ABSTRACT
Objectives: To determine the serotype distribution of pneumococcus causing invasive pneumococcal disease (meningitidis, bacteremia and empyema) in children in Turkey, and to observe potential changes in this distribution in time to guide effective vaccine strategies.
Methods: We surveyed S. pneumoniae with conventional bacteriological techniques and with real-time polymerase chain reaction (RT-PCR) in samples of cerebrospinal fluid (CSF), blood and pleural fluid. S. pneumoniae strains were isolated from 33 different hospitals in Turkey, which are giving health services to approximately 60% of the Turkish population.
Results: A total of 167 cases were diagnosed with invasive pneumococcal disease between 2015 and 2018. We diagnosed 52 (31.1%) patients with meningitis, 104 (62.2%) patients with bacteremia, and 11 (6.6%) patients with empyema. Thirty-three percent of them were less than 2 years old and 56% less than 5 years old. Overall PCV13 serotypes accounted for 56.2% (94/167). The most common serotypes were 19 F (11.9%), 1 (10.7%) and 3 (10.1%).
Conclusions: Besides the increasing frequency of non-vaccine serotypes, vaccine serotypes continue to be a problem for Turkey despite routine and high-rate vaccination with PCV13 and significant reduction reported for the incidence of IPD in young children. Since new candidate pneumococcal conjugate vaccines with more serotype antigens are being developed, continuing IPD surveillance is a significant source of information for decision-making processes on pneumococcal vaccination.
Introduction
Diseases caused by Streptococcus pneumoniae (pneumococcus) are a global public health concern. It is estimated that about one million children die of pneumococcal disease each year.Citation1 Over 90 different pneumococcal serotypes have been identified and it has been reported that serotype distribution of the pneumococcus changes due to various factors including clonal enlargement, capsular transformation, mass pneumococcal vaccination, socioeconomic conditions, immune status and changes in antibiotic use in population.Citation2–4 Therefore, obtaining information about local serotype distribution and changes over time is essential for effective vaccination strategies.Citation5 Currently, there are three different pneumococcal vaccines (PPSV23, PCV10 and PCV13), each of them has their own complications and successes.Citation6 Underdeveloped specific splenic B cell subsets, as well as the composition of antibody and T cell receptor repertoires, are the basis for the poor immunogenicity of PPV23 in infants. Conjugate vaccines composed of polysaccharide antigen linked to a carrier protein have been developed to elicit higher levels of protective antibodies, thus overcoming this problem.Citation7
Although there are variations in regional pneumococcal serotype distribution for the common serotypes, the first PCV which is 7-valent PCV (PCV7) containing 7 (4, 6B, 9 V, 14, 18 C, 19 F, 23 F) common serotypes encountered in childhood was recommended by WHO to be included in national immunization programs (NIP) in 2007.Citation8 Routine PCV7 vaccination had a major impact on the incidence of invasive and noninvasive pneumococcal diseases in children worldwide.Citation9 More serotypes were added to the same seven serotypes to expand the serotype coverage. These higher valency PCVs are 10-valent PCV (PCV10) (contains serotypes 1, 5, and 7 F plus PCV7 serotypes) and 13-valent PCV (PCV13) (includes PCV10 serotypes plus 3, 19A, and 6A).Citation10 Turkey implemented PCV7 vaccination in NIP in 2009. It was changed to PCV13 with the same 3 + 1 vaccination schedule (2, 4, 6 months and a booster at 12 months) in 2011.Citation11 Recently, it was modified to a 2 + 1 schedule (3rd dose was removed from the schedule).
We aimed to assess the effect of PCV13 vaccination on the serotype distribution of pneumococci causing invasive pneumococcal diseases (meningitidis, bacteremia and empyema) in children in Turkey.
Method
This multicenter, hospital-based, epidemiological study was conducted in Turkey among children younger than 18 y. Specimens were collected between January 2015 and December 2018. All patients treated for invasive infections attributable to S. pneumoniae were screened in 33 hospitals, located in all geographical regions of Turkey providing health services to approximately 60% of the Turkish population. Cases were considered eligible for evaluation if S. pneumoniae was isolated from a normally sterile body site (cerebrospinal fluid [CSF], blood or pleural fluid) and was identified based on typical colony morphology on blood agar as well as Gram strain and optochin sensitivity.
Duplicate isolates from the same patient were not included in the study. The presence of S. pneumoniae in isolate was confirmed at the central study laboratory (Department of Microbiology and Infection Disease, Istanbul Faculty of Medicine). We obtained isolates of S. pneumoniae as a part of the routine clinical diagnostic practice, from blood-culture system (Bactec 9050, Becton Dickinson, Temse, Belgium), lung aspirate by inoculation of culture media at the patients’ bedside, and CSF, using standard microbiological procedures. We surveyed S. pneumoniae using the conventional bacteriological techniques and real-time polymerase chain reaction (rt-PCR) in samples.Citation12
Tests for susceptibility to antimicrobial agents were performed using the standard disc diffusion method on Müller-Hinton agar, supplemented with 5% ship blood. The susceptibility to penicillin was detected with a 1 ml oxacillin disc. The minimal inhibitory concentration (MIC) of the antibiotics was detected using the E-test.Citation13 E tests were performed according to the guidelines outlined by the Clinical and Laboratory Standards Institute (2012). An inoculum density equivalent to 0,5 MacFarland Standard was prepared in Müller-Hinton broth.Citation14 Serotyping was performed by the Quelling reaction using serotype-specific antisera according to the manufacturer`s instructions (Statens Serum Institute, Copenhagen, Denmark). Minimal inhibitory concentrations (MICs) for penicillin and ceftriaxone were performed and interpreted by CLSI guidelines (2015). For non-meningitis cases, intermediate resistance to penicillin is defined as MIC between 2 and 8 µg/ml, high-level resistance as MIC>8 µg/ml and susceptible as MIC ≤2 µg/ml. Susceptibility to cefotaxime was defined as MIC≤1.0 µg/ml. For meningitis cases, isolates were considered susceptible to parenteral penicillin if MICs were ≤0.06 µg/ml or to cefotaxime if MICs were ≤0.5 µg/ml.Citation15 Vaccine-type strains included serotypes 4, 6B, 9 V, 14, 18 C, 19 F, 23 F, 1, 5, 7 F, 3, 6A and 19A. All other serotypes were considered as non-vaccine types.
The study was approved by the İstanbul University Istanbul Faculty of Medicine Clinical Research Ethics Committee (2012/1676-1269).
Statistical analysis
All statistical analyses were performed using SPSS version 21.0 (IBM Corporation, Armonk, NY, USA). Descriptive statistics were used to summarize the participants’ baseline characteristics for continuous variables and frequency distributions for categorical variables. Categorical variables were expressed as frequencies and proportion.
Result
Streptococcus pneumoniae strains causing invasive pneumococcal disease were isolated and serotyped in 167 samples between 2015 and 2018. Of these, 33% of the cases were under 2years old and 56% of them were under 5 years old. Among the cases, the site of infection showed that the most common manifestation was bacteremia followed by meningitis and empyema. We diagnosed 104 (62.2%) patients with bacteremia, 52 (31.1%) patients with meningitidis and 11 (6.6%) with empyema (). PCV13 serotypes accounted for 55.8% of bacteremic isolates and 57.1% of non-bacteremic isolates. The rates of PCV13 and non-PCV13 serotypes were not statistically different between bacteremia and non-bacteremia isolates.
Table 1. The site of infection according to the years
The total number of PCV13, the most common non-PCV13 serotypes, the distribution of each serotype and the number of PCV13 or PCV7 vaccine doses before IPD are shown in . Children born in an after 2009 were considered as properly vaccinated for their age, with the PCV in use for the NIP. PCV13 serotype isolates accounted for 56.2% (94 out of 167) over the period of this study; 32 (34%) of the children who had IPD due to a PCV13 serotype isolate received only one dose of PCV13 or none before their IPD.
Table 2. Serotype distribution of Streptococcus pneumoniae according to clinical samples and vaccination status
The most common vaccine serotypes were 19 F (n = 20, 11.9%), 1 (n = 18, 10.7%) and 3 (n = 17, 10.1%). These three serotypes accounted for 58.5% of the PCV13 serotypes and 32.9% of IPD in children. The most common non-PCV13 serotypes were 15B (n = 6, 3.6%) and 8, 12 F and 10A (n = 5, 2.9% in each) and 11A, 15 C, 15 F and 20 (n = 3, 1.7% in each) ().
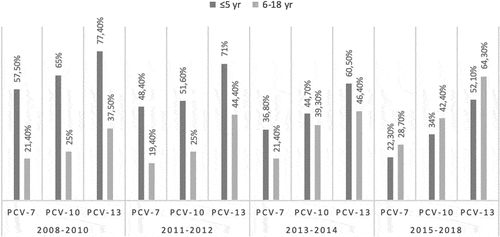
During the first 5 years of age, the potential serotype coverage rates of PCV7, PCV10, and PCV13 were 22.3%, 34%, and 52.1%, respectively; the coverage rates of these vaccines were 28.7%, 42.4%, and 64.3% for the 5−18 years age group. Data regarding the vaccine serotype coverage rates for PCV7, PCV10 and PCV13 before and after the inclusion of PCV7 and PCV13 in Turkey’s NIP according to the years is depicted in .
Figure 1. Vaccine serotype coverage rates for PCV7, PCV10 and PCV13 before and after the inclusion of PCV7 and PCV13 in Turkey’s NIP according to the years
About 61 children (36.5%) in our study were not fully vaccinated with PCV7 or PCV13, with no doses or only one dose reported, while 106 (63.4%) patients were vaccinated with either PCV7 or PCV13 at least two doses. Among the 61 (36.5%) children who were not fully vaccinated, seven had received one dose before their IPD and 54 had no vaccination, 42 were of an age at the time of IPD for which PCV was recommended according to our NIP, they were born before 2007. Only 28 (16.7%) children had received four doses of PCV13.
Of the 106 cases who had at least two doses of vaccine, 72 were diagnosed with bacteremia, 24 with meningitis and 10 with empyema. In this vaccinated group, the most common isolated serotypes were 19 F (n = 17), 1 (n = 13) and 3 (n = 12). Among the 61 children who were not fully vaccinated, 32 of them were diagnosed with bacteremia, 28 with meningitis and one with empyema. We detected non-PCV13 serotypes in 42.4% (n = 45) of patients who had been vaccinated with at least two doses of either PCV7 or PCV13.
In our previous studies, the penicillin-resistant Streptococcus pneumonia (PRSP) ratio was 16.5% in 2008–2010 and 33.7% in 2011–2014. In this study, PRPS was 32.9% in invasive isolates. The ceftriaxone susceptibility rate of the PID isolates was 84.7%. The proportion of PRSP is 38.4% for non-PCV13 serotypes and 28.5% for PCV13 serotypes. The resistance rate of serotypes from higher to lower was 12A, 18, 18 C, 2, 6B, 24A, 24 F, 28A, 33 C, 6B, 6D, 9 N (100% for each), 23 F (66.6%), 12, 15A, 15B, 4, 6 C, 7 F (50% for each), and 19A (42.9%) (). The proportion of PRPS isolates potentially covered by the PCV7, PCV10 and PCV13 vaccines was 22.4%, 32.6% and 46.9%, respectively.
Table 3. Penicillin-resistance rate of serotypes
Discussion
Successful vaccination policies for protection from invasive pneumococcal diseases are crucial for every country. Therefore, the present study evaluated data on the serotyping of the pneumococci associated with IPD in Turkey between 2015 and 2018 after the beginning of PCV13 vaccination. We found that PCV13 serotypes (56.2%) accounted for more than non-PCV13 serotypes (43.7%); in our previous studies, the ratio of non-PCV13 serotypes was 27.2% between 2008 and 2010 and 37.6% between 2011 and 2014.Citation12,Citation16 According to the data from three sequential periods, it was detected that the number of cases with IPD due to non-vaccine serotypes increased whereas the number of cases with IPD caused by the vaccine serotypes decreased, which might be attributable to the impact of NIP with PCVs. Similarly, a study evaluating pneumococcal serotypes in Western Europe reported that marked reduction in diseases related to vaccine serotypes has been observed while the nonvaccine serotypes have increased.Citation17,Citation18
By the way, PCV13 serotypes still account for a considerable proportion of IPD cases in our study despite the high vaccination rate in Turkey (96%).Citation19 In the literature, several cases were reported of those who had IPD due to PCV13 serotypes despite having received at least two doses of PCV vaccination.Citation20–28 There is a lack of information about the risk of IPD with vaccine-types after completing the recommended course of PCV immunization. After the vaccination, the changes of antibody titers against serotypes affect the serotype distribution of IPD besides the serotype carriage.Citation24 The immunogenicity of the vaccine serotypes and the underlying conditions that altered host defenses against IPD could result in vaccine failure. There is a need for a more systematic vaccine failure reporting system in every country.Citation24,Citation29
In this study period, the most common serotypes in order of frequency were 19 F, 1 and 3. Consistent with our findings, the most common serotypes causing IPD were 3, 19A and 19 F after the early years of introduction of the PCV13 vaccine in the United States with the difference of seroepidemiology of 19A.Citation30 Additionally, studies from Europe, America, and Western Pacific after the introduction of PCVs showed a dominant contribution of 19A to the pediatric cases with IPD;Citation31 however, a recent review found that there was a low prevalence of serotype 19A while serotypes 1, 14 and 19 F were common in South Asian countries.Citation32 Similarly, 19A did not seem to be a major problem in Turkey in the vaccine era for the beginning of the study period. The Center for Disease Control and Prevention Active Bacterial Core surveillance program on the molecular characterization of serotype 19A strains showed that the number of isolates within the specific serotype 19A`s clonal complexes has fluctuated significantly in the United States. These changes have resulted in declines and increase clonal complex prevalence.Citation33 The difference in the seroepidemiology of serotype 19A could be attributable to the distribution of the clonal complex.
In this study period, the most common manifestation was bacteremia followed by meningitis and empyema, as noted in previous study periods. The rates of PCV13 and non-PCV13 serotypes and common serotypes were similar between bacteremia and non-bacteremia cases.
Several predominant non-PCV13 serotypes were reported from different countries all over the world.Citation18,Citation31,Citation34 Non-PCV13 serotypes contributed to 43.7% of IPD cases in our study. Regional differences are seen in other countries (57.8% in North America, 71.9% in Europe, 45.9% in Western Pacific, and 28.5% in Latin America).Citation31 Therefore, based on the common serotypes not covered by PCV13, candidate PCVs are being developed by adding the following serotypes to the PCV13 serotypes: 15-valent (22 F, 33 F) and 20-valent (8, 10A, 11A, 12 F, 15B, 22 F, 33 F).Citation35,Citation36 The most common non-PCV13 serotypes in our study were 15B, 8, 12 F, 10A, 11A, 15 C, 15 F and 20. Neither 22 F nor 33 F, which are included as new serotypes to PCV15, is common pathogen in our country. It seems that non-PCV13 serotypes will continue to be a problem due to the regional differences in serotype distribution.
Antibiotic resistance is an increasing challenge that affects the successful management of the pneumococcal diseases.Citation37 Serotypes 6B, 6A, 9 V, 14, 15A, 19 F, 19A, and 23 F were found to have the highest antibiotic resistance rates in the world.Citation38 In our study, penicillin-resistant Streptococcus pneumonia (PRPS) was 32.9% in invasive isolates and consistently, the most resistant isolates were serotypes 23 F, 12, 15A, 15B, 4, 6 C, 7 F and 19A. This may be related to the fact that PCVs are extremely effective in reducing resistant infections by reducing the carriage of antibiotic-resistant serotypes and through an overall reduction in antibiotic use.Citation39
Our study has several limitations. First, we do not know the incidence of IPD because there is no case surveillance in Turkey. Second, we could not evaluate the immune status or underlying diseases of children and the effect of serotype distribution according to underlying diseases. Despite these limitations, our project has provided useful and significant insight into the serotype distribution of S. pneumoniae in Turkey.
In conclusion, besides the increasing nonvaccine serotypes, vaccine serotypes continue to be a problem for our country despite routine immunization with PCV13 with a 96% vaccination rate since 2011.Citation19 In contrast to many other countries, Turkey has not seen an increase in IPD cases with serotype 19A. Serotypes 22 F and 33 F, which are problems in many developed countries and are added to developing conjugated pneumococcal vaccines, were not among the commonly isolated serogroups in our study. Our ongoing IPD surveillance is a significant source of information for the overall impact of pneumococcal vaccination programs as well as appropriate decision-making processes for NIP in Turkey.
Disclosure of potential conflicts of interest
The authors declare that they have no other conflicts of interest.
Additional information
Funding
References
- World Health Organization. Pneumococcal diseases (updated 2015 Nov 16). https://www.who.int/biologicals/vaccines/pneumococcal/en/
- Pletz MW, Maus U, Krug N, Welte T, Lode H. Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents. 2008;32:199–206. doi:10.1016/j.ijantimicag.2008.01.021.
- Feikin DR, Klugman KP. Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clin Infect Dis. 2002;35:547–55. doi:10.1086/341896.
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73. doi:10.1016/S0140-6736(10)62225-8.
- Ozsurekci Y, Ceyhan M, Gurler N, Öksüz L, Aydemir S, Özkan S, Yüksekkaya S, Emiroğlu MK, Gültekin M, Yaman A, et al. Serotype distribution of Streptococcus pneumoniae in children with invasive diseases in Turkey: 2008–2014. Hum Vaccin Immunother. 2016;12:308–13. doi:10.1080/21645515.2015.1078952.
- Wantuch PL, Avci FY. Current status and future directions of invasive pneumococcal diseases and prophylactic approaches to control them. Hum Vaccin Immunother. 2018;14:2303–09. doi:10.1080/21645515.2018.1470726.
- Westerink MA, Schroeder HW, Nahm MH. Immune Responses to pneumococcal vaccines in children and adults: rationale for age-specific vaccination. Aging Dis. 2012;3:51–67.
- World Health Organization. Pneumococcal conjugate vaccine for childhood immunization – WHO position paper. Weekly epidemiological report. Weekly Epidemiological Rec. 2007;82:93–104.
- Weil-Olivier C, van der Linden M, de Schutter I, Dagan R, Mantovani L. Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective. BMC Infect Dis. 2012;12:207. doi:10.1186/1471-2334-12-207.
- Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O’Brien KL. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7:e1000348. doi:10.1371/journal.pmed.1000348.
- Ceyhan M, Ozsurekci Y, Gurler N, Ozkan S, Sensoy G, Belet N, Hacimustafaoglu M, Celebi S, Keser M, Dinleyici EC, et al. Serotype distribution of Streptococcus pneumoniae causing parapneumonic empyema in Turkey. Clin Vaccine Immunol. 2013;20:972–76. doi:10.1128/CVI.00765-12.
- Ceyhan M, Gurler N, Ozsurekci Y, Keser M, Aycan AE, Gurbuz V, Salman N, Camcioglu Y, Dinleyici EC, Ozkan S, et al. Meningitis caused by Neisseria meningitidis, haemophilus influenzae type B and streptococcus pneumoniae during 2005-2012 in Turkey: A multicenter prospective surveillance study. Hum Vaccin Immunother. 2014;10:2706–12. doi:10.4161/hv.29678.
- Yalcin I, Gurler N, Alhan E, Yaman A, Turgut M, Celik U, Akcakaya N, Camcioglu Y, Diren S, Yildirim B. Serotype distribution and antibiotic susceptibility of invasive Streptococcus pneumoniae disease isolates from children in Turkey, 2001-2004. Eur J Pediatr. 2006;165:654–57. doi:10.1007/s00431-006-0128-x.
- Performance standards for antimicrobial disk susceptibility tests; approved standard-eleventh edition. Wayne (PA): Clinical and Laboratory Standards Institute; 2016. p. 9–10. [accessed Jan 31] http://www.antimicrobianos.com.ar/ATB/wp-content/uploads/2012/11/01-CLSI-M02-A11-2012.pdf
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing—twenty-fifth informational supplement. Wayne (PA): Clinical and Laboratory Standards Institute; 2015.
- Ceyhan M, Gurler N, Yaman A, Ozturk C, Oksuz L, Ozkan S, Keser M, Salman N, Alhan E, Esel D, et al. Serotypes of Streptococcus pneumoniae isolates from children with invasive pneumococcal disease in Turkey: baseline evaluation of the introduction of the pneumococcal conjugate vaccine nationwide. Clin Vaccine Immunol. 2011;18:1028–30. doi:10.1128/CVI.00526-10.
- Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis. 2010;14:e197–209. doi:10.1016/j.ijid.2009.05.010.
- Htar MTH, Christopoulou D, Schmitt HJ. Pneumococcal serotype evolution in Western Europe. BMC Infect Dis. 2015;15:419. doi:10.1186/s12879-015-1147-x.
- Turkey Ministery of Health. [ accessed 2019 Dec 20]. https://www.saglik.gov.tr/tr,11588/istatistik-yilliklari.html
- Antachopoulos C, Tsolia MN, Tzanakaki G, Xirogianni A, Dedousi O, Markou G, Zografou SM, Eliades A, Kirvassilis F, Kesanopoulos K, et al. Parapneumonic pleural effusions caused by Streptococcus pneumoniae serotype 3 in children immunized with 13-valent conjugated pneumococcal vaccine. Pediatr Infect Dis J. 2014;33:81–83. doi:10.1097/INF.0000000000000041.
- Madhi F, Godot C, Bidet P, Bahuaud M, Epaud R, Cohen R. Serotype 3 pneumococcal pleural empyema in an immunocompetent child after 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2014;33:545–46. doi:10.1097/INF.0000000000000252.
- Angoulvant F, Levy C, Grimprel E, Varon E, Lorrot M, Biscardi S, Minodier P, Dommergues MA, Hees L, Gillet Y, et al. Early impact of 13-valent pneumococcal conjugate vaccine on community acquired pneumonia in children. Clin Infect Dis. 2014;58:918–24. doi:10.1093/cid/ciu006.
- Pírez MC, Algorta G, Chamorro F, Romeo C, Varela A, Cedres A, Giachetto G, Montano A. Changes in hospitalizations for pneumonia after universal vaccination with pneumococcal conjugate vaccines 7/13 valent and haemophilus influenzae type b conjugate vaccine in a pediatric referral hospital in uruguay. Pediatr Infect Dis J. 2014;33:753–59. doi:10.1097/INF.0000000000000294.
- Godot C, Levy C, Varon E, Picard C, Madhi F, Cohen R. Pneumococcal meningitis vaccine breakthroughs and failures after routine 7-valent and 13-valent pneumococcal conjugate vaccination in children in France. Pediatr Infect Dis J. 2015;34:e260–e263. doi:10.1097/INF.0000000000000818.
- Moraga-Llop F, Garcia-Garcia JJ, Díaz- Conradi A, Ciruela P, Martinez-Osorio J, Gonzalez-Peris S, Hernandez S, de Sevilla MF, Uriona S, Izquierdo C, et al. Vaccine failures in patients properly vaccinated with 13- valent pneumococcal conjugate vaccine in Catalonia, a region with low vaccination coverage. Pediatr Infect Dis J. 2016;35:460–63. doi:10.1097/INF.0000000000001041.
- Sütcü M, Aktürk H, Karagozlü F, Somer A, Guler N, Salman N. Empyema due to streptococcus pneumoniae serotype 9V in a child immunized with 13-valent conjugated pneumococcal vaccine. Balkan Med J. 2017;34:74–77. doi:10.4274/balkanmedj.2015.0937.
- Diawara I, Zerouali K, Elmdaghri N, Abid A. A case report of parapneumonic pleural effusion caused by Streptococcus pneumoniae serotype 19A in a child immunized with 13-valent conjugate pneumococcal vaccine. BMC Pediatr. 2017;17:114. doi:10.1186/s12887-017-0872-2.
- Tanır Basaranoglu S, Karadag Oncel E, Aykac K, Ozsurekci Y, Cengiz AB, Kara A, Ceyhan M. Invasive pneumococcal disease: from a tertiary care hospital in the post-vaccine era. Hum Vaccin Immunother. 2017 Apr;3(13):962–64. doi:10.1080/21645515.2016.1256519.
- Oligbu G, Hsia Y, Folgori L, Collins S, Ladhani S. Pneumococcal conjugate vaccine failure in children: A systematic review of the literature. Vaccine. 2016;34:6126–32. doi:10.1016/j.vaccine.2016.10.050.
- Kaplan SL, Barson WJ, Lin PL, Romero JR, Bradley JS, Tan TQ, Pannaraj PS, Givner LB, Hulten KG. Invasive Pneumococcal Disease in Children’s Hospitals: 2014-2017. Pediatrics. 2019;144:e20190567. doi:10.1542/peds.2019-0567.
- Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis. PLoS One. 2017;12:e0177113. doi:10.1371/journal.pone.0177113.
- Jaiswal N, Singh M, Das RR, Jindal I, Agarwal A, Thumburu KK, Kumar A, Chauhan A. Distribution of serotypes, vaccine coverage, and antimicrobial susceptibility pattern of Streptococcus pneumoniae in children living in SAARC countries: a systematic review. PLoS One. 2014;9:e108617. doi:10.1371/journal.pone.0108617.
- Gregory JT. The Changing Epidemiology of Streptococcus pneumoniae Serotype 19A Clonal Complexes. J Infect Dis. 2011;203:1345–47. doi:10.1093/infdis/jir056.
- Steens A, Bergsaker MA, Aaberge IS, Rønning K, Vestrheim DF. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine. 2013;31:6232–38. doi:10.1016/j.vaccine.2013.10.032.
- Greenberg D, Hoover PA, Vesikari T, Peltier C, Hurley DC, McFetridge RD, Dallas M, Hartzel J, Marchese RD, Coller BG, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Vaccine. 2018;36:6883–91. doi:10.1016/j.vaccine.2018.02.113.
- Thompson A, Lamberth E, Severs J, Scully I, Tarabar S, Ginis J, Jansen KU, Gruber WC, Scott DA, Watson W. Phase 1 trial of a 20-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2019;37:6201–07. doi:10.1016/j.vaccine.2019.08.048.
- Improving global health by preventing pneumococcal disease. Report from the All-Party Parliamentary Group on Pneumococcal Disease Prevention in the Developing World. www.gavi.org/Library/Documents/AMC/All-Party-Parlimentary-Group-on-Pneumococcal-Disease-Report
- Liñares J, Ardanuy C, Pallares R, Fenoll A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect. 2010;16:402–10. doi:10.1111/j.1469-0691.2010.03182.x.
- Dagan R, Klugman KP. Impact of conjugate pneumococcal vaccines on antibiotic resistance. Lancet Infect Dis. 2008;8:785–95. doi:10.1016/S1473-3099(08)70281-0.
