ABSTRACT
While national authorities recommended and provided reimbursement for men who have sex with men under 27 in 2016 and 2017, respectively, we aimed to comprehensively analyze human papillomavirus (HPV) vaccine uptake in French men over a 4-year period surrounding these changes. Data regarding HPV vaccine sales to men in all French pharmacies from 2015 through 2018 were retrieved through query made to the national public insurance database. Data were classified according to the age of patients and the time of dispensation so as to display aggregate uptake according to age over time. Time-series analysis was conducted and an exponential smoothing extrapolation was selected to analyze the impact of the reimbursement. Overall, 12,814 HPV vaccines were dispensed in men over the study period. Age was available for the majority of cases (12,793; 99.8%), averaging 29. Dispensation data for each year were the following: 1,917 (2015), 1,921 (2016), 2,643 (2017), 6,312 (2018). Age analysis showed that vaccine uptake among men over 26 was substantial (n = 5974; 46.7%). The exponential increase in the number of vaccines sold started after the second quarter of 2017. In conclusion, we found that HPV vaccine uptake among French men is partly misaligned with recommendations and reimbursement in terms of age, and still moderate overall even though we found signs of marked increase in uptake over the most recent period, suggesting an effective impact of insurance coverage.
Introduction
Human papillomavirus (HPV) vaccination has been shown to be safe and effective in men.Citation1,Citation2 It has been assessed as moderately effective against persistent lesions in already infected men, and highly effective as prevention in HPV-naïve men.Citation2 Therefore, many countries have promoted HPV vaccination for boys or for men who have sex with men (MSM) so as to pursue the public health objective to eliminate HPV infection and its complications.Citation3,Citation4 However, actual uptake in men is incompletely known and seems to vary among countries. A report from Scotland has measured that a little less than two-thirds of targeted MSM had adhered to a dedicated national vaccine program during its first year of implementation.Citation4 Another local report from an Australian sexual health clinic showed a higher figure for uptake, with a 73.2% coverage rate among eligible MSM to whom the vaccine was offered by their clinician, yet with an overall coverage (i.e. all eligible MSM) considered as unsatisfactory, measured at 42.6%.Citation3
No such data exist for France. Whereas HPV vaccine uptake in teenage girls has been particularly low in our country since its inception in 2007,Citation5 on February 19, 2016, a report from the French Advanced Council for Public Health (Haut Conseil de la Santé Publique) recommended to propose and cover HPV vaccine to MSM under 27 (with no lower age threshold mentioned in its report).Citation6 This recommendation was followed in April 2017 by a governmental body (Direction Générale de la Santé) in charge of designing and executing national public health policy, which effectively granted reimbursement for HPV vaccine in MSM under 27. However, there is currently no published nor governmental information regarding actual uptake of the HPV vaccine in French men. Also, we have no data regarding “off-label” vaccination, that is vaccination in men over 26.
Therefore, we aimed to report national HPV vaccine uptake in French men over a four-year period surrounding the inception of national reimbursement. In particular, we aimed to analyze whether there had been an increase in uptake following recommendation and expanded reimbursement in young men and whether actual vaccination complies with national recommendations in terms of age.
Methods
French MSM can decide to get vaccinated on their own or following some medical advice but a prescription from a physician is mandatory to retrieve the vaccine in pharmacies. Only men aged 26 or less are reimbursed while others need to fund their vaccination out of pocket. No additional national program was implemented.
We retrieved dispensation data for men regarding the two vaccines (Cervarix® from GlaxoSmithKline, Gardasil® from Merck) available in France at the time of our study (January 1, 2015-December 31, 2018). These data were obtained through query made to the national public insurance database, covering the entire French population. This database contains exhaustive data regarding vaccine sales in French community pharmacies. Data were classified according to the age of patients and the quarter of dispensation over the study period. Numbers of dispensed boxes were summed so as to display aggregate semesterly uptake and yearly uptake according to age. Use of anonymized patient data does not require any approval from an ethics committee according to the French ethic law.
Statistical analysis
Time-series analysis was conducted using IBM SPSS Statistics 24 (SPSS Inc., Chicago, IL). A linear regression model was performed including the number of vaccines sold as main outcome and the number of quarters as predictor, entered as both a linear (T) and a quadratic term (TCitation2). Exponential smoothing extrapolation was selected to estimate the predicted values with a corresponding 95% confidence interval. A p value <.05 was considered significant.
Results
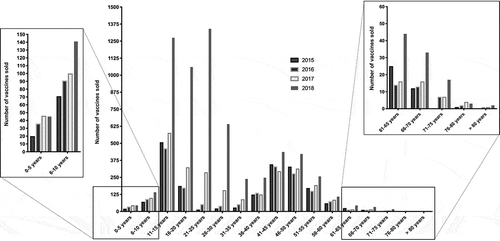
Overall, 12,814 HPV vaccines were dispensed to men over the study period. For 12,793 of them (99.8%), the age of the patient was available, averaging 29 years. Vaccination started at the age of 1 and the oldest patient was 80. Dispensation data for each year were the following: 1917 (2015), 1921 (2016), 2643 (2017), 6312 (2018). Almost half (49.3%) of the vaccines that were sold during the study period were used in 2018. displays the number of vaccines sold each year according to subgroups of patients depending on their age range (5 years interval), exhibiting two peaks: 11–25 years (n = 6263 vaccines; 48.9%) and 41–50 years (n = 2755 vaccines; 21.5%). Among individuals younger than 10 years, very few vaccines were dispensed (n = 550; 4.3%), with however a 50% increase in sales between 2015–2016 and 2017–2018. For patients aged from 11 to 30, the increase was of 287.3% (1462 vaccines in 2015–2016 vs. 5662 in 2017–2018). For middle-aged patients (31–60 years old), the number of vaccines only mildly increased across the study period (35% growth from 2086 in 2015–2016 to 2818 in 2017–2018). Vaccination in people over 60 was very limited (n = 218; 1.7%).
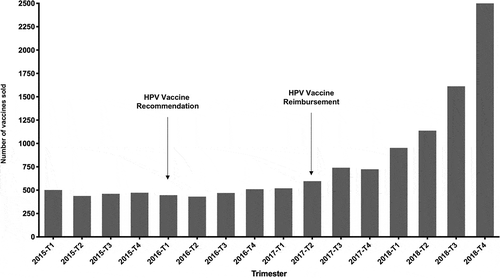
The linear regression identified a concave relationship over time in the curve shape between the number of vaccines sold and quarters (p = .002 for T and p < .001 for T2, respectively). represents the observed sales during the study period. The exponential increase in the number of vaccines sold started after the second quarter of 2017.
In 2017 and even more in 2018, a third peak was observed around 24-25-26 years. In 2018, these three periods gathering 9 years of lifespan represented 11.3% of all sales that were measured for this year.
Discussion
Our report regarding HPV vaccine uptake in French men is comprehensive and spans over four years. It is therefore, to our knowledge, one of the most quantitative assessments to date regarding HPV vaccination in men. The strengths come from the exhaustive and national scope of our data, the methods we used, and the fact that the analysis surrounds supplemental recommendation and reimbursement in men, allowing to assess its likely impact on uptake in this population.
Our study suggests at least two messages of interest in public health and public policy. First, national vaccine uptake in French men is showing signs of acceleration. As of April 2017, actual coverage by public national insurance was effective for MSM under 27, and there has been a marked increase in sales in 2017 and even more dramatically in 2018. Time-series analysis, therefore, showed a significant impact of the reimbursement of the vaccine by the French Authorities on the number of vaccines sold. Although encouraging, it seems likely that uptake is still insufficient. Indeed, even if there is no official count of homosexuality in our country, a landmark study has estimated that approximately 1% to 4% of the population was likely to experience homosexual intercourse.Citation7 According to the 2019 data from the National Institute for Statistics and Economic Studies (INSEE, Institut National de la Statistique et des Etudes Economiques), there are currently 3,468,772 French men whose age ranges from 18 to 26.Citation8 We, therefore, estimate that the target population may range from approximately 34,700 to 138,700 men depending on the quantitative assumption regarding the actual prevalence of homosexuality. Thus, with a cumulative number of 12,814 dispensed boxes and given that 3 injections are needed and generally performed, it seems plausible that the target population is undertreated, from approximately 3% to 12% of the coverage depending on assumptions. Second, vaccine uptake does not fully comply with reimbursement recommendation. Our data show that uptake was already present in 2015, while there was no recommendation nor insurance coverage in men. Foremost, age analysis revealed that vaccine uptake goes well beyond recommendations, since men over 26 were highly represented among the whole national sample (n = 5974; 46.7%) while they need to pay out-of-pocket the full price of the vaccine. Perhaps even more surprising is the fact that children and at the other end of the spectrum, elderly people were represented among the national sample. We have no explanation for those findings.
Our study is limited by the administrative nature of its data. Except for age and gender, we did not have access to clinical data regarding the indication of the prescription, the immune and HIV status of patients, and their sexual behaviors. Also, since we analyzed sales and did not have access to patient identification, we were not capable to say how many men were dispensed. Given that hospital pharmacies were not included in our dataset, our study may also have underestimated vaccine uptake although vaccination through hospital is extremely rare in France, and foremost hospital pharmacies have no stockpile of vaccines, and therefore, patients that get vaccinated in hospitals need to go in community pharmacies to buy it first. As such, our completeness is thought to be very high. Last, we cannot affirm that all dispensed vaccines were indeed administered even though we believe that this was the case for the vast majority of patients. However, there are currently no other data available to our knowledge in France, particularly no individual data from registries or clinical studies, and those we used, therefore, provide the best material for estimation to date.
In conclusion, our 4-year national time-series analysis shows that HPV vaccine uptake in French men is exhibiting signs of increase even though likely still below target and partly misaligned with national recommendations with respect to age. Our findings yet suggest an effective impact of reimbursement on vaccine uptake. Efforts should be pursued, such as national campaigns and longitudinal surveys in sexual clinics, so as to further increase coverage and monitor it respectively.
Disclosure of potential conflicts of interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Zeitoun reports being an advisor for several consulting firms in link with the pharmaceutical industry (Cepton, Oliver Wyman, Roland Berger, TBWA, Havas). He also reports speaking fees from a manufacturers’ professional association, consulting fees from Ferring, Pierre Fabre, AbbVie, Astra Zeneca, Biogen, Boehringer Ingelheim, and Johnson & Johnson. He is a personal investor in approximately 20 digital companies, medtech companies or biotech companies, and as a limited partner in an investment fund. He is also a shareholder and advisory board member in several medtech companies. He reports being cofounder and shareholder of Inato, a digital company involved in clinical research and whose customers are pharmaceutical companies. Dr. de Parades had speaking fees outside the submitted work from AbbVie and Tillotts. Dr. Duclos has no competing interest to report. Dr. Lefèvre reports being invited to an international medical congress by Sanofi (2015), Eumedica (2015), Biomup (2018), receiving fees from Takeda (2018), Ethicon (2018) and as a consultant from Safeheal (2018–2020). No other disclosures were reported.
Acknowledgments
Dr. Zeitoun would like to thank Claude Gissot, Evelyne Toustou, and Emmanuel Stranadica for their help in retrieving the data, and Arnaud Alessandrin and Patrick Papazian for their advice.
References
- Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr., Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–85. doi:https://doi.org/10.1056/NEJMoa1010971.
- Harder T, Wichmann O, Klug SJ, MAB VDS, Wiese-Posselt M. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: a systematic review. BMC Med. 2018;16:110. doi:https://doi.org/10.1186/s12916-018-1098-3.
- McGrath L, Fairley CK, Cleere EF, Bradshaw CS, Chen MY, Chow EPF. Human papillomavirus vaccine uptake among young gay and bisexual men who have sex with men with a time-limited targeted vaccination programme through sexual health clinics in Melbourne in 2017. Sex Transm Infect. 2019 May;95(3):181–86. doi:https://doi.org/10.1136/sextrans-2018-053619.
- Pollock KG, Wallace LA, Wrigglesworth S, McMaster D, Steedman N. HPV vaccine uptake in men who have sex with men in Scotland. Vaccine. 2019 Sep 3;37(37):5513–14. doi:https://doi.org/10.1016/j.vaccine.2018.11.081.
- Lefevre H, Schrimpf C, Moro MR, Lachal J. HPV vaccination rate in French adolescent girls: an example of vaccine distrust. Arch Dis Child. 2018;103:740–46. doi:https://doi.org/10.1136/archdischild-2017-313887.
- Publique HCdlS. Recommandations vaccinales contre les infections à papillomavirus humains chez les hommes. 2017.
- Bajos N, Bozon M Enquête sur la sexualité en France, Pratiques, genre et santé La Découverte, 2008.
- INSEE. Bilan démographique 2018. 2019.