ABSTRACT
The varicella vaccine passage extension (VAR-PE) process was undertaken to extend the availability of varicella zoster virus (VZV)-containing vaccines. This study (V210-A03; NCT03239873) assessed the immunogenicity, safety, and tolerability of VAR-PE process in comparison with varicella vaccine commercial product 2016 (VAR) randomized 1:1 in 600 healthy children 12 to 23 months of age administered concomitantly with measles-mumps-rubella (MMR) vaccine. The VZV seroconversion rate at 6 weeks Postdose 1 in the PP population was 100% for both groups. VZV antibody response rates and GMTs of VZV antibodies to VAR-PE induced and were non-inferior to those induced by VAR 6 weeks Postdose 1. From Day 1 through Day 42, adverse events (AEs) were reported by 81.3% of participants Postdose 1 and 67.9% Postdose 2. From Day 1 through Day 42 Postdose 1, injection-site AEs related to varicella vaccine were reported by 31.1% and 29.7% of participants in VAR-PE and VAR, respectively, and Postdose 2, by 25.7% and 25.5% of participants in the VAR-PE and VAR groups, respectively. Systemic AEs were generally comparable for the 2 vaccination groups, with the exception of pyrexia and otitis media higher in VAR-PE, and diarrhea and teething higher in VAR. The incidence of systemic AEs was generally lower Postdose 2 compared with Postdose 1.
Introduction
Varicella (chickenpox) is a common and highly contagious childhood infectious disease caused by primary infection with varicella zoster virus (VZV), with seasonal occurrence and peak incidence in temperate climates during late winter and early spring.Citation1 In the prevaccination era, 90% of the cases occurred in children 1 to 14 years of age, with 60% of these cases among children 5 to 9 years, and during this time it was estimated that varicella resulted in ~10,000 hospitalizations and 100 deaths per year, primarily in immunocompetent children and adults.Citation2 Typically, varicella is a self-limiting illness characterized by fever, malaise, and generalized papulovesicular rash, with subsequent crusting and resolution over 5 to 6 days.Citation1,Citation2 During primary infection, VZV establishes latent infection in the dorsal root ganglia; reactivation may result in the development of herpes zoster.Citation1,Citation2 The most common complication is bacterial superinfection of skin lesions; less common though more severe complications include viral or bacterial pneumonia, septic shock, secondary bacterial arthritis and fasciitis, thrombocytopenia, nephritis, uveitis, orchitis, purpura fulminans, and Reye Syndrome.Citation3–6
Varicella vaccine (VARIVAX™: Varicella Virus Vaccine Live [Oka/Merck], Merck & Co., Inc., Kenilworth, NJ, USA) was first licensed in the US in 1995. Following licensure, vaccine coverage has increased to an estimated 90% of the pediatric population in the US, thereby reducing varicella incidence by up to 91.6% and varicella-related hospitalizations by 75%–88%.Citation7–10 During the first 12 years after licensure of varicella vaccine in the US, varicella-related mortality decreased by 88%, including a 97% reduction in individuals younger than 20 years of age, and a 96% reduction in individuals younger than 50 years of age.Citation11 Additionally, epidemiologic data have demonstrated that varicella vaccine not only affords long-term protection over 15 years against varicella, but also imparts community protection to unvaccinated individuals in settings where vaccine adoption is widespread.Citation12,Citation13
Following the recommendation of a 2-dose varicella vaccination schedule by the Advisory Committee on Immunization Practices, and the approval of other VZV-containing products (ZOSTAVAX™ and ProQuad™, Merck & Co., Inc., Kenilworth, NJ, USA), the demand for VZV-containing vaccines has increased. The varicella vaccine passage extension (VAR-PE) process will extend the availability of VZV (Oka/Merck)-containing vaccines and is not expected to change the safety profile or effectiveness of the vaccine. The purpose of this Phase 3, randomized, double-blind, multicenter, controlled study (V210-A03; NCT03239873) was to assess the immunogenicity, safety, and tolerability of VAR-PE process in comparison with varicella vaccine commercial product 2016 (VAR) in healthy children who are between 12 to 23 months of age administered concomitantly with measles-mumps-rubella (MMR) vaccine (M-M-R™ II; Merck & Co., Inc., Kenilworth, NJ, USA).
Results
Participants
Overall, 599/600, (99.8%) of randomized participants received Dose 1 of study vaccine, 93.2% (558/599) received both Dose 1 and Dose 2 (). Approximately 90.2% of participants completed the study (received both doses, had all blood samples collected, and completed the 42-day safety data after each vaccination). Overall, 9.8% of participants discontinued the study with the most common reasons for discontinuation being lost to follow-up (n = 31; 5.2%) and withdrawal by parent/guardian (n = 25; 4.2%).
Figure 1. Participant disposition
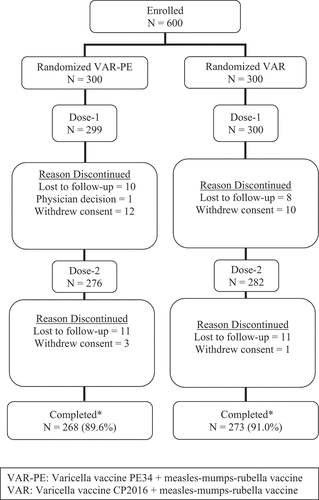
Participants across both groups were similar with respect to gender, age, and race (). The majority of vaccinated participants in each vaccination group (VAR-PE: 88.0%; VAR: 86.0%) were seronegative at baseline with an initial VZV antibody titer <1.25 glycoprotein enzyme-linked immunosorbent assay (gpELISA) units/mL. Overall, 37.5% and 42.8% of participants received any medical therapy within 14 days prior to Doses 1 and 2, respectively; the most common medication classes being analgesics (12.0% before Dose 1; 13.8% before Dose 2), antibacterials (10.8% before Dose 1; 8.4% before Dose 2), and anti-inflammatory/antirheumatic products (7.5% before Dose 1; 12.0% before Dose 2). Comparable proportion of participants across both vaccination groups received any medical therapy during the study.
Table 1. Demographics
Immunogenicity
The results demonstrate non-inferiority of VAR-PE compared to VAR based on the VZV antibody response rate at 6 weeks Postdose 1 in the Per-Protocol (PP) population, as the lower bound of the 2-sided 95% CI for the treatment difference in response rate (VAR-PE – VAR) excluded a decrease of 10 percentage points or more (p < .001) (). Acceptability of the immune response was demonstrated based on the VZV antibody response at 6 weeks Postdose 1 in the PP population, as the lower bound of the one-sided 95% CIs of the antibody response rate was greater than 76% (p < .001) (). In the PP population, antibody geometric mean titers (GMTs) for VZV at 6 weeks Postdose 1 in the PP population were comparable (). The VZV seroconversion rate (defined as the proportion of participants with baseline VZV antibody titer <1.25 gpELISA units/mL and with postvaccination VZV antibody titer ≥1.25 gpELISA units/mL) at 6 weeks Postdose 1 in the PP population was 100% for both groups.
Table 2. Summary of VZV response rates and geometric mean titers (GMTs) at 6 weeks postdose 1
A total of 71 participants (VAR-PE: 31; VAR:40) were identified as seropositive at baseline (VZV antibody ≥1.25 gpELISA units/mL) and excluded from the PP analysis. The range of antibody titers for these participants at baseline was 1.252 to 26.43 gpELISA units/mL. Postvaccination, results were comparable between the 2 vaccination groups in the antibody response rates (proportion of participants ≥5 gpELISA units/mL), GMTs, geometric mean fold rise (GMFR), and proportion of participants with ≥4-fold rise at 6 weeks Postdose 1 compared to baseline (Supplemental Table 1).
Immunogenicity was also assessed in a supportive analysis which included all participants with valid serology results regardless of protocol deviations, and results were comparable to the PP population with respect to antibody response rate and GMTs.
Where postvaccination immunogenicity was not able to be assessed due to discontinuation or due to a missing serology result, the baseline antibody titer range was (<0.625, 6.095).
Safety
Adverse events (AEs) were reported by 81.3% of participants Postdose 1 and 67.9% Postdose 2 (). The incidence of participants with injection-site or vaccine-related systemic AEs was comparable for the vaccination groups. A total of 12 participants, 6 in each vaccination group, reported a total of 15 serious AEs (SAEs) from Day 1 through the end of the study (Day 180 Postdose 2) (Supplemental Table 2). No SAEs were considered by the investigator to be vaccine-related and no participants discontinued study vaccine or died during the primary safety follow-up period (Day 1 to Day 42 Postdose 1 or 2).
Table 3. Adverse Experience (AE) Summary (Days 1 to 42 Postdose 1 and 2)
All injection-site AEs were considered related to the vaccination. From Day 1 through Day 42 Postdose 1, injection-site AEs related to varicella vaccine were reported by 33.8% and 32.3% of participants in VAR-PE and VAR, respectively, and Postdose 2, by 28.3% and 27.7% of participants in the VAR-PE and VAR groups, respectively (). The incidences of solicited injection-site AEs from Day 1 to Day 5 Postdose 1 and Postdose 2 were comparable ().
Table 4. Summary of participants with VRC-solicited injection site varicella vaccine related adverse events
From Day 1 through 42 Postdose 1, systemic AEs were reported for the majority of participants and the incidences of systemic AEs were generally comparable for the 2 vaccination groups, with the exception of pyrexia and otitis media which were higher in VAR-PE, and diarrhea and teething which were higher in VAR (). Overall, 22.5% of participants experienced vaccine-related systemic AEs from Day 1 to Day 42 Postdose 1. The proportions of participants with vaccine-related systemic AEs were comparable for the vaccination groups (VAR-PE: 23.1%; VAR: 22.0%). The most commonly reported vaccine-related systemic AEs for each vaccination group were pyrexia (7.5%) and irritability (7.2%).
Table 5. Summary of Participants with Systemic Adverse Events (incidence ≥10% in either group)
The incidence of systemic AEs was generally lower Postdose 2 compared with Postdose 1 (). From Day 1 through Day 42 Postdose 2, the most frequently reported systemic AEs were pyrexia (VAR-PE: 15.9%; VAR: 12.1%) and rhinorrhea (VAR-PE: 10.1%; VAR: 5.7%). Overall, 10.9% of participants experienced vaccine-related systemic AEs from Day 1 to Day 42 Postdose 2. The proportions of participants with vaccine-related systemic AEs were comparable for the vaccination groups (VAR-PE: 10.1%; VAR: 11.7%). The most commonly reported vaccine-related systemic AEs for each vaccination group were pyrexia (3.8%) and irritability (3.2%).
Across all day ranges, the incidence of fever, defined as temperature ≥102.2°F (≥39.0°C) oral equivalent, was comparable for the 2 vaccination groups and no significant differences were observed (). The incidence of fever was lower Postdose 2 compared with Postdose 1. The highest rate of fever was observed between Day 6 to Day 14 Postdose 1 and between Days 29 to 42 Postdose 2.
Table 6. Analysis of rates of fever by Day Range between vaccination groups (all participants as treated population)
The incidence of vaccine-specific rashes was low and comparable for the vaccination groups from Day 1 to Day 42 Postdose 1 (VAR-PE: 3.3%; VAR: 3.3%) and Postdose 2 (VAR-PE: 2.2%; VAR: 0.7%) with no significant differences observed for the 2 vaccination groups. The incidence of vaccine-specific rashes was generally higher Postdose 1 compared with Postdose 2 (Supplemental Table 3). None of these rashes were considered a SAE by the study investigator. No participants in either vaccination group experienced mumps-like symptoms from Day 1 to Day 42 Postdose 1 or Postdose 2.
Discussion
Varicella vaccine is indicated for active immunization for the prevention of varicella in individuals 12 months of age and older. The Varicella Passage Extension process is part of a multi-stage process designed to ensure ongoing supply of VZV (Oka/Merck)-containing vaccines.Citation14 This process change was not expected to impact the safety profile or effectiveness of the vaccine. Strengths of the trial include the randomized, double-blind, comparator-controlled design in order to assess both immunogenicity, safety, and tolerability. Another strength was that this trial was performed in healthy children who were between 12 to 23 months of age, which was the recommended age group for the first dose of VZV-containing vaccine. Immmunogenicity was assessed twice during the study, prior to enrollment and Postdose 1, but not Postdose 2. This may be viewed as a limitation; however, this schema was chosen as not to be overly prohibitive for the participants and their parents/guardians, and importantly, PD1 immunogenicity data supports broad use globally. In a previous study comparing one versus two injections of VAR in healthy children 1–12 years of age, the seroconversion rate was 98.2% after one injection and 99.9% after two injections. Additionally, >99% of these children who received either one or two doses of VAR maintained antibody to varicella at 1 year postvaccination with geometric mean titers of 19.5 and 31.2, respectively.Citation15
The results of this study demonstrate non-inferiority of immune responses induced by vaccinations with VAR-PE compared to VAR, including both antibodies assessed by gpELISA, as well as GMTs, 6 weeks postvaccination. The immune responses met the statistical criteria for acceptability. Vaccination with VAR-PE process product is well tolerated and has a safety profile comparable to that of VAR.
The results from this study demonstrate that VAR-PE is non-inferior with respect to immunogenicity and has a comparable safety and tolerability profile compared to VAR. The results support the passage extension manufacturing change in order to ensure the availability of VZV (Oka/Merck)-containing vaccines.
Methods
Design
This was a randomized, double-blind clinical trial conducted in 35 sites within the US from October 2017 to April 2019. The protocol was conducted in accordance with principles of Good Clinical Practice (GCP), including obtaining written informed consent from each participant’s parent(s) or legal guardian(s) prior to study entry, and was approved by the Institutional Review Board applicable to each study site. Randomization occurred centrally using an automated web/phone system. Subjects were assigned randomized treatment in a 1:1 ratio to VAR-PE or VAR, without stratification.
Participants
Healthy children 12 to 23 months of age with a negative history for varicella, herpes zoster, measles, mumps, and rubella, and without prior immunization against these diseases were eligible for the study. Exclusion criteria included receipt of any inactivated vaccine within 14 days, or any live vaccine within 30 days, prior to study entry; history of seizure disorder; febrile illness within 72 hours prior to study entry; or any congenital or acquired immune deficiency, neoplastic disease, or immunosuppression.
Participants were allocated to a vaccination group using a randomized schedule generated by the study statistician. Participants were randomized to receive either two 0.5 ml subcutaneous doses of VAR-PE vaccine 3 months apart or two 0.5 ml subcutaneous doses of VAR vaccine 3 months apart, concomitantly with MMR vaccine. The study was designed to have approximately 600 participants randomized in a 1:1 ratio to either one of the two groups. Based on approximately 300 participants per group, and with an expected evaluability rate of 80%, the study provided 94.1% power for demonstrating noninferiority of the response rate, 95.8% power for demonstrating noninferiority of the GMT, and >99.9% power for demonstrating acceptability of the response rate at an overall 2-sided 5% α-level.
Vaccines
Varicella vaccine is manufactured using MRC-5 cells inoculated with the Oka/Merck strain of the VZV. The virus is harvested into a stabilizer solution followed by sonication and clarification by filtration. The dispensed drug substance is diluted with stabilizer solution, filled into vials and lyophilized. The lyophilized vaccine pellet is reconstituted with sterile diluent prior to administration. VAR-PE is manufactured using an additional passage extension which establishes an additional stock seed, which is then the process input to manufacture the varicella drug substance at increased passage level. The end result is an increase of the seed supply to be used to manufacture VZV (Oka/Merck)-containing vaccines. Both VAR-PE and VAR have the same dose, as VAR-PE is formulated to contain the same potency as VAR at the time of release. Additionally, this manufacturing change is not expected to change the immunogenicity or safety profile of the vaccine.
Blinded, single-dose vials of VAR-PE and VAR were supplied to the clinical sites, were indistinguishable in appearance, and stored at 2º to 8ºC. Open-label, single-dose vials of MMR-II vaccine and sterile diluent for reconstitution were also supplied to the clinical sites and handled according to the package inserts.
Immunogenicity
The primary immunogenicity analyses were based on the Per-Protocol (PP) population. The PP population was defined as participants who received 1 dose of vaccine according to their vaccination group assignment, adhered to study instructions, and provided serum samples within the appropriate day ranges. The PP population excluded participants who had important protocol deviations with the potential to impact the immunogenicity analyses (not compliant with immunogenicity specimen collection, n = 8; receipt of systemic steroids 7 days prior to vaccination or during the 42-day safety follow-up period, n = 6; receipt of improperly stored study treatment, n = 2; improper reconstitution of vaccine, n = 1), as well as participants who did not receive any study vaccine, n = 1, did not have postvaccination immunogenicity assessments, n = 45, or had seropositive status at baseline, n = 77.
Serum samples collected before and 6 weeks Postdose 1 were tested for VZV antibody titers for varicella evaluated by gpELISA method.Citation16–18 Immunogenicity was evaluated by response rates at each time point and GMTs of antibodies to each virus were evaluated 6 weeks Postdose 1. Serum samples were not collected 6 weeks Postdose 2 for antibody measurement.
The primary immunogenicity objectives were: (1) to demonstrate that a single dose of VAR-PE process induces VZV antibody responses 6 weeks Postdose 1 that are noninferior to those induced by VAR; and (2) to demonstrate that a single dose of VAR-PE process induces an acceptable VZV antibody response 6 weeks Postdose 1. Response rate was defined as the proportion of participants with VZV antibody titer ≥5 gpELISA units/mL 6 weeks Postdose 1, among participants who were seronegative to VZV (antibody titer <1.25 gpELISA units/mL) at baseline.
The secondary immunogenicity objective was to summarize the VZV antibody responses after a single dose of VAR-PE and after a single dose of VAR. The endpoint for participants who were seronegative to VZV (antibody titer <1.25 gpELISA units/mL) at baseline, the seroconversion rate was defined as the proportion of participants with VZV antibody titer ≥1.25 gpELISA units/mL 6 weeks Postdose 1. The endpoint for participants who were seropositive to VZV (antibody titer ≥1.25 gpELISA units/mL) at baseline was defined as the GMFR and the proportion of participants achieving ≥4-fold rise in antibody titer from baseline to Postdose 1.
Safety
All participants who received a vaccine dose were included in the safety analysis. The primary safety objective was to assess the safety and tolerability of the first and second doses of VAR-PE compared to VAR. Using an electronic Vaccination Report Card (eVRC), parents and/or guardians recorded solicited injection-site reactions (redness, swelling, and pain/tenderness) of any intensity or size were recorded from Day 1 through Day 5 postvaccination. Additionally, unsolicited injection-site AEs, systemic AEs, elevated temperatures (≥102.2°F [≥39.0°C] oral equivalent), and rashes (varicella-like, zoster-like, measles-like, or mumps-like) were recorded on the eVRC through 42 days following each dose. All participants were followed for serious AEs (SAEs) throughout the trial duration and medically-attended AEs from Day 133 through Day 271 (Day 180 Postdose 2).
Statistical methods
For the primary immunogenicity hypothesis based on antibody response rate, VAR-PE was considered non-inferior to VAR if the lower bound of the 2-sided 95% CI (Miettinen and Nurminen unconditional asymptotic method) for the difference in rates (VAR-PE minus VAR) excluded a decrease of 10 percentage points or more. For the primary immunogenicity hypothesis based on GMT ratios, VAR-PE was considered non-inferior to VAR if the lower bound of the 2-sided 95% CI of the GMT ratio (VAR-PE/VAR) was >0.67. For the primary safety hypothesis, VAR-PE was considered acceptable if the lower bound of the 2-sided 95% CI for the response rate was above 76.0%.
Abbreviations
| AE | = | Adverse experience |
| CI | = | Confidence interval |
| VAR | = | Varicella vaccine commercial product 2016 |
| GCP | = | Good Clinical Practices |
| GMFR | = | Geometric mean fold rise |
| GMT | = | Geometric mean titer |
| gpELISA | = | Glycoprotein enzyme-linked immunosorbant assay |
| HZ | = | Herpes zoster |
| MMR | = | Measles, mumps, and rubella vaccine |
| VAR-PE | = | Varicella vaccine passage extension 34 product |
| PP | = | Per-Protocol |
| SAE | = | Serious adverse experience |
| US | = | United States |
| eVRC | = | electronic Vaccination Report Card |
| VZV | = | Varicella-zoster virus |
Author contributions
Peter Silas and Edward Zissman: enrollment of participants and/or data collection, analysis and interpretation of data, and preparation of manuscript. Julie Gardner, Shanjun Helian, Andrew Lee, and Heather Platt: study concept and design, analysis and interpretation of data, and preparation of manuscript.
Disclosure of potential conflicts of interest
Peter Silas and Edward Zissman were investigators for the sponsor supported by research grants.
Julie Gardner, Shanjun Helian, Andrew Lee, and Heather Platt are employees of the sponsors and may hold stock and/or stock options from the sponsors.
Supplemental Material
Download MS Word (36.5 KB)Acknowledgments
The authors would like to thank: All the participants who participated in this study and their parents or legal guardian; the Protocol A03 Study group: G Adams, DM Brandon, SG Christensen, L Chu, RA Clifford, MG Cruz, O deValle, CA Duffy, BK Eberhard, JE Ervin, DJ Finn, M Harper, RD Hartvickson, AY Ituriaga, WH Johnston, KA Julien, JL Kirstein, ML Leonardi, J Ley, K Palapurwala, JT Peterson, AG Pruitt, MA Rausch, B Rizzardi, KG Rouse, RE Rupp, R Schumacher, SD Senders, JS Shepard, PE Silas, L Soylu, V Statler, WP Stewart, MA Turner, LB Weiner, JL Williams, PP Wisman, and EN Zissman; and Jon E. Stek and Karyn Davis of Merck & Co., Inc., Kenilworth, NJ, USA for their editorial assistance.
Supplementary Material
Supplemental data for this article can be accessed online at http://doi.org/10.1080/21645515.2020.1743122.
Additional information
Funding
References
- Straus SE, Ostrove JM, Inchauspé G, Felser JM, Freifeld A, Croen KD, Sawyer MH. NIH conference. Varicella-zoster virus infections. Biology, natural history, treatment, and prevention. Ann Intern Med. 1988;108(2):221–37. doi:10.7326/0003-4819-108-2-221.
- Choo PW, Donahue JG, Manson JE, Platt R. The epidemiology of varicella and its complications. J Infect Dis. 1995;172(3):706–12. doi:10.1093/infdis/172.3.706.
- Guess HA, Broughton DD, Melton LJ, Kurland LT. Population-based studies of varicella complications. Pediatrics. 1986;78:723–27.
- Wharton M. The epidemiology of varicella-zoster virus infections. Infect Dis Clin North Am. 1996;10(3):571-&. doi:10.1016/S0891-5520(05)70313-5.
- Galil K, Brown C, LIN F, Seward J. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr Infect Dis J. 2002;21(10):931–35. doi:10.1097/00006454-200210000-00009.
- Meyer PA, Seward JF, Jumaan AO, Wharton M. Varicella mortality: trends before vaccine licensure in the United States, 1970–1994. J Infect Dis. 2000;182(2):383–90. doi:10.1086/315714.
- Guris D, Jumaan AO, Mascola L, Watson BM, Zhang JX, Chaves SS, Gargiullo P, Perella D, Civen R, Seward JF. Changing varicella epidemiology in active surveillance sites–United States, 1995–2005. J Infect Dis. 2008;197(Suppl 2):S71–5. doi:10.1086/522156.
- Luman ET, Ching PL, Jumaan AO, Seward JF. Uptake of varicella vaccination among young children in the United States: a success story in eliminating racial and ethnic disparities. Pediatrics. 2006;117(4):999–1008. doi:10.1542/peds.2005-1201.
- Zhou F, Harpaz R, Jumaan AO, Winston CA, Shefer A. Impact of varicella vaccination on health care utilization. JAMA. 2005;294(7):797–802. doi:10.1001/jama.294.7.797.
- Centers For Disease Control. Prevention of varicella: recommendations of the advisory committee on immunization practices (ACIP). MMWR. 2007;56(RR–4):1-CE-4.
- Marin M, Zhang JX, Seward JF. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics. 2011;128(2):214–20. doi:10.1542/peds.2010-3385.
- Baxter R, Ray P, Tran TN, Black S, Shinefield HR, Coplan PM, Lewis E, Fireman B, Saddier P. Long-term effectiveness of varicella vaccine: a 14-Year, prospective cohort study. Pediatrics. 2013;131(5):e1389–96. doi:10.1542/peds.2012-3303.
- Centers For Disease Control And Prevention. Recommended immunization schedules for persons aged 0–18 years-United States, 2007. MMWR. 2007;55(51):Q1–Q4.
- Senders SD, Bundick ND, Li J, Zecca C, Helmond FA. Evaluation of immunogenicity and safety of VARIVAX New Seed Process (NSP) in children. Hum Vaccin Immunother. 2018;14(2):442–49. doi:10.1080/21645515.2017.1388479.
- Ngai AL, Staehle BO, Kuter BJ, Cyanovich NM, Cho I, Matthews H, Keller P, Arvin AM, Watson B, White CJ. Safety and immunogenicity of one vs. two injections of Oka/Merck varicella vaccine in healthy children. Pediatr Infect Dis J. 1996;15(1):49–54. doi:10.1097/00006454-199601000-00011.
- Keller PM, Lonergan K, Neff BJ, Morton DA, Ellis RW. Purification of individual varicella-zoster virus (VZV) glycoproteins gpI, gpII, and gpIII and their use in ELISA for detection of VZV glycoprotein-specific antibodies. J Virol Methods. 1986;14(2):177–88. doi:10.1016/0166-0934(86)90048-0.
- Wasmuth EH, Miller WJ. Sensitive enzyme-linked-immunosorbent-assay for antibody to varicella-zoster virus using purified vzv glycoprotein antigen. J Med Virol. 1990;32(3):189–93. doi:10.1002/()1096-9071.
- Provost PJ, Krah D, Kuter B, Morton D, Schofield T, Wasmuth E, White C, Miller W, Ellis R. Antibody-assays suitable for assessing immune-responses to live varicella vaccine. Vaccine. 1991;9(2):111–16. doi:10.1016/0264-410X(91)90266-9.
