ABSTRACT
Conserved Moraxella catarrhalis (Mcat) proteins, oligopeptide permease (Opp)A, hemagglutinin (Hag), outer membrane protein (OMP) CD, Pilin A clade 2 (PilA2), and Moraxella surface protein (Msp) 22 have been studied as vaccine candidates. Children who experience frequent acute otitis media (AOM) confirmed with pathogen identification by tympanocentesis are referred to as stringently-defined otitis prone (sOP). Synchrony of serum antibody responses against 5 Mcat proteins, OppA, Hag, OMP CD, PilA2, and Msp22 resulting from nasopharyngeal colonization and AOM was studied for 85 non-otitis prone (NOP) children and 34 sOP children. Changes in serum IgG were quantitated with ELISA. Serum IgG antibody levels against OppA, Hag, OMP CD, and Msp22 rose in synchrony in NOP and sOP children; that is, the proteins appeared equally and highly immunogenic in children at age 6 to 22–25 months old and then leveled off in their rise at 22–25 to 30 months old. In contrast, rises of PilA2 were slow from 6 months old and kept constant and did not level off significantly before 30 months old. OppA, Hag, OMP CD, and Msp22 elicited a synchronous acquisition of naturally-induced serum antibody in young children. A multi-valent Mcat protein vaccine combining OppA, Hag, OMP CD, and Msp22 may exhibit less antigen competition when administered as a combination vaccine in young children.
1. Introduction
Moraxella catarrhalis (Mcat) is a Gram-negative diplococcus that causes acute otitis media (AOM), otitis media with effusion, acute sinusitis and acute exacerbations of chronic bronchitis. AOM is the most common infectious disease among children that induces parents to seek medical care for their child and receive antibiotics. Mcat was the third most common cause of AOM after Streptococcus pneumoniae (Spn) and nontypeable Haemophilus influenzae (NTHi) in children and adults for decades;Citation1–3 but in 2014 for the first time ever Mcat caused more AOM than Spn or NTHi in children in our study cohort.Citation4 Similarly, Mcat was recently identified as the most common otopathogen in Finish children.Citation5 Mcat nasopharyngeal (NP) colonization rates have significantly increased since the introduction of the pneumococcal glycoconjugate vaccines in children.Citation6 Coincidentally, Mcat-caused AOM morbidity (107 million-142 million global cases each year) is high and increasing.Citation7 In the United States alone, the economic burden of otitis media exceeded 5 USD billion/year in 1997 in medical treatment, surgical management, and loss of income for working parents.Citation8 In addition, Mcat is responsible for approximately 10% (up to 3 million cases) of exacerbations of chronic obstructive pulmonary disease (COPD) in adults annually in the U.S.Citation9,Citation10 COPD is the third leading cause of death in the U.S., affecting at least 24 million people and costs 50 USD billion in healthcare expenses each year.Citation11 Mcat produces beta-lactamase thereby rendering the organism resistant to the recommended first-line antibiotics to treat children with AOM. Therefore, there are pressing needs for the development of a Mcat vaccine.
A candidate Mcat vaccine antigen should possess some essential characteristics such as exposed on the surface, conserved among strains, highly immunogenic, functioning for Mcat pathogenesis such as adhesion or virulence. A number of potential vaccine antigens of Mcat have been identified with significant immunogenicity and protective effectiveness in various animal models.Citation12–15 Some studies have detected antibody responses to Mcat proteins in humans.Citation12–14,Citation16,Citation17 We have been investigating 5 Mcat proteins as possible ingredients to be included in a multi-component vaccine. We expect that a multi-component vaccine could be more efficacious than a single-valent vaccine because multiple antigens may produce a more synergistic immune response with broader coverage of the Mcat strains. The 5 proteins studied have been oligopeptide permease (Opp)A (an oligopeptide binding protein),Citation11 hemagglutinin (Hag, an adhesin and transporter),Citation18 outer membrane protein (OMP) CD (a porin and adhesin),Citation19 Pilin A clade 2 (PilA2, a major pilin subunit)Citation20 and Moraxella surface protein (Msp) 22 (a putative outer membrane lipoprotein).Citation21
We have been examining serum and mucosal antibody responses to the 5 protein vaccine candidates following natural NP colonization and AOM in young children.Citation22–24 We expected that immunogens prepared in pure form and adjuvanted may stimulate an immune response in young children when natural exposure to the protein would not stimulate a response. However, natural priming and boosting of the immune system play an important role in successful vaccination and sustaining immunogenicity and protection from disease.Citation25 Therefore, we hypothesized that among the antigens available, selection of those that were more immunogenic upon natural immunization at the youngest ages enhanced the chances of their success as vaccines.
Our group has been particularly interested to find a vaccine to prevent AOM caused by Mcat and specifically in young children who experience repeated, closely spaced AOM infections, termed otitis prone (OP) children, since they experience the greatest morbidity and financial costs to the health care system.Citation26 We have adopted a standard definition of otitis prone as ≥3 AOM episodes in 6 months or ≥4 AOM episodes in 12 months. Our group introduced the concept of the stringently defined otitis prone (sOP) child for which every AOM episode is confirmed by culture of bacterial otopathogens from middle ear fluid collected by using tympanocentesis.Citation26,Citation27 Children who have 0–2 AOM episodes in 6 months or 0–3 AOM episodes in 12 months are termed non-otitis prone (NOP).Citation27–30 Our studies of relative immunogenicity in infants and toddlers following NP colonization and AOM in sOP children identified OppA, Hag and OMP CD as the best candidates to consider in a multi-component vaccine.Citation23,Citation24
It has been shown that immunogenicity can be reduced when multiple vaccine ingredients are combined into a multi-component product compared to immunogenicity elicited when the components are administered as single ingredients.Citation31 Various mechanisms have been proposed to account for reduced immunogenicity in combination vaccines but common among the results has been a reduction in immunogenicity for the least immunogenic ingredient in a combination.Citation31 We previously successfully used a novel generalized additive model (GAM) to show that naturally-induced serum antibodies against three Spn protein vaccine candidates pneumococcal histidine triad protein (Pht)D, PhtE, and pneumolysin (Ply) rise in a synchronous pattern in young children at age 6–25 months old.Citation32 Our analyses demonstrated that the three Spn antigens are similarly immunogenic and are compatible to be formulated in a single trivalent vaccine.Citation32 In this study we apply a different linear mixed effects model for analyzing the immunogenicity of the five Mcat protein vaccine candidates as a means to predict an increased likelihood of equivalent immunogenicity of a multi- component vaccine. Our results show that there is a synchrony in the serum antibody responses to OppA, Hag, OMP CD, and Msp22 antigens but not with PilA2 in both NOP and sOP children.
2. Materials and methods
2.1. Subjects and sampling
The samples collected and analyzed were obtained during a prospective study supported by the National Institute of Deafness and Communication Disorders, as previously described.Citation33,Citation34 Healthy children without previous episodes of AOM were enrolled at 6 months of age from a middle class, suburban socio-demographic pediatric practice in Rochester, NY during years 2006–2016 (). Spn, NTHi, and Mcat were identified as the main colonizing potentially otopathogenic bacteria in the nasopharynx and main pathogens causing AOM by analyzing all collected nasal wash, NP, oropharygneal, middle ear fluid (MEF) samples using culture and Polymerase chain reaction (PCR) tests as in prior studies by our group.Citation33,Citation35,Citation36 Comprehensive NP colonization results and AOM rates of the 3 pathogens have been previously reported in detail for the same cohort of children by our group.Citation35 Children are defined as NOP if they have 0–2 AOM episodes in 6 months or 0–3 AOM episodes in 12 months and sOP if have ≥ 3 AOM episodes within 6 months or ≥ 4 AOM in one year.Citation27–30
Table 1. Demographics of the subjects
For this study we assessed a total of 119 children followed prospectively until 30 months of age (). These children were selected based on detected Mcat colonization of the nasopharynx at 1 or more of 7 prospective visits (at age 6, 9, 12, 15, 18, 24, and 30 months). Serum, nasal wash, and nasopharyngeal (NP) and oropharyngeal cultures were collected at each visit during the study period. Whenever children experienced an AOM episode a confirmatory tympanocentesis was performed and MEF was microbiologically assessed (an essential component to the definition of “stringently-defined” AOM) since virtually all prior studies have relied on only a clinical diagnosis that is known to be variably accurate. Serum, nasal wash, NP, oropharyngeal and MEF sampling was conducted as previously describedCitation23,Citation33 and the samples were analyzed to identify bacterial pathogens by using standard culture and PCR assay.Citation33 The study was approved by the Rochester General Hospital Research Subjects Review Boards. Written informed consent was obtained for participation and all procedures are in accordance with the Declaration of Helsinki.
2.2. Enzyme-linked immunosorbent assay
Protein-specific antibody titers were determined using an Enzyme-linked Immunosorbent Assay (ELISA) as previously described.Citation22–24 The serum antibodies to the Mcat proteins were determined by ELISA using purified recombinant proteins of OppA, Hag5-9 (truncated Hag protein), OMP CD, PilA2, and Msp22NL (non-lipidated Msp22).Citation22–24 All the recombinant proteins have been used to immunize mice to produce anti-sera. The binding specificity of the 5 Mcat protein antigens on the surface of the Mcat with corresponding mouse anti-sera have been confirmed by using flow cytometry (data not shown) although the selected children’s sera were not tested in whole Mcat cell ELISA assay for both NOP and sOP children in this study.
2.3. Statistical analysis
All antibody titers were logarithm transformed. Estimates of functional relationship between mean log10 concentration response and age (months) were obtained using a linear mixed effects model.Citation37–39 The model takes into account the longitudinal effect of some children being tested multiple time at subsequent visits. In addition, 95% confidence bands were constructed for the response functions.
Models were fit using the lme4 package, part of the R statistical computing environment.Citation39 This function allows inclusion of random effects in the model. This permits modeling of appropriate correlation structure induced by the use of subject-level repeated measures.
3. Results
3.1. Serum IgG antibody response to Mcat proteins in NOP children
The children studied here were selected from a large cohort based on the fact that they experienced culture-confirmed NP colonization with Mcat repeatedly such that an immune response most likely would be stimulated. To confirm that antibody concentrations to these 5 proteins reflected a child’s exposure to Mcat and not a response to other natural NP organisms that cause AOM, such as Spn, NTHi, and Staphylococcus aureus, we performed a protein homology search analysis using the basic local alignment search tool (BLASTp) algorithm. We found that the conserved 5 Mcat proteins studied did not share significant homology with the other common organisms that cause AOM.
The antigen-specific serum antibody analysis reported in this study describes the change of antibody concentration over time among the children in the specified cohorts for each antigen tested as shown in the accompany . For the NOP children, significant change of serum IgG against OppA (P < .001, ), Hag5-9 (P < .001, ), OMP CD (P < .001, ), PilA2 (P < .001, ), and Msp22NL (P < .001, ) were measured over time. The antibody levels against OppA, Hag5-9, OMP CD, and Msp22NL peaked at age 24–30 months old. Serum IgG concentrations to OppA, Hag5-9, OMP CD, and Msp22NL were in synchrony in NOP children. This is evident by examination of the slope for the 4 antigens (). In contrast, serum IgG against PilA2 () did not peak over time and had a different rate of rise from those of OppA, Hag5-9, OMP CD, and Msp22NL.
Figure 1. Serum IgG antibody response to Mcat proteins OppA (A), Hag5-9 (truncated Hag protein, B), OMP CD (C), PilA2 (D) and Msp22NL (non-lipidated Msp22, E) in NOP children age 6–30 months. Serum IgG antibody concentrations (ng/mL) were determined with a quantitative ELISA and then logarithm transformed. Mean (solid lines) and 95% confidence intervals (dashed lines) are shown. Functional estimates of log10 concentration against age were obtained using a linear mixed effects model. A total of 149 time points from 85 subjects were analyzed. Small circles represent IgG data
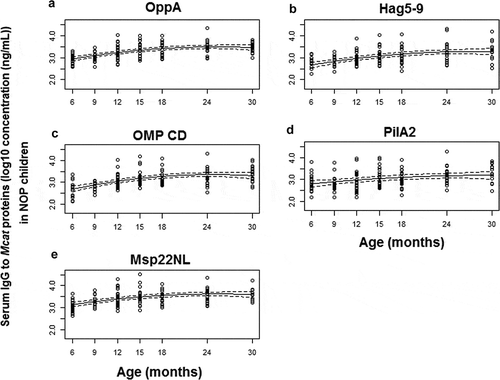
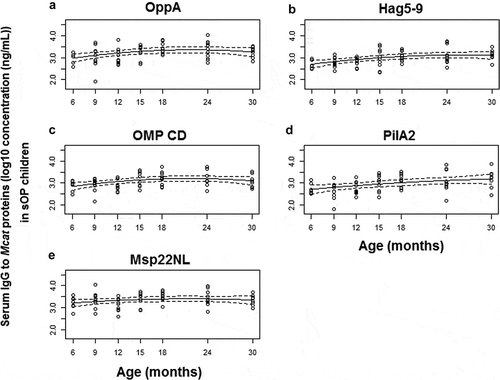
Figure 2. Serum IgG antibody response to Mcat proteins OppA (A), Hag5-9 (truncated Hag protein, B), OMP CD (C), PilA2 (D) and Msp22NL (non-lipidated Msp22, E) in sOP children age 6–30 months. Serum IgG antibody concentrations (ng/mL) were determined with a quantitative ELISA and then logarithm transformed. Mean (solid lines) and 95% confidence intervals (dashed lines) are shown. Functional estimates of log10 concentration against age were obtained using a linear mixed effects model. A total of 66 time points from 34 subjects were analyzed. Small circles represent IgG data
The mean rate of increase for serum IgG against OMP CD was the highest among 5 Mcat proteins at age 6–18 months (), indicating that OMP CD was more immunogenic than OppA, Hag5-9, Msp22NL, and PilA2 at an earlier age. However, the antibody levels against Hag5-9 and PilA2 continued to rise through age 6–30 months, suggesting a longer-lasting immunogenicity in young children than OppA, OMP CD, Msp22NL, for which the antibody levels started to decrease earlier at age 25–27 months old ().
3.2. Serum IgG antibody response to Mcat proteins in sOP children
For sOP children, significant change was measured in serum IgG against OppA (P = .012, ), Hag5-9 (P = .001, ), and OMP CD (P = .008, ) and PilA2 (P = .004, ), but not Msp22NL (P = .26, ) at age 6–30 months. Serum IgG concentrations to OppA, Hag5-9, OMP CD, and Msp22NL were in synchrony in sOP children. This is evident by examination of the slope for the 4 antigens (). In contrast the slope in antibody concentration for PilA2 kept constant across the full time of observations (), which was not similar to OppA, Hag5-9, OMP CD or Msp22NL.
3.3. Mcat NP colonization and AOM effects on serum antibody response to Mcat proteins in NOP and sOP children
We have detected serum antibodies against the 5 Mcat protein antigens in combined NOP and sOP children with or without NP colonization of Mcat and compared them in children without any obvious medical history of AOM to children with AOM history. We did 35 comparisons of those two populations across 7 visits (at 6 to 30 months) and 5 Mcat protein antigens. Out of all 35 cases, only two P-values were below 0.05 (0.027 and 0.035). However, in order to obtain the joint alpha level of 0.05 for all 35 cases, one should use 0.05/35 = 0.0014 P-value threshold for each individual comparison. Hence, we conclude that there is no statistically significant difference between those two populations.
We previously found that 49% of sOP children with AOM had Mcat-caused AOM, which was significantly higher than 24% of NOP children with AOM (P < .0001). The relative risk of Mcat-caused AOM for sOP:NOP was thus twofold higher.Citation23 However, there was no statistically significant difference in serum IgG levels against the 5 Mcat protein antigens between children colonized with Mcat who never had AOM versus the children colonized with Mcat who developed AOM during age 6–30 months when NOP and sOP children were pooled together for analysis. We did 35 comparisons of those two populations across 7 visits (at 6 to 30 months) and 5 Mcat protein antigens. In all 35 cases, the results were not significant (P > .05).
4. Discussion
Mcat protein antigens OppA, Hag, and OMP CD all have been shown to be highly immunogenic to elicit age-dependent natural acquisition of serum and mucosal antibody increase in young children in our previous studies.Citation22–24 Here we demonstrated that the age-dependent rise of naturally-induced serum antibody responses against OppA, Hag, and OMP CD, and Msp22 were in synchrony in both NOP and sOP children. The current data suggest that OppA, Hag, and OMP CD, and Msp22 are similarly immunogenic and would be less likely to result in diminished antibody responses caused by antigenic competition if the antigens are given as a multi-component product. Further studies comparing the human immune efficacy among the different combinations of these antigens and each individual component with or without adjuvants are warranted to confirm the observations in the current study. However, the current work supports that OppA, Hag, and OMP CD, and Msp22 are compatible to be formulated in a single multi-valent vaccine.
We applied a statistical model to understand age-dependent immunogenicity of multiple vaccine candidates in this study. The serum antibodies against OppA, Hag, and OMP CD, and Msp22 reached a plateau around age 24 months with a baseline gradient of increase for both NOP and sOP children. This may suggest a peaked serum humoral immune response to the Mcat antigens in young children. Such analysis corroborated the synchrony of responses. A dynamic change of natural acquisition of serum antibody against Mcat in young children was depicted by the plots.
We previously have shown that sOP children had lower natural acquisition of serum and mucosal antibody responses to Mcat antigens OppA, Hag, and OMP CD, and PilA2 than NOP children upon NP colonization and middle ear infection of Mcat.Citation23,Citation24 sOP children also mount lower serum antibody responses to NTHi vaccine antigens P6, protein D, and OMP26,Citation28 and serum and mucosal antibodies to Spn vaccine antigens PhtD, PcpA, and PlyD1Citation35,Citation40 in our prior studies. However, our current data suggest that Mcat antigens OppA, Hag, and OMP CD, and Msp22 are able to elicit a significantly synchronous rise of natural serum antibody responses in sOP children. sOP children is the most vulnerable population to the morbidity of AOM and any vaccination strategy for AOM must especially target that population. By eliciting synchronous antibody rises at younger ages, a multi-component vaccine containing all or partial of these four antigens might be expected to confer more efficacious protection than any individual antigen in sOP children.
The current study did not show synchrony of serum antibody response to PilA2 with serum antibody response to OppA, Hag, and OMP CD, and Msp22 because PilA2 produced low and later-developing antibody responses with age. The current findings are encouraging because the synchronous serum antibody responses to the Mcat proteins OppA, Hag, and OMP CD, and Msp22 were identified not only in NOP children but also in sOP children although NOP children generally produce a higher serum antibody response to these proteins than sOP children, as we have found previously.Citation23,Citation24 It is noteworthy that a linear mixed effects model was used to compare the difference of the serum antibody responses between NOP and sOP children in our prior studyCitation24 and a different linear mixed effects model was used to separately analyze the synchrony of the serum antibody responses in both NOP and sOP children in the current work although the subjects of both studies are from the same population.
Our studies thus far suggest that deficient serum and mucosal antibody responses in sOP children are associated with the susceptibility of this population to recurrent otitis media.Citation23,Citation24,Citation28,Citation35,Citation40 Vaccination with the combined Mcat protein antigens eliciting synchronous immune responses may confer effective protection against Mcat-caused otitis media. However, the protective levels of the serum antibodies against each Mcat protein have not been determined. Efficacy clinical trials would be necessary to address all these concerns in the future.
Serum samples were collected from children initially at age 6 months. Serum antibody changes in children from 6 months to 30 months were compared relative to baseline at age 6 months and rates of change in antibody concentrations over time did not depend on the initial values at 6 months. This means that maternal IgGs did not interfere the assay/calculations as such. Antibodies to Mcat proteins measured at 6 months likely are a mixture of maternal and infant-produced. Maternal IgG in infants wanes around 6–9 months after birth. We are not able to determine the amount of maternal IgG in infants age 6 months old nor to determine if sOP children have different levels of protective maternal antibodies compared to NOP children.
Our study has limitations. The current work assessed the samples of subjects with Mcat NP colonization positive confirmed by culture during the 7 periodic healthy visits. We may miss some Mcat NP colonization events out of the sampling visits. However, the dynamics of the antibody development over time can be reliably predicted by using the linear mixed effects model. We did not undertake studies of Mcat protein antigens in in vivo conditions in this work. Future studies on immunizations with combined vaccines comprising these antigens may reveal mechanisms underlying the synchronous serum immune responses to these proteins in humans especially the main target population, young children. Our study design does not include collection of blood from mother or child before 6 months of age. Therefore, we are not able to determine the maternal IgG level or in infants younger than 6 months old due to absence of serum samples available for analysis, which is a limitation of this work. Future studies are warranted to investigate the role of maternal antibodies and antibodies in infants younger than 6 months old in the pathogenesis of AOM in young children.
5. Conclusions
Serum IgG antibody responses against OppA, Hag, and OMP CD, and Msp22 were in synchrony in both NOP and sOP children, which suggests that they are similarly immunogenic and therefore compatible to be combined in a multi-component Mcat protein vaccine.
Disclosure of potential conflicts of interest
Timothy F. Murphy has patents for vaccines for M. catarrhalis. Michael E. Pichichero has received research grants from Pfizer, Sanofi Pasteur, and Merck. No other potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Dr. Anthony A. Campagnari and Dr. Nicole Luke-Marshall at University at Buffalo, The State University of New York for their kindness to provide the expression plasmid and bacterial strains for production of PilA2 recombinant protein. We are indebted to Jill Mangiafesto and Konnor Shares for their ELISA work. We thank Dr. Janet Casey and the nurses and staff of Legacy Pediatrics and the collaborating pediatricians from Sunrise Pediatrics, Westfall Pediatrics, Lewis Pediatrics and Long Pond Pediatrics and the parents who consented and the children who participated in this challenging study. We also thank the support of materials and methods from R01 DC012200 to Dr. Timothy F. Murphy.
Additional information
Funding
References
- Casey JR, Kaur R, Friedel VC, Pichichero ME. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatr Infect Dis J. 2013;32:805–09.
- Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr Infect Dis J. 2004;23:824–28. doi:https://doi.org/10.1097/01.inf.0000136871.51792.19.
- Pichichero ME, Pichichero CL. Persistent acute otitis media: I. Causative pathogens. Pediatr Infect Dis J. 1995;14:178–83. doi:https://doi.org/10.1097/00006454-199503000-00002.
- Casey JR, Kauer R, Pichichero M Otopathogens causing otitis media in the 13-valent pneumococcal conjugate vaccine era. 18th international symposium on recent advances in otitis media. Gaylord National Resort and Convention Center, Maryland, USA; 2015. p. 211.
- Sillanpaa S, Oikarinen S, Sipila M, Kramna L, Rautiainen M, Huhtala H, Aittoniemi J, Laranne J, Hyöty H, Cinek O, et al. Moraxella catarrhalis might be more common than expected in acute otitis media in young finnish children. J Clin Microbiol. 2016;54(9):2373–79. doi:https://doi.org/10.1128/JCM.01146-16.
- Revai K, McCormick DP, Patel J, Grady JJ, Saeed K, Chonmaitree T. Effect of pneumococcal conjugate vaccine on nasopharyngeal bacterial colonization during acute otitis media. Pediatrics. 2006;117:1823–29. doi:https://doi.org/10.1542/peds.2005-1983.
- Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, Grasso D, Barbiero C, Tamburlini G. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7(4):e36226. doi:https://doi.org/10.1371/journal.pone.0036226.
- Kaplan B, Wandstrat TL, Cunningham JR. Overall cost in the treatment of otitis media. Pediatr Infect Dis J. 1997;16:S9–11. doi:https://doi.org/10.1097/00006454-199702001-00003.
- Murphy TF, Brauer AL, Grant BJ, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med. 2005;172:195–99. doi:https://doi.org/10.1164/rccm.200412-1747OC.
- Seemungal TA, Hurst JR, Wedzicha JA. Exacerbation rate, health status and mortality in COPD–a review of potential interventions. Int J Chron Obstruct Pulmon Dis. 2009;4:203–23. doi:https://doi.org/10.2147/COPD.S3385.
- Jones MM, Johnson A, Koszelak-Rosenblum M, Kirkham C, Brauer AL, Malkowski MG, Murphy TF. Role of the oligopeptide permease ABC transporter of Moraxella catarrhalis in nutrient acquisition and persistence in the respiratory tract. Infect Immun. 2014;82:4758–66. doi:https://doi.org/10.1128/IAI.02185-14.
- Su YC, Singh B, Riesbeck K. Moraxella catarrhalis: from interactions with the host immune system to vaccine development. Future Microbiol. 2012;7:1073–100. doi:https://doi.org/10.2217/fmb.12.80.
- Mawas F, Ho MM, Corbel MJ. Current progress with Moraxella catarrhalis antigens as vaccine candidates. Expert Rev Vaccines. 2009;8:77–90. doi:https://doi.org/10.1586/14760584.8.1.77.
- Tan TT, Riesbeck K. Current progress of adhesins as vaccine candidates for Moraxella catarrhalis. Expert Rev Vaccines. 2007;6:949–56. doi:https://doi.org/10.1586/14760584.6.6.949.
- Ren D, Pichichero ME. Vaccine targets against Moraxella catarrhalis. Expert Opin Ther Targets. 2016;20:19–33. doi:https://doi.org/10.1517/14728222.2015.1081686.
- Smidt M, Battig P, Verhaegh SJ, Niebisch A, Hanner M, Selak S, Schüler W, Morfeldt E, Hellberg C, Nagy E, et al. Comprehensive antigen screening identifies Moraxella catarrhalis proteins that induce protection in a mouse pulmonary clearance model. PLoS One. 2013;8:e64422. doi:https://doi.org/10.1371/journal.pone.0064422.
- Verhaegh SJ, de Vogel CP, Riesbeck K, Lafontaine ER, Murphy TF, Verbrugh HA, Jaddoe VWV, Hofman A, Moll HA, van Belkum A, et al. Temporal development of the humoral immune response to surface antigens of Moraxella catarrhalis in young infants. Vaccine. 2011;29(34):5603–10. doi:https://doi.org/10.1016/j.vaccine.2011.06.019.
- LaFontaine ER, Snipes LE, Bullard B, Brauer AL, Sethi S, Murphy TF. Identification of domains of the Hag/MID surface protein recognized by systemic and mucosal antibodies in adults with chronic obstructive pulmonary disease following clearance of Moraxella catarrhalis. Clin Vaccine Immunol. 2009;16:653–59. doi:https://doi.org/10.1128/CVI.00460-08.
- Murphy TF, Kirkham C, Liu DF, Sethi S. Human immune response to outer membrane protein CD of Moraxella catarrhalis in adults with chronic obstructive pulmonary disease. Infect Immun. 2003;71:1288–94. doi:https://doi.org/10.1128/IAI.71.3.1288-1294.2003.
- Luke-Marshall NR, Sauberan SL, Campagnari AA. Comparative analyses of the Moraxella catarrhalis type-IV pilus structural subunit PilA. Gene. 2011;477:19–23. doi:https://doi.org/10.1016/j.gene.2011.01.010.
- Ruckdeschel EA, Kirkham C, Lesse AJ, Hu Z, Murphy TF. Mining the Moraxella catarrhalis genome: identification of potential vaccine antigens expressed during human infection. Infect Immun. 2008;76:1599–607. doi:https://doi.org/10.1128/IAI.01253-07.
- Ren D, Almudevar AL, Murphy TF, Lafontaine ER, Campagnari AA, Luke-Marshall N, Casey JR, Pichichero ME. Serum antibody response to Moraxella catarrhalis proteins OMP CD, OppA, Msp22, Hag, and PilA2 after nasopharyngeal colonization and acute otitis media in children. Vaccine. 2015;33:5809–14. doi:https://doi.org/10.1016/j.vaccine.2015.09.023.
- Ren D, Murphy TF, Lafontaine ER, Pichichero ME. Stringently defined otitis prone children demonstrate deficient naturally induced mucosal antibody response to Moraxella catarrhalis proteins. Front Immunol. 2017;8:953. doi:https://doi.org/10.3389/fimmu.2017.00953.
- Ren D, Almudevar AL, Murphy TF, Lafontaine ER, Campagnari AA, Luke-Marshall N, Pichichero ME. Serum antibody response to Moraxella catarrhalis proteins in stringently defined otitis prone children. Vaccine. 2019;37:4637–45. doi:https://doi.org/10.1016/j.vaccine.2017.07.027.
- Pichichero ME. Booster vaccinations: can immunologic memory outpace disease pathogenesis? Pediatrics. 2009;124:1633–41. doi:https://doi.org/10.1542/peds.2008-3645.
- Pichichero ME, Casey JR, Almudevar A. Reducing the frequency of acute otitis media by individualized care. Pediatr Infect Dis J. 2013;32:473–78. doi:https://doi.org/10.1097/INF.0b013e3182862b57.
- Pichichero ME. Ten-year study of the stringently defined otitis-prone child in Rochester, NY. Pediatr Infect Dis J. 2016;35:1033–39. doi:https://doi.org/10.1097/INF.0000000000001217.
- Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine. 2011;29:1023–28. doi:https://doi.org/10.1016/j.vaccine.2010.11.055.
- Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis. 2011;204:645–53. doi:https://doi.org/10.1093/infdis/jir340.
- Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. J Infect Dis. 2012;205:1225–29. doi:https://doi.org/10.1093/infdis/jis179.
- Pichichero ME. New combination vaccines. Pediatr Clin North Am. 2000;47:407–26. doi:https://doi.org/10.1016/S0031-3955(05)70214-5.
- Ren D, Almudevar AL, Pichichero ME. Synchrony in serum antibody response to conserved proteins of Streptococcus pneumoniae in young children. Hum Vaccin Immunother. 2015;11:489–97. doi:https://doi.org/10.4161/21645515.2014.990861.
- Pichichero ME, Kaur R, Casey JR, Sabirov A, Khan MN, Almudevar A. Antibody response to Haemophilus influenzae outer membrane protein D, P6, and OMP26 after nasopharyngeal colonization and acute otitis media in children. Vaccine. 2010;28:7184–92. doi:https://doi.org/10.1016/j.vaccine.2010.08.063.
- Pichichero ME, Kaur R, Casey JR, Xu Q, Almudevar A, Ochs M. Antibody response to Streptococcus pneumoniae proteins PhtD, LytB, PcpA, PhtE and Ply after nasopharyngeal colonization and acute otitis media in children. Hum Vaccin Immunother. 2012;8:799–805. doi:https://doi.org/10.4161/hv.19820.
- Xu Q, Casey JR, Newman E, Pichichero ME. Otitis-prone children have immunologic deficiencies in naturally acquired nasopharyngeal mucosal antibody response after streptococcus pneumoniae colonization. Pediatr Infect Dis J. 2016;35:54–60. doi:https://doi.org/10.1097/INF.0000000000000949.
- Ren D, Xu Q, Almudevar AL, Pichichero ME. Impaired proinflammatory response in stringently defined otitis-prone children during viral upper respiratory infections. Clin Infect Dis. 2019;68:1566–74. doi:https://doi.org/10.1093/cid/ciy750.
- Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. New Jersey, USA: Wiley; 2011.
- Pinheiro JC, Bates DM. Mixed effects models in S and S-Plus. New York, NY: Springer-Verlag; 2000.
- Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. doi:https://doi.org/10.18637/jss.v067.i01.
- Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J. 2011;30:645–50. doi:https://doi.org/10.1097/INF.0b013e31821c2d8b.
