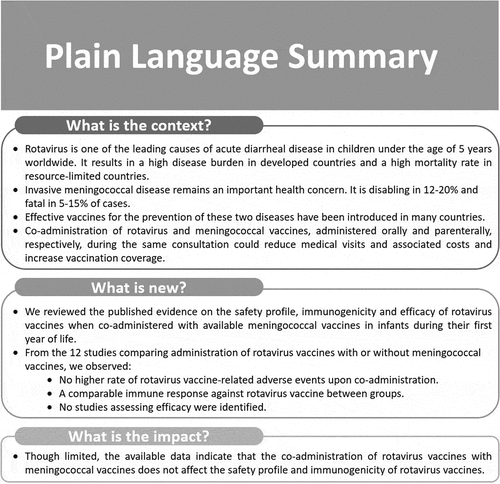
This study is aimed to review the published evidence on safety, immunogenicity, and efficacy of rotavirus vaccines when co-administered with meningococcal vaccines in infants. A systematic literature search was performed in four databases containing peer-reviewed articles and conference abstracts. In total, twelve articles were included in the review; 11 provided information on safety and five on the immunogenicity of rotavirus vaccines following co-administration. No paper was found on efficacy. Additional routine vaccines were administered in all studies. The safety analysis was mainly focused on fever, vomiting, diarrhea, intussusception, and changes in eating habits. Overall, safety profiles and immune responses associated with rotavirus vaccination were comparable between infants co-administered with rotavirus and meningococcal vaccines and infants receiving rotavirus vaccines without meningococcal vaccines. Although data are limited, co-administration of rotavirus and meningococcal vaccines does not appear to interfere with the safety or immunogenicity of rotavirus vaccines.
PLAIN LANGUAGE SUMMARY
What is the context?
Rotavirus is one of the leading causes of acute diarrheal disease in children under the age of 5 years worldwide. It results in a high disease burden in developed countries and a high mortality rate in resource-limited countries.
Invasive meningococcal disease remains an important health concern. It is disabling in 12-20% and fatal in 5-15% of cases.
Effective vaccines for the prevention of these two diseases have been introduced in many countries.
Co-administration of rotavirus and meningococcal vaccines, administered orally and parenterally, respectively, during the same consultation could reduce medical visits and associated costs and increase vaccination coverage.
What is new?
We reviewed the published evidence on the safety profile, immunogenicity and efficacy of rotavirus vaccines when co-administered with available meningococcal vaccines in infants during their first year of life.
From the 12 studies comparing administration of rotavirus vaccines with or without meningococcal vaccines, we observed:
No higher rate of rotavirus vaccine-related adverse events upon co-administration.
A comparable immune response against rotavirus vaccine between groups.
No studies assessing efficacy were identified.
What is the impact?
Though limited, the available data indicate that the co-administration of rotavirus vaccines wih meningococcal vaccines does not affect the safety profile and immunogenicity of rotavirus vaccines.
Introduction
Rotavirus (RV) is a common cause of severe and fatal acute diarrhea in young children throughout the world.Citation1 In the European Union, 300–600 children per 100,000 under the age of 5 years are hospitalized due to RV gastroenteritis (RVGE) annually.Citation2 Many countries worldwide have already included oral RV vaccines in their National Immunization Programs (NIP) as recommended by the World Health Organization (WHO).Citation3,Citation4 Following RV vaccine introduction, significant reduction in infant diarrheal deaths, RVGE-related hospitalization, and cases of RVGE was observed.Citation1 Currently, there are two globally available oral vaccines for the prevention of RVGE in the first year of life. The human live-attenuated RV vaccine containing G1P[8] strain (HRV, Rotarix) is administered as a two-dose schedule, with a minimum interval of 4 weeks between doses, the first dose being administered from 6 weeks of age. The schedule must be completed by 24 weeks of age.Citation5,Citation6 The human-bovine live-attenuated reassortant pentavalent RV vaccine (HBRV, RotaTeq) is administered as a three-dose regimen, with an interval of at least 4 weeks between doses, the first dose being administered between 6 and 12 weeks of age. The schedule must be completed by 32 weeks of age.Citation7,Citation8 Additionally, new live-attenuated naturally reassorted monovalent (nHRV, Rotavac) and human-bovine reassortant pentavalent (BRV-PV, Rotasiil) RV vaccines have been recently licensed.Citation9,Citation10 All these four vaccines are now WHO prequalified.Citation11
Invasive meningococcal disease (IMD), characterized by high mortality and morbidity, is mainly caused by six (MenA, MenB, MenC, MenW, MenY, and MenX) of the 12 serogroups of Neisseria meningitidis. Epidemiology of IMD varies considerably geographically, with socio-economic setting, and over time.Citation12 Different meningococcal vaccines are available to prevent IMD. Following recommendations of these vaccines in immunization programs, disease incidences decreased significantly in many countries worldwide.Citation13,Citation14 In young children, meningococcal vaccines are generally administered from 2 to 24 months of age: recombinant four-component MenB vaccine (4CMenB, Bexsero), Hib and MenC (Menitorix) and MenC and MenY (Hib-MenCY-TT, MenHibrix, discontinued in 2016) tetanus toxoid conjugated vaccines, tetravalent MenA, MenC, MenW-135, MenY diphtheria toxin (MenACWY-CRM, Menveo, and Menactra) and tetanus toxin conjugated vaccines (Nimenrix), MenA (MenAV, MenAfriVac) and MenC (MenCC, NeisVac-C) tetanus toxoid conjugated vaccines, and MenC diphtheria toxin conjugated vaccines (MenC-CRM, Meningitec, and Menjugate).Citation15
Since the vaccination schedules for RV and some meningococcal vaccines overlap, co-administration of these vaccines as part of the routine immunization is likely.Citation16,Citation17 Given the increased recommendations of meningococcal vaccines worldwide, a summary of data on the safety, immunogenicity, and efficacy of their co-administration with RV vaccines is of interest to demonstrate the compatibility of these vaccines. This evidence could more easily prompt healthcare practitioners to boost parental attitude toward vaccination and to co-administer the vaccines, thus increasing RV and meningococcal vaccine coverage amongst children <5 year-olds, while saving on costs and number of medical visits required to comply with the routine vaccination schedule.Citation18
Methods
The objectives of this systematic literature search were to identify the published worldwide evidence on the safety profile, immunogenicity, and efficacy of RV vaccines when co-administered (at the same clinical visit) with meningococcal vaccines in infants during the first year of life.
Identification, selection, and data extraction
The literature search was carried out in PubMed, Embase, Cochrane Library, and LILACS (Latin America) databases for peer-reviewed articles published between 1 January 2000 and 04 January (PubMed), 11 January (Embase), 13 February (Cochrane and LILACS) 2019. In addition, international conference abstracts published between 2014 and 2018, selected from the hits from the Embase search, were searched to identify studies not yet published in peer-reviewed journals. A gray literature search (03 March 2019) was also conducted to identify data for the remaining gaps. The websites of Pan American Health Organization (PAHO), Centers for Disease Control and Prevention (CDC), European Center for Disease Prevention and Control (ECDC), WHO, and regional agencies (National Health Service England and Ministries of Health of Spain, Brazil, and Italy) were searched for relevant data. In addition, an internet search was performed using the terms “rotavirus vaccines,” “meningococcal vaccines,” and “coadministration.” Search strings are provided in Supplementary Table S1.
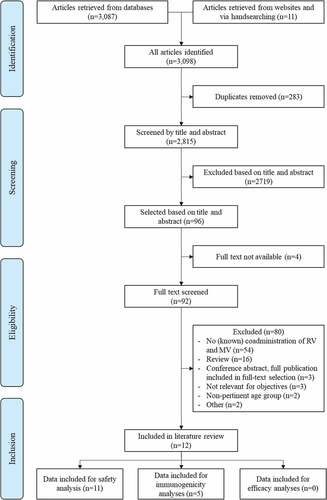
Relevant studies were selected by a three-step selection procedure checking against a list of inclusion and exclusion criteria (). In a first step, a screening of titles and abstracts was performed in duplicate by two epidemiologists and relevant papers were selected for full-text screening. In case of doubt, the article was included in the full-text screening step. In a second step, full-text papers were screened in duplicate by two epidemiologists. Critical appraisal of full-text articles was performed only if the article provided information for one of the review objectives. Further scrutiny of articles was carried out during the data-extraction step (third step) to identify any additional information that might be relevant to be included in the current assessment. The reference lists of selected articles, narratives, and systematic reviews were checked manually for potentially relevant studies. Data from the selected articles were summarized using a standardized data-extraction spreadsheet in Excel. If needed, percentages of safety and immunogenicity parameters were calculated from the original data. If the article referred to a registration number at clinicaltrials.gov, the website was checked and relevant data were extracted.
Table 1. Systematic literature review: inclusion and exclusion criteria during the selection phases
Study quality assessment
The original research articles eligible for data extraction were assessed using the SIGN checklists; this included criteria of the Cochrane checklists and the most important criteria of PRISMA and STROBE guidelines.Citation19 The studies were rated as “high” quality if there was little or no risk of bias and the results were not likely to be changed by further research, “acceptable” quality if some flaws in the study were present with an associated medium risk of bias and results may change in the light of further studies, and “low” quality if significant flaws associated with high risk of bias were present. The final decision whether the quality of a study was sufficient or not for inclusion was based on the expertise of one epidemiologist, keeping the results of the checklist and the objectives of the review in mind. In case of doubt, the methodological quality of the study was discussed with the second epidemiologist.
Results
In total, 3,098 references (including 342 conference abstracts) were retrieved from the databases and websites or identified by manual searching. Of the 2,815 unique records, twelve articles were finally included in the review (). No additional articles were found through the gray literature search. Study design and characteristics are presented in . Quality of selected studies was mostly “acceptable.” After the initial screening, seven conference abstracts were selected for in-depth analysis. Three eligible abstracts referred to data published in full reports; all three reports were added to the full-text screening. The remaining four abstracts were considered not relevant with respect to objectives or did not report data on co-administration of RV and meningococcal vaccines. Eleven studies provided information on the safety profile of co-administration of RV vaccines and meningococcal vaccinesCitation20-30 and five studies reported data on immunogenicity.Citation21,Citation22,Citation24,Citation29,Citation31 No studies presented data on vaccine efficacy.
Table 2. Description of studies included in the literature review
Safety
Co-administration of RV and meningococcal vaccines compared to sequential administration
One randomized trial evaluated the co-administration of the HBRV vaccine and a tetanus toxoid conjugate MenCC vaccine (at 10–11 and 20–21 weeks of age, Group 1) compared to their sequential administration at alternating visits, at least four weeks apart (HBRV administered at 6–7 and 15–16 weeks of age, Group 2) in healthy infants aged 6–7 weeks.Citation29 The third dose of HBRV was administered at 24–25 weeks of age in both groups. Both groups received additional routine vaccines (Supplementary Table S2). In the first 6 days after the first co-administered dose, proportion of participants with a solicited systemic adverse events (AEs) in Group 1 was 23.3% (diarrhea), 19.8% (vomiting), and 8.6% (fever), while 44.0% was assessed as related to HBRV (). In the 13-day period after their second dose, 42.6% of the children in Group 1 experienced at least one systemic AE related to HBRV. In Group 2, none of the systemic AEs were reported by >10% of the participants during the 13-day period following the second and third HBRV doses. Two serious adverse events (SAEs) occurred, one in each group: an episode of epilepsy of moderate intensity, starting 13 days after the second vaccination (Group 1) and a severe viral infection, starting 9 days after the third vaccination (Group 2). These events were considered not related to either study vaccine by the study investigators. One infant in each group experienced non-serious, mild hematochezia that was considered related to HBRV in Group 1 and to MenCC in Group 2.
Table 3. Systemic adverse events (%) related to co-administration of HBRV and MenCC or their sequential administrationCitation28.
Routine vaccination including co-administration of RV and meningococcal vaccines compared to routine vaccines including RV without meningococcal vaccines
Five phase III clinical studies investigated the co-administration of HRV or HBRV vaccines and conjugated meningococcal vaccines as part of routine immunization compared to vaccination without meningococcal vaccine, but including RV vaccines.Citation22,Citation25-28 The results could not be stratified for the AEs related to RV vaccines as other routine vaccines were also administered in all studies. We focused on diarrhea, vomiting, intussusception, change in eating habits, and fever ().
Table 4. SAE and a selection of solicited systemic adverse events (AEs) during concomitant administration of rotavirus vaccine and meningococcal vaccines plus other routine vaccines or individual administration of rotavirus vaccine plus other routine vaccines (n = 5 studies)
One phase 3 study assessed the immunogenicity and safety of 3 + 1 doses of Hib-MenCY-TT vaccine when co-administered with routine vaccines including HRV.Citation22 In the co-administration group, enrolled infants received 2 doses of HRV at 2 and 4 months of age and 3 primary doses of Hib-MenCY-TT at 2, 4, and 6 months of age and a booster dose at 12–15 months of age, along with other routine vaccines. In the control group, infants were administered with 2 HRV doses at 2 and 4 months of age, Hib vaccine without a meningococcal component at 2, 4, and 12–15 months of age, and other routine vaccines. Fever was reported by 41.8% of infants in the co-administration group and 48.5% in the control group; vomiting was reported by 4.7% and 6.6% of participants, diarrhea by 8.8% and 5.9%, change in eating habits by 59.6% and 66.0% of participants, respectively (). No cases of intussusception were reported. None of the reported SAEs was assessed as vaccination-related by the investigator.Citation22
Three articles were identified on HBRV.Citation25,Citation26,Citation28 Immunogenicity and safety of MenACWY-CRM, Menveo co-administered with routine vaccines including HBRV was assessed in a large phase 3 study conducted in the United States (US)Citation28 and in Colombia and Argentina.Citation26 After three infant doses of MenACWY-CRM and three HBRV doses at 2, 4, and 6 months of age in US infants, fever was reported by 5%, vomiting by 5%, diarrhea by 7%, and change in eating habits by 15% of study participants.Citation28 When the same 3-dose vaccination schedule was evaluated in Colombia and Argentina, the incidence of solicited systemic AEs was 13.3% (fever), 14.2% (vomiting), 15.4% (diarrhea), and 17.1% (change in eating habits) after the first vaccination (at month 2).Citation26 Co-administration of two primary doses of MenACWY-CRM (at 2 and 6 months of age) with 3 HBRV doses was also assessed in the Latin American study population. At month 2, fever, vomiting, diarrhea, and change of eating habits were reported by 5.0%, 8.3%, 14.0%, and 12.3% of participants, respectively.Citation26 In both studies, comparable results were obtained in the control group who received routine vaccines without MenACWY-CRM. In the US population, three SAEs reported in the co-administration group were considered to be at least possibly related to vaccination: Kawasaki disease (29 days after the third dose), partial complex seizures (31 days after the second dose), and two episodes of febrile convulsions (8 and 29 days after the third dose).Citation28
In the third article assessing the co-administration with HBRV, three doses of hexavalent combination vaccine (DTaP-IPV-HB-PRP-T, Hexaxim, at 2, 3, and 4 months of age) and two doses of MenCC vaccines (at 2 and 4 months of age) (Group 1) were compared to three doses of DTaP-IPV-HB-PRP-T vaccine without MenCC (Group 2). HBRV was administered in both groups at 2, 3, and 4 months of age together with 13-valent pneumococcal conjugate vaccine (PCV13, Prevenar, two doses at 2 and 4 months of age).Citation25 Fever, vomiting, diarrhea, and change in eating habits were reported by 72.4%, 36.2%, 6.3%, and 56.3% of participants, respectively, in Group 1. Comparable values were obtained in Group 2 (). One participant in Group 1 experienced an SAE (fever ≤39.5°C on the day of first vaccination) that was considered related to study vaccines. No cases of intussusception were reported in any of the three articles.
The fifth study in this category compared the immunogenicity and safety of three (ACWY3 group, at 2, 4 and 12 months of age) or four doses (ACWY4 group, at 2, 4, 6 and 12 months of age) of MenACWY-CRM when co-administered with routine vaccines, including an RV vaccine.Citation27 The control group received routine vaccines without MenACWY-CRM. Reactogenicity and safety profiles for all study groups were comparable, fever being reported by 23.1% (ACWY3), 21.8% (ACWY4), and 17.9% (control) of participants, vomiting by 28.6%, 21.8%, and 23.6%, diarrhea by 37.4%, 28.9%, and 29.7%, and change in eating habits by 43.7%, 43.7%, and 40.6% of participants, respectively.Citation27 No cases of intussusception or vaccination-related SAEs were reported.
Additionally to the five aforementioned phase III clinical studies, safety data on the concomitant administration of 4CMenB and HRV were retrieved from a prospective surveillance study conducted among children up to 18 months of age who received 4CMenB as part of the NIP in the United Kingdom (UK).Citation30 The study revealed no significant safety concerns within 20 months following 4CMenB introduction. Evaluation of immunization records for HRV vaccine collected before and after 4CMenB introduction suggested that 4CMenB reactogenicity did not affect compliance with the recommended vaccination schedule, including the administration of HRV.Citation30
Co-administration of RV and meningococcal vaccines, no comparative group
Four studies examined the co-administration of HRV or HBRV and meningococcal vaccines, however, no comparison was made with the administration of RV vaccines without meningococcal vaccines.Citation20,Citation21,Citation23,Citation24 Additional vaccines were also provided in all four studies as part of the local immunization program.
Two of the four studies were conducted in Spain to assess the immunogenicity and safety of several hexavalent and pentavalent combination vaccines administered in a mixed primary series concomitantly with other recommended vaccines.Citation20,Citation24 In a phase 3, single-arm study, the mixed 2-4-6-month schedule of hexavalent DTaP5-HB-IPV-Hib (Vaxelis) and pentavalent DTaP5-IPV-Hib (Pediacel) vaccines was co-administered with three HBRV doses (at 2, 4, 6 months of age) and MenCC and PCV13 vaccines (at 2 and 4 months of age). Solicited vaccine-related systemic AEs within 1–5 days following vaccinations at 2, 4, and 6 months of age were reported in 73.5%, 59.0%, and 42.7% of children, respectively. Of them, fever was reported in 4.9%, 6.2%, and 3.6%, vomiting in 12.7%, 8.1%, and 6.5%, and changing in eating habits in 36.6%, 22.9%, and 17.7% of participants.Citation20
The second Spanish open-label study assessed the co-administration of HBRV (2, 4, 6 months of age), MenCC (2 months of age) and PCV13 (2, 4, 6 months of age) with DTaP-IPV-HB-PRP-T (Hexaxim) and DTaP-IPV//PRP-T (Pentaxim) (2, 4, 6 months of age). Any solicited systemic AE was reported in 97.4% of children, including 17.4% severe (grade 3) systemic AEs. Fever and vomiting were reported in 58.9% and 35.5% of children during the first 7 days after vaccination.Citation24 There were no SAEs that were considered related to the study vaccines in either studies.
Although HBRV is recommended as a 3-dose primary vaccination series in infants from 6 weeks to 32 weeks of age, an additional HBRV dose co-administered with MenAV, measles, and yellow fever vaccines in 9–11-month-old infants was evaluated in Mali.Citation21 Gastrointestinal illness was reported in 13.0% of the participants, including vomiting, diarrhea, or gastroenteritis, within 28 days after vaccination. No vaccination-related SAE and no cases of intussusception were reported.Citation21
A review of the 4CMenB clinical development program reported results from a pooled sub-group analysis of two pivotal trials (5,515 participants) in which 303 infants had received at least one dose of RV vaccine (HRV or HBRV according to local recommendations) concomitantly with 4CMenB and other recommended vaccines.Citation23 The study revealed comparable reactogenicity profiles in infants who did or did not receive RV vaccine. A systemic AE was reported for 80.5% of infants in the co-administration group and 75.3% of infants not receiving RV vaccines. Severe systemic reactions were recorded for 19.5% and 24.7% of participants, respectively. Frequency of fever cases was also comparable between group, high fever (≥39.5°C) being reported by 2.2–4.2% in the co-administration group and 2.6–4.5% in the group not receiving RV vaccines.Citation23
Although letters to the editor were excluded from this systematic literature review (see ), one of them was considered to contain relevant safety information and is described below. The letter reported solicited and unsolicited AEs from a study conducted in hospitalized preterm infants who received HRV vaccine either before or after the introduction of the 4CMenB vaccination into the NIP in the UK.Citation32 Of 17 infants who completed the study, 8 received the 4CMenB vaccine. Overall, 4CMenB vaccine recipients were significantly more likely to have any temperature instability compared to infants receiving HRV without 4CMenB (50% versus 0%, P = .029). Decreased feeding and reduced activity were more common in the 4CMenB group, whereas irritability and crying occurred more frequently in the infants who did not receive the 4CMenB vaccine.
Immunogenicity
Immunogenicity results were identified in five studies.Citation21,Citation22,Citation24,Citation29,Citation31 Geometric mean concentrations and titers are summarized in , seroconversion and seroresponse rates are presented in . Seroconversion rate was defined as the percentage of infants with anti-RV immunoglobulin A and G (IgA and IgG) ≥20 U/mL post-vaccination (measured by enzyme-linked immunosorbent assay) who had antibody titer below this threshold pre-vaccination. Seroresponse rates were defined as at least threefold increase in IgA response from pre- to post-vaccination. Two studies did not present data for a comparator group with individual RV vaccination.Citation21,Citation24
Table 5. Rotavirus immunogenicity measured as geometric mean titer or geometric mean concentration (n = 4 studies)
Table 6. Seroconversion and seroresponse rates following rotavirus and meningococcal vaccination (n = 5 studies)
In a phase 3b, placebo-controlled study conducted across six European countries (the Czech Republic, Finland, France, Germany, Italy, and Spain), HRV or placebo were co-administered with routine vaccines recommended in the respective countries. Immunogenicity results were available from 794 infants in the HRV group and 422 infants in the placebo group. Spain was the only country administering a meningococcal vaccine (MenC-CRM, Meningitec) during routine immunizations, thus, descriptive results for Spain compared to other countries are provided. The seroconversion rate was 85.5% after co-administration of HRV and MenC-CRM in Spain and ranged from 82.1% to 94.6% after administration of HRV in other countries ().Citation31
Another study conducted in the US demonstrated non-inferiority of two primary doses of HRV co-administered with Hib-MenCY-TT and other recommended vaccines compared to similar vaccination series without meningococcal vaccine in terms of anti-RV IgA antibody concentrations.Citation22 Two months post-vaccination, anti-RV IgA concentrations ≥20 U/mL were detected in 81.3% of infants in the co-administration group and 80.1% in those receiving HRV without meningococcal vaccineCitation22 ().
Immunogenicity data for co-administration of HBRV and MenCC were retrieved from two studies.Citation24,Citation29 Seroconversion rates were 88.4% in the co-administration group in one study (with no comparative group)Citation24 while in the second study, seroresponse rates were 96.9% in the co-administration group and 98.1% in the group receiving the vaccines sequentiallyCitation29 ().
The study evaluating the immunogenicity of an additional HBRV dose co-administered with MenAV vaccine in children who completed the recommended 3-dose primary HBRV vaccination schedule reported seroconversion rates for 56.9% (anti-RV IgA) and 83.5% (anti-RV IgG) of participants and seroresponse rates for 44.9% and 57.3%, respectively ().Citation21
Efficacy
No studies reporting efficacy results of RV vaccination and meningococcal vaccination were identified by this systematic review.
Discussion
This systematic review aimed to retrieve and summarize the currently available evidence on the safety profile, immunogenicity, and efficacy of RV vaccines when co-administered with available meningococcal vaccines in infants. The limited number of studies presenting a comparative evaluation of RV vaccines when administered with or without meningococcal vaccines, as well as the heterogeneity in study designs and local immunization programs led to a descriptive analysis of the data without applying meta-analysis methods. Other routine injectable vaccines were also administered in all studies, thus no direct comparison between RV vaccine alone and RV and meningococcal vaccines together could be made. However, the concomitant administration of other routine vaccines is representative of the current vaccination practice. The quality of studies included was generally acceptable. In most of the studies, investigators were not blinded for the study allocation groups, which could be a limitation in the perspective of the assessment of AEs. Our search identified only one study with immunogenicity data in low- and middle-income countries. This is considered another limitation since the disease burden of both RV and meningitis is high in this setting.Citation33
Safety analysis was mainly focused on AEs that might be associated with RV vaccination, such as fever, diarrhea, vomiting, change in eating habits, and intussusception, and found no new safety concerns upon co-administration with meningococcal vaccines. In the only study comparing directly the co-administration of HBRV and MenCC vaccines with their sequential administration, the incidence of gastrointestinal symptoms tended to be lower in the sequential group than in the co-administration group.Citation29 Although the difference was not considered clinically significant, the authors point out that the convenience of co-administration of HBRV and MenCC may outweigh the slight increase in risk of mild diarrhea and mild vomiting associated with co-administration.Citation29 In studies comparing routine co-administration of RV and meningococcal vaccines with vaccination schedules without meningococcal vaccines, similar rates of solicited systemic AEs were found.Citation22 ,Citation25-28,Citation30 Studies reporting safety data for co-administration of RV and 4CMenB vaccines were mainly focused on 4CMenB-related safety aspects, but reactogenicity of 4CMenB was not impacted by the concomitant administration of RV vaccines, supporting their concomitant use in routine practice.Citation23,Citation30 Following the introduction of 4CMenB to the NIP in the UK in 2015, no significant safety concerns were observed.Citation30
There is a theoretical concern that the co-administration of multiple antigens may lead to immune interference.Citation34 The reviewed literature revealed that anti-RV IgA response rates were comparable between groups of children receiving RV and meningococcal vaccines versus those receiving RV vaccines alone or subsequently, regardless of the administration of other routine vaccines.Citation22,Citation29,Citation31 Moreover, serotype-specific rotavirus neutralizing antibody response rates were similar for children receiving HBRV and MenCC either concomitantly or separately.Citation29 This suggests the absence of immune interference, but might also be explained by the differences in the route of administration (oral for RV vaccines versus intramuscular for meningococcal vaccines) along with vaccine type and composition.Citation35,Citation36 Only one study reported immunogenicity data for 4CMenB when co-administered with RV. Immune responses of 4CMenB did not appear to interfere with the immunogenicity of any of the vaccines tested, including RV.Citation23
Oral poliovirus vaccine (OPV), the only oral vaccine commonly co-administered with RV vaccine, may slightly reduce the immune response to RV vaccine, but clinical protection against severe RVGE is maintained.Citation37,Citation38 Since OPV was not administered in any of the studies included in this review, we could not evaluate the co-administration of RV vaccines, meningococcal vaccines, and OPV.
Worldwide, several national and international agencies recommend the co-administration of RV vaccines with other infant vaccines against diphtheria, tetanus, pertussis, Hib, poliovirus, Hepatitis B, Pneumococcal, and Meningococcal disease.Citation2,Citation16,Citation17 Co-administration of vaccines could contribute to better vaccine coverage and could reduce medical visits and associated costs. It also helps to provide protection for children during the vulnerable early months of their lives.
A summary contextualizing the results and their potential clinical relevance is provided in to assist communications to the parents.
Conclusions
Despite the limited data, co-administration of RV and meningococcal vaccines does not appear to interfere with the safety or immunogenicity of the two globally available RV vaccines.
Disclosure of potential conflicts of interest
PP, BB and LM are employees of the GSK groups of companies. PP and BB hold shares in the GSK group of companies.
Trademark statement
Rotarix, Menjugate, Menveo, MenHibrix, and Bexsero are trademarks owned by the GSK group of companies. RotaTeq is a registered trademark of Merck&Co, Inc. Rotavac is a registered trademark of Bharat Biotech. Rotasiil and MenAfriVac are registered trademarks of Serum Institute of India Ltd. NeisVac-C is a registered trademark of Baxter International Inc. Meningitec is a registered trademark of Nuron Biotech. Menactra is a registered trademark of Sanofi.
Author contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. All authors reviewed and commented critically drafts of the manuscript for important intellectual content and gave final approval to submit for publication. The corresponding author had final responsibility to submit for publication. Drafts were developed by a professional publication writer according to the recommendations and documentation. All authors were involved in the study conception and data interpretation. PP managed the collection and generation of the study data and performed the study and PP and BB provided materials.
Supplemental Material
Download MS Word (57.1 KB)Acknowledgments
The authors acknowledge Pallas for the systematic literature search development. Authors thank the Modis platform for editorial assistance and manuscript coordination, on behalf of GSK. Botond Nagy provided medical writing support and Julie Mellery coordinated the manuscript development and editorial support.
SUPPLEMENTARY MATERIAL
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2020.1739485
Additional information
Funding
References
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62(Suppl 2):S96–S105. doi:10.1093/cid/civ1013.
- ECDC. Expert opinion on rotavirus vaccination in infancy; 2017 [accessed 2019 Sept 24]. https://ecdc.europa.eu/en/publications-data/expert-opinion-rotavirus-vaccination-infancy#no-link
- Rotavirus vaccines. WHO position paper; 2013 January [accessed 2019 Sept 24]. https://www.who.int/wer/2013/wer8805.pdf
- WHO. Statement on risks and benefits of rotavirus vaccines Rotarix and RotaTeq; 2019 [accessed 2019 Sept 10]. https://www.who.int/vaccine_safety/committee/topics/rotavirus/rotarix_and_rotateq/statement_May_2015/en/
- European public assessment report (EPAR) summary for Rotarix; [accessed 2019 Sept 10]. https://www.ema.europa.eu/en/medicines/human/EPAR/rotarix.
- Prescribing information for Rotarix; [accessed 2019 Sept 10]. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Rotarix/pdf/ROTARIX-PI-PIL.PDF
- European public assessment report (EPAR) summary for Rotateq; [accessed 2019 Sept 10]. https://www.ema.europa.eu/en/medicines/human/EPAR/rotateq.
- Prescribing information for Rotateq; [accessed 2019 Sept 10]. https://www.merck.com/product/usa/pi_circulars/r/rotateq/rotateq_pi.pdf
- WHO prequalified vaccines: Rotavac; [accessed 2019 Sept 10]. https://extranet.who.int/gavi/PQ_Web/PreviewVaccine.aspx?nav=0&ID=314
- WHO prequalified vaccines: Rotasiil; [accessed 2019 Sept 10]. https://extranet.who.int/gavi/PQ_Web/PreviewVaccine.aspx?nav=0&ID=355&AspxAutoDetectCookieSupport=1
- WHO prequalified vaccines listing; 2019 Sept 18. [accessed 2019 Sept 24]. https://extranet.who.int/gavi/PQ_Web/.
- Borrow R, Alarcon P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echaniz-Aviles G, Findlow J, Head C, et al. The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16:313–28. doi:10.1080/14760584.2017.1258308.
- Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59:S3–S11. doi:10.1016/j.jadohealth.2016.04.012.
- Burman C, Serra L, Nuttens C, Presa J, Balmer P, York L. Meningococcal disease in adolescents and young adults: a review of the rationale for prevention through vaccination. Hum Vaccin Immunother. 2019;15:459–69. doi:10.1080/21645515.2018.1528831.
- WHO vaccine-preventable diseases: monitoring system. global summary; 2019. http://apps.who.int/immunization_monitoring/globalsummary/schedules
- Public Health England, UK immunisation schedule: the green book, chapter 11; September 2019 [accessed 2019 Nov 05]. https://www.gov.uk/government/publications/immunisation-schedule-the-green-book-chapter-11.
- Moreno-Pérez D, Álvarez García FJ, Álvarez Aldeán J, Cilleruelo Ortega MJ, Garcés Sánchez M, García Sánchez N, Hernández Merino Á, Méndez Hernández M, M MM, Montesdeoca Melián A, et al. Immunisation schedule of the Spanish association of paediatrics: 2019 recommendations. Anales De Pediatría (English Edition). 2019;90:56.e51–56.e59. doi:10.1016/j.anpede.2018.11.001.
- Smith LE, Amlôt R, Weinman J, Yiend J, Rubin GJ. A systematic review of factors affecting vaccine uptake in young children. Vaccine. 2017;35:6059–69. doi:10.1016/j.vaccine.2017.09.046.
- Scottish intercollegiate guidelines network, critical appraisal notes and checklists; [accessed 2019 Sept 10]. https://www.sign.ac.uk/checklists-and-notes.html
- Martinon-Torres F, Boisnard F, Thomas S, Sadorge C, Borrow R. Immunogenicity and safety of a new hexavalent vaccine (DTaP5-IPV-HB-Hib) administered in a mixed primary series schedule with a pentavalent vaccine (DTaP5-IPV-Hib). Vaccine. 2017;35:3764–72. doi:10.1016/j.vaccine.2017.05.043.
- Haidara FC, Tapia MD, Sow SO, Doumbia M, Coulibaly F, Diallo F, Traore A, Kodio M, Kelly CL, Fitzpatrick M, et al. Evaluation of a booster dose of pentavalent rotavirus vaccine coadministered with measles, yellow fever, and meningitis a vaccines in 9-month-old malian infants. J Infect Dis. 2018;218:606–13. doi:10.1093/infdis/jiy215.
- Klein NP, Abu-Elyazeed R, Baine Y, Cheuvart B, Silerova M, Mesaros N. Immunogenicity and safety of the Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine co-administered with human rotavirus, hepatitis A and 13-valent pneumococcal conjugate vaccines: results from a phase III, randomized, multicenter study in infants. Hum Vaccin Immunother. 2019;15:327–38. doi:10.1080/21645515.2018.1526586.
- O’Ryan M, Stoddard J, Toneatto D, Wassil J, Dull PM. A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program. Drugs. 2014;74:15–30. doi:10.1007/s40265-013-0155-7.
- Martinon-Torres F, Diez-Domingo J, Feroldi E, Jordanov E, B’Chir S, Da Costa X. Evaluation of a hexavalent-pentavalent-hexavalent infant primary vaccination series followed by a pentavalent booster vaccine in healthy infants and toddlers. Pediatr Infect Dis J. 2019;38:317–22. doi:10.1097/inf.0000000000002231.
- Vesikari T, Borrow R, Da Costa X, Richard P, Eymin C, Boisnard F, Lockhart S. Concomitant administration of a fully liquid, ready-to-use DTaP-IPV-HB-PRP-T hexavalent vaccine with a meningococcal serogroup C conjugate vaccine in infants. Vaccine. 2017;35:452–58. doi:10.1016/j.vaccine.2016.11.053.
- Tregnaghi M, Lopez P, Stamboulian D, Grana G, Odrljin T, Bedell L, Dull PM. Immunogenicity and safety of a quadrivalent meningococcal polysaccharide CRM conjugate vaccine in infants and toddlers. Int J Infect Dis. 2014;26:22–30. doi:10.1016/j.ijid.2014.03.1390.
- Block SL, Shepard J, Garfield H, Xie F, Han L, Dull PM, Smolenov I. Immunogenicity and safety of a 3- and 4-dose vaccination series of a meningococcal ACWY conjugate vaccine in infants: results of a phase 3b, randomized, open-label trial. Pediatr Infect Dis J. 2016;35:e48–59. doi:10.1097/inf.0000000000000965.
- Klein NP, Reisinger KS, Johnston W, Odrljin T, Gill CJ, Bedell L, Dull P. Safety and immunogenicity of a novel quadrivalent meningococcal CRM-conjugate vaccine given concomitantly with routine vaccinations in infants. Pediatr Infect Dis J. 2012;31:64–71. doi:10.1097/INF.0b013e31823dce5c.
- Vesikari T, Karvonen A, Borrow R, Kitchin N, Baudin M, Thomas S, Fiquet A. Results from a randomized clinical trial of coadministration of RotaTeq, a pentavalent rotavirus vaccine, and NeisVac-C, a meningococcal serogroup C conjugate vaccine. Clin Vaccine Immunol. 2011;18:878–84. doi:10.1128/cvi.00437-10.
- Bryan P, Seabroke S, Wong J, Donegan K, Webb E, Goldsmith C, Vipond C, Feavers I. Safety of multicomponent meningococcal group B vaccine (4CMenB) in routine infant immunisation in the UK: a prospective surveillance study. Lancet Child Adolesc Health. 2018;2:395–403. doi:10.1016/s2352-4642(18)30103-2.
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Thollot F, Garcia-Corbeira P, Damaso S, Han HH, Bouckenooghe A. Immunogenicity and safety of the human rotavirus vaccine Rotarix co-administered with routine infant vaccines following the vaccination schedules in Europe. Vaccine. 2010;28:5272–79. doi:10.1016/j.vaccine.2010.05.057.
- Sadarangani M, Barlow S, Anthony M, Pollard AJ. Four component meningococcal capsular group B vaccine in preterm infants. Pediatr Infect Dis J. 2017;6:309–10. doi:10.1093/jpids/pix013.
- GBD Meningitis Collaborators.Global, regional, and national burden of meningitis, 1990–2016: a systematic analysis for the Global burden of disease study 2016. Lancet Neurol. 2016;2018(17):1061–82. doi:10.1016/s1474-4422(18)30387-9.
- Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: A review. Vaccine. 2010;28:5513–23. doi:10.1016/j.vaccine.2010.06.026.
- CDC, Principles of vaccination - immunology and vaccine-preventable diseases; [accessed 2019 Sept 10]. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/prinvac.pdf
- Zhang L, Wang W, Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev Vaccines. 2015;14:1509–23. doi:10.1586/14760584.2015.1081067.
- Emperador DM, Velasquez DE, Estivariz CF, Lopman B, Jiang B, Parashar U, Anand A, Zaman K. Interference of monovalent, bivalent, and trivalent oral poliovirus vaccines on monovalent rotavirus vaccine immunogenicity in rural Bangladesh. Clin Infect Dis. 2015;62:150–56. doi:10.1093/cid/civ807.
- Tregnaghi MW, Abate HJ, Valencia A, Lopez P, Da STR, Rivera L, Rivera Medina DM, Saez-Llorens X, Gonzalez ASE, De LT, et al. Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J. 2011;30:e103–108. doi:10.1097/INF.0b013e3182138278.