 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Lymphocyte recirculation within the human body is essential for efficient pathogen detection and immune responses. So far, immune cell migration has been investigated largely using ovine and murine models, with little evidence in humans. Here, we analyzed peripheral blood of healthy individuals following primary vaccination with the Yellow Fever vaccine YF-17D. We found that the number of leukocytes was transiently and sharply reduced in blood as detected on day 7 after vaccine administration. The T cell drop was restricted to cells expressing the lymph node-homing chemokine receptor CCR7. Interestingly, the vaccine-induced drop positively correlated with the expression of CD69 by the T cells before vaccination. This suggests that CCR7+ T cells are being trapped within the lymph nodes through CD69-induced suppression of egress. Strikingly, we further found that the T cell drop negatively correlated with CD8 T cell activation and with production of neutralizing antibodies. In conclusion, early and transient T cell depletion in blood negatively correlated with protective immune response events induced by YF-17D vaccination. Our data highlight baseline CD69 expression and early drop in T cells as potential biomarkers of the Yellow Fever vaccine response.
1. Introduction
Lymphocytes are mobile cells, continually recirculating between blood and secondary lymphoid tissues such as lymph nodes (LNs) and spleen.Citation1 Initially, rats and sheep have been used as experimental systems to study the pattern of lymphocyte recirculation, then mouse models have further revealed tissue-specific expression of migratory molecules and tissue-resident T cell subsets.Citation2–4 Lymphocytes thus migrate to and reside in different regions of the body depending on their type, their origin and their activation status.Citation4–7 In the event of pathogen invasion, circulating lymphocytes are called to inflamed sites and migrate to LNs for efficient priming. After activation, exit of lymphocytes from LNs is regulated by the egress mediator Sphingosine 1-Phosphate Receptor 1 (S1PR1) on lymphocytes.Citation8 When S1PR1 is internalized, lymphocytes are transiently sequestered in LNs, a process termed “LN trapping.” LN trapping is thought to contribute to increase the time available for T cells to efficiently encounter antigen-presenting cells in the LNs, and several signals induce S1PR1 internalization in T cells.Citation8–10
The molecular interactions between receptors and associated ligands allowing selective entry and exit of lymphocytes into tissues have been widely characterized.Citation11,Citation12 Experimental evidence largely derives from non-human animal models, given technical and ethical reasons that limit the analysis of tissues to study lymphocyte migration in humans. Furthermore, studies generally focused on the analysis of localization in LNs and tissues, and do not include the analysis of what occurs in blood. Consequently, there is limited evidence on events occurring in peripheral blood versus other tissues during immune responses.
In this work, we studied various lymphocyte populations in peripheral blood of healthy individuals following YF-17D vaccination using flow cytometry. We showed that early and transient reduction of circulating T cells following YF-17D injection is negatively associated with protective immune response parameters.
2. Materials and methods
2.1. Study design, population, and ethics statement
Blood samples were obtained in the framework of a clinical longitudinal study (protocol 324/13) approved by the Human Research Ethics Committee of the Canton of Vaud with healthy volunteers participating under written informed consent. The study subjects (n = 10) were HLA-A*0201 healthy volunteers aged 21 to 50 years, who for traveling purposes were about to receive the YF-17D vaccine (Stamaril, Sanofi Pasteur) at the Center de Vaccination et Médecine des Voyages (Policlinique Médicale Universitaire, Lausanne). Exclusion criteria were: immunosuppressive treatment, HIV-, hepatitis B- or C-infection, pregnancy or breastfeeding, hemoglobin level under 125 g/L. PBMCs, complete blood counts, and plasma were obtained before vaccination (21 to 46 days before YF-17D injection to comply with allowance of blood withdrawal volumes and intervals) and at various time points following vaccination: days 3, 7, 14, 28 ± 2 days, months 3 ± 2 weeks and 6 ± 2 weeks. The summary of metadata is provided in Table S1.
2.2. Peripheral blood collection and processing
Peripheral blood was immediately processed to read complete blood cell counts and for cryopreservation of plasma and cells awaiting experimental use. Plasma samples were obtained from the supernatant of EDTA-coated blood after centrifugation at 1ʹ000 g for 15 min at room temperature (RT) followed by a second centrifugation at 8ʹ000 g for 10 min at 4°C. For cryopreservation of cells, whole blood was heparinated and processed to fractionate Peripheral blood mononuclear cells (PBMCs) by dilution 1:1 in phosphate buffered saline (PBS), overlay on Lymphoprep for density gradient fractionation (30 min at 400 g without break) and cryopreservation in complete RPMI 1640 supplemented with 40% fetal calf serum (FCS) and 10% dimethyl sulfoxide.
2.3. Flow cytometry analysis
All stainings were performed using PBS with 5 mM EDTA, 0.2% bovine serum albumin, and 20 mM sodium azide [fluorescence-activated cell sorting (FACS) buffer]. For antigen-specific CD8 T cell analysis, PBMC were enriched for CD8 T cells by negative selection (EasySepTM Human CD8 + T cell enrichment kit, Stem Cell, cat. no. 19053) and stained with a PE-labeled multimer HLA-A*0201/LLWNGPMAV (NS4b214-222) (TCmetrix Sàrl) for 30 min at 4°C. For the B lymphocytes, cells were incubated with 20% FcR blocking reagent (Miltenyi Biotec). The following surface antibodies were analyzed in this study: CD3 Krome Orange (Beckman Coulter, clone B00068), CD4 BV785 (Biolegend, clone OK-T4), CD8 APC-AF750 (Beckman Coulter, clone B9.11), CD69 BV650 (Biolegend, clone FN50), CD38 A700 (eBioscience, clone HIT2), CD45RA ECD (Beckman Coulter), CCR7 BV421 (Biolegend, clone G043H7), CD95 PE-Cy7 (Biolegend, clone DX2, CD58 PE (BD Biosciences), CD19 FITC (Beckman Coulter). Surface antibody staining was followed by staining with LIVE/DEAD-Fixable-Aqua (Invitrogen), each step at 4°C for 30 min. Cells were fixed overnight in 1X Fix/Perm solution from the Foxp3 staining kit (eBioscience). Samples were acquired using an LSRII-SORP flow cytometer (BD Bioscience). Cytometer Set-up and Tracking (CSnT) settings were ran in order to quality control the performance of the machine and to calibrate the voltages such that we normalize the sensitivity of the machine across experiments.Citation13 The data were analyzed with FlowJo (Tree Star Inc., v9.5.2) and absolute counts were calculated from the complete blood count analysis. The gates were defined according to the negative and positive populations of each antibody.
2.4. Quantification and statistical analysis
Flow cytometry data analyzed with FlowJo were quantified based on tabulated exports of the frequencies and events in the gates of interest. Summary statistics and data vizualisations were obtained using the softwares Microsoft Excel 14.2.0 and GraphPad Prism 8.1.0, as detailed in each figure legend. The population fold change was defined as
2.5. Plaque reduction neutralizing test (PRNT)
Serum or plasma samples were heated to 56°C for 30 min to inactivate complement. YFV neutralizing antibodies were measured by PRNT. In brief, two independent titers (technical duplicates) of the sera starting at 1/20 were incubated overnight at 4°C with 1000 plaque-forming units of YFV. Monolayers of 5ʹ000 Vero cells were incubated with the titers-virus mixture for 1 h at 37°C (final volume of the PRNT culture was 200 μl). The wells were overlaid with agarose and 2XM199 medium and plaques were counted 3–4 days later using neutral red. As controls, wells with Vero cell monolayers with or without YFV were used (in absence of sera), as the 100% of plaques or 0% of plaques controls, respectively. PRNT50 was calculated, as the dilutions were 50% of the plaques were observed.
3. Results and discussion
To study the immune response to primary YF-17D vaccination, we collected blood before and at several time-points following vaccine injection as described previously.Citation14 We determined the complete blood cell counts based on standard hemograms performed on whole blood and found that the numbers of leukocytes sharply declined 7 days after vaccination, increasing back to baseline levels after 14 days (). The drop concerned to varying extents all cell types that were counted except basophils, which were less visible. In contrast to primary vaccination, we did not observe a decrease of cell counts after recall vaccination (10 years after the primary vaccination against Yellow fever).Citation15 Subsequently, we performed detailed analyses by flow cytometry on PBMC. Amongst the mononuclear cell types analyzed, we focused in particular on the classical T (CD4 and CD8) and B lymphocytes and found that these cells transiently dropped in blood after primary YF-17D vaccination (). Monocytes and NK cells did not display such dropping in cell numbers.Citation15 The extent of CD8 T cell drop correlated with the CD4 T cell drop (), while we found no correlation between the T and B cell drops (). The absence of correlation between the reductions of T and B lymphocytes may be due to the distinct mechanisms accounting for the homing and circulation for each of these cell types.
Figure 1. Circulating CCR7-expressing CD8 and CD4 T cells transiently decrease after primary YF-17D vaccination
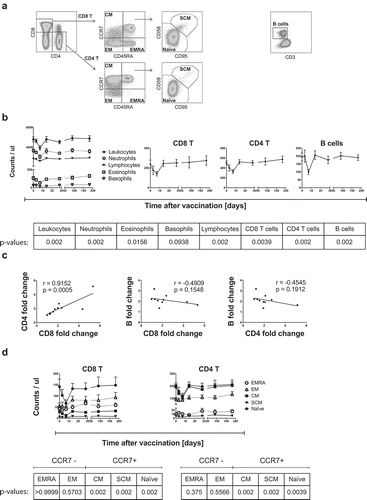
Interestingly, a study in humans on the early signatures of protective immunity in YF-17D vaccination shows data on T and B cell drops occurring in blood, however without particular mention nor investigation on its relevance or correlation to immunogenicity.Citation16 Here we found that the reduction of CD8 and CD4 T cells after YF-17D vaccination was restricted to cells expressing the chemokine receptor CCR7 (). This molecule is critical for naïve CD8 T cell migration to the LNs and splenic white pulp, and memory CD8 T cell localization into secondary lymphoid organs.Citation17–19 Amongst differentiation subsets, Central Memory (CM: CCR7+ CD45RA−) and naïve (CCR7+ CD45RA+ CD95−) subsets significantly decreased, whereas Effector Memory (EM: CCR7− CD45RA−) and Effector Memory CD45RA positive (EMRA: CCR7− CD45RA+) populations remained stable (). The Stem Cell-like Memory (SCM: CCR7+ CD45RA+ CD95+) subset was minor and its drop was less striking yet statistically significant. This CCR7-biased dropping in circulating T cells suggested that early upon YF-17D vaccination, naïve and memory populations (CM and SCM) home to LNs, whereas effector memory cells (EM) and effector memory CD45RA+ cells (EMRA) remain in the periphery. However, our data do not allow to exclude that the reduction of circulating T cells might be caused by apoptosis rather than recruitment to the LNs.
In addition to chemotaxis, another important aspect of T cell homing is retention in the LNs. The early activation marker CD69 is up-regulated on activated T cells and binds to the egress mediator S1PR1 inducing its internalization and consequently sustaining T cell trapping in the LN.Citation8 We observed that some healthy volunteers expressed an increased percentage of CCR7+ CD69+ in T cells at baseline (Fig S1A). This increased co-expression was neither associated with age nor with gender (Fig S1B,C). The absence of correlation between CCR7 CD69 co-expression and CD38 expression at baseline is compatible with the notion that there is no principle difference in the levels of baseline inflammation (Fig S1D).
Interestingly, we found a significant positive correlation between CCR7 and CD69 co-expression levels in CD8 T cells at baseline and the fold-drop in CD8 T cell number after primary YF-17D vaccination (, left panel). For CD4 T cells, the fold-drop in cell numbers tended to correlate positively with the co-expression of CCR7 and CD69 level at baseline (, right panel). This suggests that preexisting levels of CD69 may determine the extent of T cell dropping upon YF-17D vaccination. Similarly, the co-expression of CCR7 and CD69 in CD8 T cells at day 7 after YF-17D vaccination (the peak of CD69 expression) tended to correlate positively with the fold change in CD8 T cell numbers (, left panel). We also observed a significant positive correlation between CCR7 and CD69 co-expression in CD4 T cells at day 7 and the fold-drop in CD4 T cell numbers (, right panel).
Figure 2. CCR7 and CD69 co-expression correlates with the reduction of T lymphocytes in the blood. A. Linear correlations between the CD8 T cell drop and the percentage of CD8 T cells expressing CD69 at baseline (n = 10) and between the CD4 T cell drop and the percentage of CD4 T cells expressing CD69 at baseline (n = 10). B Linear correlations between the CD8 T cell drop and the percentage of CD8 T cells expressing CD69 at day 7 after YF-17D primary vaccination (n = 10) and between the CD4 T cell drop and the percentage of CD4 T cells expressing CD69 at day 7 after YF-17D primary vaccination (n = 10). The Spearman r value and the p-value are indicated on each graph
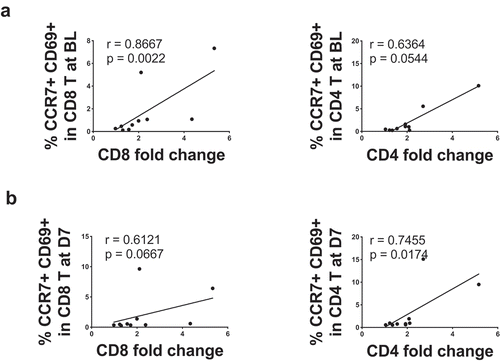
Studies on CD4 T cells in mice suggested that CD69 expression promotes T cell retention in the LNs by downregulating the migratory response to S1P1. CD69 physically interacts with S1PR1, and modulation of CD69 expression is important for the migration of naïve CD4 T cells.Citation20 Our findings suggest that the T cell drop observed after primary YF-17D vaccination results from both the migration (via the CCR7-CCL19/CCL21 interaction) and the CD69-mediated trapping of T cells from the periphery into secondary lymphoid organs.
Next, we wondered whether the drop of B and T lymphocytes in the blood was related to immunogenicity parameters of the YF-17D vaccine, including T cell activation as well as immune protection represented by neutralizing antibodies (nAbs). We analyzed nAb titers at day 28 (peak of antibody induction), and T cell activation based on CD38 expression in total CD8 T cells as well as the frequency of YF-specific CD8 T cells mounted against the immunodominant epitope (HLA-A2/LLWNGPMAV, hereafter A2/LLW) at day 14, i.e., at the peak of the CD8 T cell responseCitation15 (). Of note, all individuals participating in the study are HLA-A*02-positive.
Figure 3. Inter-relation between T and B lymphocyte drops, T cell responses and neutralizing antibodies after primary YF-17D vaccination
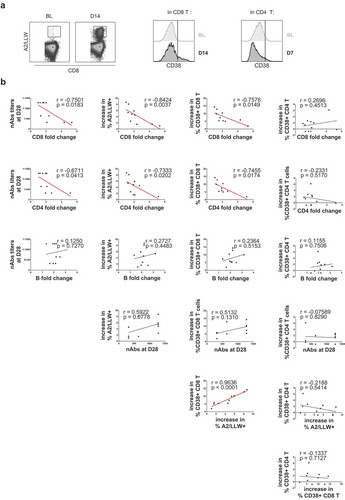
Surprisingly, we found a negative correlation between the extent of CD8 T cell drop (fold change at day 7) and the titer of nAbs at day 28 after YF-17D vaccination (, first line): more CD8 T cell dropping at day 7 resulted in less nAbs at day 28. In addition, we observed that the CD8 T cell drop negatively correlated with the activation of total CD8 T cells (, first line). The T cell drop also negatively correlated with the magnitude of the YF-specific CD8 T cell response (, first line). Regarding CD4 T cells, only the correlation between the CD4 fold change and the increase of the CD38+ CD8 T cell frequency was significant (, second line). Nevertheless, the correlations between the CD4 T cell drop and CD8 T cell activation and the antibody response were close to significance (, second line).
In contrast, the B cell drop did not show any correlation to either nAbs nor T cell activation events (, third line). However, due to the limited number of individuals analyzed and the shorter dynamic range of the B cell drop, we cannot fully exclude a link between these two parameters that could eventually become apparent with larger cohorts. Thus, the observed reduction of nAbs may be related to the drop of particular CD4 T cell subsets and/or to other factors related to B cell biology that remain to be determined.
Altogether, higher CD69 expression in T cells at baseline may lead to a sharper vaccine-induced drop in T cell numbers in peripheral blood, suggesting more pronounced recruitment and trapping of T cells in LNs. A sharper T cell drop correlated with less CD8 T cell activation, a lower magnitude of YF-antigen specific CD8 T cells, and with lower nAb responses to vaccine. These data suggest that individuals with high CD69 expression already before vaccination might have a predisposition for excessive trapping of T cells into LNs that would reduce the overall immunogenicity of the YF-17D vaccine.
Similar to the modulation of S1PR1 by CD69, several studies have shown that LN egress can be blocked with the drug Fingolimod (FTY720) through its action on S1PR1 expressed on T cells.Citation21 FTY720 induces sequestration of lymphocytes in LNs, thereby inhibiting lymphocyte migration to sites of disease.Citation22 Due to its effect to decrease lymphoid cell counts in peripheral blood, FTY720 is remarkably effective in models of transplantation and autoimmunity, and provides therapeutic benefits in patients with multiple sclerosis by reducing the infiltration of lymphocytes into the central nervous system, thereby lowering neuroinflammation.Citation23–26 One could hypothesize that the presence of a higher number of T cells in the secondary lymphoid organs leads to a better B cell activation and antibody production. However, we observed the opposite as both the CD8 and CD4 T cell drops negatively correlated with the production of nAbs after Yellow Fever vaccination. Alternatively, we hypothesize that the impeded recirculation of lymphocytes compromises the priming of lymphocytes and potentially consequently the T–B interaction, leading to poorer humoral responses. Although the exact mechanism of our findings remains to be elucidated, this hypothesis is supported by the observation that the FTY720 compound was shown to impair T-dependent antibody production due to the suppression of germinal center reaction in secondary lymphoid organs.Citation27
Since FTY720 shows immunosuppressive activity, the use of this compound in humans has been associated with a higher incidence of severe infections.Citation28,Citation29 Along those lines, we here highlight the potential relationship between LN trapping of T lymphocytes and the immunogenicity of YF-17D vaccination. However, since this YF-17D vaccination mediates protection of the vast majority of vaccinees, it is unlikely that the observed reduction in immune cells and nAbs alone widely cause clinically significant disease susceptibility. Nevertheless, it remains possible that these observations contributes to explain reduced and/or shorter immune protection, possibly in risk persons for which periodic booster vaccinations against Yellow Fever are recommended.Citation30,Citation31 Notably, the relationship between CD69 expression at baseline, LN trapping of T cells and immune protection that we highlighted in our study is consistent with the findings on the Dengue virus demonstrating that a higher degree of CD69 expression at early time points of disease is related to increased disease severity.Citation32 Interestingly, a clinical trial of vaccination against respiratory syncytial virus reported that the expression of CCR7 and CD69 by CD4 and CD8 T cells was higher in “non-responder” individuals before vaccination, thus supporting our findings.Citation33
In conclusion, our analysis revealed that T cell numbers drop transiently, shortly after priming with the YF-17D vaccine. It especially affected T cell populations expressing CCR7, therefore suggesting that these cells migrate to the LNs. Strikingly, the T cell drop was negatively associated with the CD8 T cell and humoral responses to the YF-17D vaccine. Interestingly, we found a positive correlation between the extent of T cell drop and the expression level of the early activation marker CD69 at baseline (before vaccination). This indicates that individuals with high CD69 expression may be predisposed to excessive LN trapping of T cells.
Our study provides only descriptive correlations and exhibits limitations inherent to research on humans. The cohort that we analyzed is small (n = 10) and the heterogeneity among individuals is large. Furthermore, most often it is only possible to study immune cells from peripheral blood but not from LNs or other organs. Finally, it remains difficult to elucidate underlying mechanisms in humans.
Altogether, our study reveals for the first time a relationship between the expression of CD69 at baseline, transient dropping of T cell counts early after vaccination and reduced ability of the host to mount a protective response following YF-17D vaccination.
Authorship
AB, DES and SAFM conceived and designed experiments. AB performed the experiments. AB and SAFM analyzed the data. AB, DES and SAFM wrote the manuscript. All authors revised and approved the final version of the manuscript.
Disclosure of potential conflicts of interest
The authors declare no existence of a conflict of interest.
Acknowledgments
We thank the collaborators of the Flow Cytometry Facility of the University of Lausanne (Switzerland) for cytometer instrument configuration and maintenance. We thank Nicole Montandon for technical assistance in processing blood specimens. We thank Hélène Maby-El Hajjami, Paula Marcos Mondéjar, Samia Abed Maillard, Blaise Genton, Francine Widmer, Pierrette Meige and the personnel of the “Centre de vaccination et médecine des voyages” at the Policlinique Médicale Universitaire in Lausanne for running the clinical vaccination study. Finally, we warmly thank all donors that volunteered and thus preciously contributed to our findings.
This study was supported by the University of Lausanne, the Quealth Foundation, and the Swiss National Science Foundation (grant 310030B_179570).
Additional information
Funding
References
- Kindt TJ, Goldsby RA, Osborne BA. Kuby Immunology (2007). New York: Freeman, W.H. and Co; 2007.
- GOWANS JL. The effect of the continuous re-infusion of lymph and lymphocytes on the output of lymphocytes from the thoracic duct of unanaesthetized rats. Br J Exp Pathol. 1957;38:67–78.
- HALL JG, MORRIS B. The origin of the cells in the efferent lymph from a single lymph node. J Exp Med. 1965;121:901–10. doi:https://doi.org/10.1084/jem.121.6.901.
- Szabo PA, Miron M, Farber DL. Location, location, location: tissue resident memory T cells in mice and humans. Sci Immunol. 2019;4:eaas9673. doi:https://doi.org/10.1126/sciimmunol.aas9673.
- Duijvestijn A, Hamann A. Mechanisms and regulation of lymphocyte migration. Immunol Today. 1989;10:23–28. doi:https://doi.org/10.1016/0167-5699(89)90061-3.
- Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–17. doi:https://doi.org/10.1084/jem.171.3.801.
- Westermann J, Ehlers E-M, Exton MS, Kaiser M, Bode U. Migration of naive, effector and memory T cells: implications for the regulation of immune responses. Immunol Rev. 2001;184:20–37. doi:https://doi.org/10.1034/j.1600-065x.2001.1840103.x.
- Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi:https://doi.org/10.1146/annurev-immunol-020711-075011.
- Klonowski KD, Marzo AL, Williams KJ, Lee S-J, Pham Q-M, Lefrançois L. CD8 T cell recall responses are regulated by the tissue tropism of the memory cell and pathogen. J. Immunol. 2006;177(6738):LP–6746. doi:https://doi.org/10.4049/jimmunol.177.10.6738.
- Olson MR, McDermott DS, Varga SM. The initial draining lymph node primes the bulk of the CD8 T cell response and influences memory T cell trafficking after a systemic viral infection. PLoS Pathog. 2012;8:e1003054. doi:https://doi.org/10.1371/journal.ppat.1003054.
- Hunter MC, Teijeira A, Halin C. T cell trafficking through lymphatic vessels. Front Immunol. 2016;7:613. doi:https://doi.org/10.3389/fimmu.2016.00613.
- Wiedle G, Dunon D, Imhof BA. Current concepts in lymphocyte homing and recirculation. Crit Rev Clin Lab Sci. 2001;38:1–31. doi:https://doi.org/10.1080/20014091084164.
- Perfetto SP, Ambrozak D, Nguyen R, Chattopadhyay P, Roederer M. Quality assurance for polychromatic flow cytometry. Nat Protoc. 2006;1:1522–30. doi:https://doi.org/10.1038/nprot.2006.250.
- Fuertes Marraco SA, Bovay A, Nassiri S, Maby-El Hajjami H, Ouertatani-Sakouhi H, Held W, Speiser DE. The human CD8 T stem cell-like memory phenotype appears in the acute phase in Yellow Fever virus vaccination. Biorxiv. 2019;808774. doi: https://doi.org/10.1101/808774
- Bovay A, Nassiri S, Maby–El Hajjami H, Marcos Mondéjar P, Akondy RS, Ahmed R, Lawson B, Speiser DE, Fuertes Marraco SA. Minimal immune response to booster vaccination against Yellow Fever associated with pre-existing antibodies. Vaccine. 2020;38(9):2172–82. doi:https://doi.org/10.1016/j.vaccine.2020.01.045.
- Kohler S, Bethke N, Böthe M, Sommerick S, Frentsch M, Romagnani C, Niedrig M, Thiel A. The early cellular signatures of protective immunity induced by live viral vaccination. Eur J Immunol. 2012;2363–73. doi:https://doi.org/10.1002/eji.201142306.
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi:https://doi.org/10.1038/44385.
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi:https://doi.org/10.1146/annurev.immunol.22.012703.104702.
- Nolz JC, Health IO. Biology, C. & Health, O. HHS Public Access. 2016;72:2461–73.
- Tomura M, Itoh K, Kanagawa O. Naive CD4+ T lymphocytes circulate through lymphoid organs to interact with endogenous antigens and upregulate their function. J Immunol. 2010;184(4646):LP–4653. doi:https://doi.org/10.4049/jimmunol.0903946.
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002;277:21453–57. doi:https://doi.org/10.1074/jbc.C200176200.
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–9. doi:https://doi.org/10.1126/science.1070238.
- Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C, Kristofic C, Kuhle J, Lindberg RLP, Kappos L, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71(1261):LP–1267. doi:https://doi.org/10.1212/01.wnl.0000327609.57688.ea.
- Mehling M, Johnson TA, Antel J, Kappos L, Bar-Or A. Clinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosis. Neurology. 2011;76:S20 LP–S27. doi:https://doi.org/10.1212/WNL.0b013e31820db341.
- Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 2006;355:1124–40. doi:https://doi.org/10.1056/NEJMoa052643.
- Brinkmann V, Lynch KR. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr Opin Immunol. 2002;14:569–75. doi:https://doi.org/10.1016/S0952-7915(02)00374-6.
- Han S, Zhang X, Wang G, Guan H, Garcia G, Li P, Feng L, Zheng B. FTY720 suppresses humoral immunity by inhibiting germinal center reaction. Blood. 2004;104:4129–33. doi:https://doi.org/10.1182/blood-2004-06-2075.
- Winkelmann A, Loebermann M, Reisinger EC, Hartung H-P, Zettl UK. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. 2016;12:217–33. doi:https://doi.org/10.1038/nrneurol.2016.21.
- Grebenciucova E, Reder AT, Bernard JT Immunologic mechanisms of fingolimod and the role of immunosenescence in the risk of cryptococcal infection: A case report and review of literature. Mult. Scler. Relat. Disord. 9, 158–62 (2016).
- SAGE Working Group. Background paper on Yellow Fever vaccine. WHO. 2013. http://www.who.int/immunization/sage/previous/en/index.html
- WHO. Vaccines and vaccination against yellow fever: WHO position paper, June 2013—Recommendations. Vaccine. 2015;33:76–77. doi:https://doi.org/10.1016/j.vaccine.2014.05.040.
- Green S, Pichyangkul S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Nisalak A, Kurane I, Rothman AL, Ennis FA. Early CD69 Expression on Peripheral Blood Lymphocytes from Children with Dengue Hemorrhagic Fever. J Infect Dis. 1999;1429–35. doi:https://doi.org/10.1086/315072.
- Lingblom CMD, Kowli S, Swaminathan N, Maecker HT, Lambert SL. Baseline immune profile by CyTOF can predict response to an investigational adjuvanted vaccine in elderly adults. J Transl Med. 2018;16:153. doi:https://doi.org/10.1186/s12967-018-1528-1.

