ABSTRACT
Background: Immune immaturity may put premature infants at increased risk for infections. DTaP-IPV-Hib-HepB vaccine (Vaxelis™), a hexavalent vaccine studied in >6,800 children, has acceptable safety and immunogenicity profiles generally similar to control vaccines. Here we evaluate safety and immunogenicity of DTaP-IPV-Hib-HepB vaccine in premature infants.
Methods: Premature infants were identified using prior medical conditions terms “premature baby/delivery” and/or “low birth weight baby”. Immunogenicity and safety data were summarized across one Phase II and four Phase III randomized, active-comparator-controlled clinical trials (Protocol 004 in Canada [Control: PENTACEL™]; Protocols 005 and 006 in the US [Control: PENTACEL™]; and Protocols 007 and 008 in the EU [Control: INFANRIX™ hexa]) and one Phase III clinical trial in the UK (PRI01C); no formal statistical comparisons were performed.
Results: Overall, 160 infants were considered premature (DTaP-IPV-Hib-HepB = 111 Control = 49). The incidence of adverse events (AEs) for DTaP-IPV-Hib-HepB was comparable between overall and premature populations for all AEs days 1–15 postvaccination (Overall = 96.3%; Premature = 97.3%;), solicited injection-site AEs days 1–5 postvaccination (Overall = 84.1%; Premature = 75.5%), and solicited systemic AEs days 1–5 postvaccination (Overall = 93.7%; Premature = 94.5%).
A high percentage of premature infants mounted protective immune responses to antigens contained in DTaP-IPV-Hib-HepB vaccine. Response rates in preterm infants for all antigens (80-99%) were in a similar range to all infants (80-99%) for both DTaP-IPV-Hib-HepB and control vaccines.
Conclusions: DTaP-IPV-Hib-HepB vaccine has a low incidence of AEs, an acceptable safety profile, and elicited satisfactory immune responses in premature infants comparable to the overall study population. These findings support vaccination with DTaP-IPV-Hib-HepB vaccine in healthy premature infants.
Introduction
The immunization schedules in the first year of life are becoming increasingly complex as new vaccines against preventable diseases are introduced into practice. In the United States, up to 23 separate injections can be necessary to complete the Recommended Childhood Immunization Schedule in the first 2 years of life.Citation1 During some office or clinic visits, the administration of up to four or five separate injections can be indicated in the first year of life. In the European Union (EU), there is an additional complexity due to the variety of immunization schedules across the member states.Citation2
Infants born prematurely (before 37 weeks gestation) are at increased risk of contracting vaccine-preventable diseases due to immature immune responses and diminished time for maternal antibody transfer.Citation3,Citation4 Vaccines are generally safe and immunogenic. While antibody responses to initial doses of vaccines may be tempered compared to those full-term infants, protective concentrations are often achieved with memory successfully induced.Citation3,Citation4 Therefore, many countries recommend vaccinating preterm infants according to their chronological age without deviation from the standard vaccine schedule in order to provide protection in this vulnerable population.Citation1-5 Despite these recommendations, the timeliness of vaccination in premature infants is often delayed, potentially due to parental or healthcare provider reluctance.Citation6,Citation7
Preterm infants are at higher risk of adverse reactions, such as cardiopulmonary events, to vaccination. This is of particular concern in very preterm (born before 32 weeks gestation) and very low birth weight (<1500 g) infants and those with underlying cardio or respiratory abnormalities.Citation8-11 The fear of adverse events, the risk of which is difficult to quantify and not always warranted, is a significant cause for delay in vaccination in premature infants,Citation12 thereby preventing the protection afforded by these vaccines.
Immune response to vaccination in a preterm infant may differ from that of the term infant due to the immaturity of the immune system. Responses to individual antigens have been studied with varried results. For certain infections with clear correlates of protection (such as diphtheria and tetanus), the overall responses appear comparable between preterm and term infants,Citation13 whereas for others such as Hemophilus influenzae type b (Hib), these responses are less consistent and, in some studies, lower in preterm infants.Citation14-16
With the development and use of combination vaccines, multiple antigens can be administered through fewer injections. Use of combination vaccines is associated with higher compliance rates in this age group with the recommended vaccination schedule, presumably due to reduction in the number of injections needed to deliver the vaccines.Citation17,Citation18 Vaxelis™ (DTaP-IPV-Hib-HepB vaccine: diphtheria, tetanus, pertussis [5 acellular components: PT, FHA, PRN and FIM 2&3], hepatitis B [rDNA], poliomyelitis [inactivated] and Haemophilus influenzae type b conjugate vaccine [adsorbed]; MCM Vaccine B.V., The Netherlands) is a preservative-free, fully liquid, ready to administer, hexavalent vaccine developed to provide active immunization against several infectious diseases caused by 6 pathogens with the convenience of one injection.Citation19 DTaP-IPV-Hib-HepB vaccine is indicated for primary and booster vaccination in infants and toddlers from the age of 6 weeks, against diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis, and invasive diseases caused by Hib. However, a lower immune response may be observed in premature infants, and the corresponding level of clinical protection is unknown.
Presented here is the immunogenicity and integrated safety and tolerability profile in premature infants of the DTaP-IPV-Hib-HepB vaccine based on 1 Phase II and 5 Phase III clinical studies (Protocols 004, 005, 006, 007, 008, and PRI01C; see Supplemental Tables 1 and ) conducted in the US, Canada, and the EU.Citation17,Citation20-24 Data from the studies were integrated and analyzed to provide a comprehensive safetyCitation25 and immunogenicity profile across a broad range of geographies, populations, and vaccination schedules.
Results
There was a total of 160 premature infants (111 in the DTaP-IPV-Hib-HepB and 49 in the control groups) identified by searching all subjects’ medical history and selecting infants with relevant terms indicating prematurity (). The terms used for identifying the premature infants were “premature baby”, “premature delivery”, “hospitalization for prematurity” and “low birth weight baby”. The vast majority of the individuals (98.2% (109/111) for DTaP-IPV-Hib-HepB; 98.0% (48/49) for Control) were identified by the terms “premature baby/delivery” and only (1.8% (2/111) and 2% (1/49) identified by “low birth weight baby” in the DTaP-IPV-Hib-HepB and control groups respectively.
Table 1. Premature infant accounting*
The clinical adverse event (AE) summary in participants who received DTaP-IPV-Hib-HepB and were born prematurely appears to be generally similar to that of the overall study population (). Systemic AEs () and injection-site AEs () days 1 to 15 following any dose vaccination were generally similar between participants who were born prematurely to that of the overall study population. Serious AEs reported days 1 to 15 after any dose (overall: 1.5%; premature: 1.8%) and AEs leading to discontinuation (overall: 0.2%; premature: 0.0%) occurred infrequently and at a similar rate in the overall study population and the premature infant population. For preterm infants receiving DTaP-IPV-Hib-HepB or the control vaccine, the incidence of adverse events (AEs) on Day 1-to-15 post-vaccination was comparable for all AEs (DTaP-IPV-Hib-HepB: 96.3%; Control: 96.9%), solicited inject-site AEs (DTaP-IPV-Hib-HepB: 84.1%; Control: 84.8%), and solicited systemic AEs (DTaP-IPV-Hib-HepB: 93.7%; Control: 94.6%). Serious AEs and AEs leading to discontinuation occurred at a similar rate for DTaP-IPV-Hib-HepB (serious AEs: 1.8%; AE discontinuation: 0.0%) and control vaccines Hib (serious AEs: 4.1%; AE discontinuation: 2.0%). There were no cases of apnea or cardiopulmonary events associated with vaccination and there were no vaccine-related deaths in any of the phase III studies. There was one unrelated death in the premature cohort in an infant who developed pneumonia >3 weeks after receiving the control and concomitant vaccines.
Table 2. Analysis of the clinical adverse events after any dose of vaccine in the overall and premature populations for DTaP-IPV-Hib-HepB and control vaccines
Figure 1. Selected systemic and injection-site adverse events days 1 to 15 following any dose of vaccination
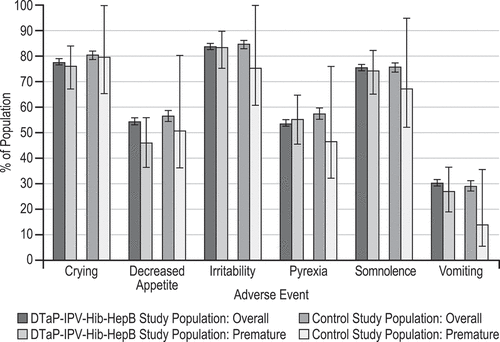
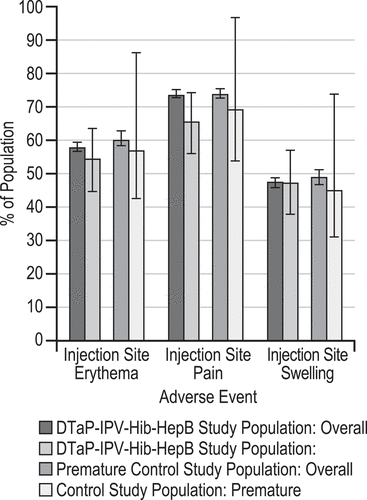
Figure 2. Selected injection-site ae days 1 to 15 following any dose vaccination
Overall, although the numbers are small, the data indicate that a high percentage of premature infants mounted protective immune responses to antigens that have a well-defined correlate of protection as well as a vaccine response against the pertussis antigens (Supplemental Table 3). Summary of immune responses to DTaP-IPV-Hib-HepB antigens at one month after the infant series in the overall study population are presented in and Supplemental Figures 1–3. The GMTs after the infant series are displayed in Supplemental Table 4.
Figure 3. Immune responses after the infant series (2, 4, and 6 months) for protocol 005 and protocol 006 combined (see Supplemental Table 3 for the response definitions by antigen)
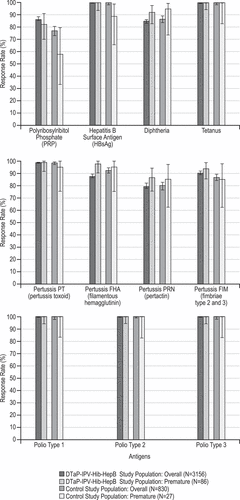
Protocols 005 and 006 have the same vaccination schedules and were conducted in a similar study population. The combined studies allow for comparison between 2713 overall study cohort infants and 70 preterm infants who received DTaP-IPV-Hib-HepB and had an available immunogenicity blood draw. As seen in the Supplemental Table 3 the post infant series show comparable immune responses between the full and preterm cohort for all antigens. Here the overall study and preterm anti-PRP responses 5.8 μg/mL (95%CI: 5.4,6.1) and 5.5 μg/mL (95%CI: 3.7,8.1) respectively, anti-HBsAg responses 1251 mIU/mL (95%CI: 1195,1310) and 1115 mIU/mL (95%CI: 821,1515) and combined responses to the Pertussis antigens, are robust, an important finding for these antigens that are at times diminished in preterm individuals.
Protocol 008, which followed a 2 + 1 infant series has a small number of preterm infants in the DTaP-IPV-Hib-HepB arm (6); however, response rates after the infant series (Supplemental Table 4) were very reassuring for all antigens.
Discussion
The DTaP-IPV-Hib-HepB vaccine Phase III studies evaluated different vaccination schedules, across North America and the European Union.Citation17,Citation20-24 The DTaP-IPV-Hib-HepB vaccine was administered to over 6,800 children and has an acceptable safety and immunogenicity profile similar to that of control vaccines.Citation17,Citation20-27
This study compared the safety of the general full-term infant group to the subset identified as premature by way of their medical history. In healthy premature infants who received DTaP-IPV-Hib-HepB vaccine, the safety profile of DTaP-IPV-Hib-HepB vaccine is generally similar to that of control vaccines [DTaP5-IPV/Hib (PENTACEL™) in the U.S. and DTaP3-IPV-HepB/Hib (INFANRIX™ hexa) in the EU] and to the overall population. Based on a low incidence of AEs with a severe intensity, vaccine-related serious AEs, and AEs leading to discontinuation, vaccination with DTaP-IPV-Hib-HepB vaccine has an acceptable safety profile in premature infants, comparable to the overall study population.
The preterm neonate is particularly vulnerable to infections early in life, and often displays a diminished response to vaccination. In this study a high percentage of premature infants mounted protective immune responses to antigens that have a well-defined correlate of protection or protocol-defined immune responses to vaccine antigens (Supplemental Table 3), as has been shown for another hexavalent combination vaccine.Citation6,Citation7 Attaining a robust post infant series response to vaccination is particularly important for protection in the months prior to the toddler booster dose. The data show these post infant series responses to be robust and generally comparable with those of the full–term population. The early and sustained Hib response induced by DTaP-IPV-Hib-HepB within the first year of life is particularly reassuring during this high-risk period for Hib disease and in this higher risk population. Furthermore, a high percentage of the premature neonates also achieved protocol-defined vaccine responses against pertussis, a disease which has been described as particularly severe in premature infants.Citation28
In several European countries the vaccine schedule requires a two-dose infant series followed by a toddler dose approximately 6–7 months later, similar that used in Protocol 008. Given there are only 2 doses for the priming series it is essential to assess this in the preterm infant who is at higher risk for low post primary responses. While the number of preterm infants in this study was small, both safety and immunogenicity between term and preterm appear comparable.
Limitations of this study include that this was a post hoc analysis of preterm infants who have been identified by medical history terms. For example, use of the term “low birth weight” would not necessarily correctly capture a preterm infant, given that low birth weight occurs frequently with, but is not synonymous with premature birth. However only 3 infants in the preterm cohort were identified primarily in this manner. The clinical trials did not include enrollment of premature infants as a study goal and as a result the numbers are small and statistical analysis is limited to descriptive analyses. Gestational age and birth weight were not collected in these studies and no a priori stratification for preterm infants planned, precluding formal statistical comparisons between DTaP-IPV-Hib-HepB and the control vaccine within preterm infants. Furthermore, because gestational age was not collected no inference on differences in safety and immunogenicity between “very preterm” (born between 28 and 32 weeks gestation) and “moderate” preterm infants (born between 32 and 37 weeks gestation) can be made. Despite these limitations, it is reassuring that there was no evidence of meaningfully diminished responses to vaccinations or increased safety concerns in the premature infants who received DTaP-IPV-Hib-HepB. As with all licensed vaccines, post-licensure pharmacovigilance and safety reporting for DTaP-IPV-Hib-HepB is ongoing.
In summary, the data presented support that DTaP-IPV-Hib-HepB vaccine can be given to premature infants and provides an option for timely vaccination of this population at heightened risk for infectious diseases.
Methods
The DTaP-IPV-Hib-HepB vaccine Phase II and III studies included healthy infants who were born prematurely, however gestational age and birthweight were not collected (Supplemental Table 1). Premature infants were identified using prior medical conditions terms of “premature baby/delivery” and/or “low birth weight baby”.
The safety and immunogenicity of DTaP-IPV-Hib-HepB vaccine was evaluated in one Phase II and five pivotal Phase III, randomized, controlled clinical trials in Canada, the EU and USA (performed in support of product licensure) (Supplemental Tables 1, 2, and 3). Reflective of the local standard-of-care, the studies differed in their vaccination schedules. Protocol 004 administered DTaP-IPV-Hib-HepB or licensed control vaccines as an infant series at 2, 4, 6 months and a toddler dose at 15 months of age. Protocols 005 and 006 were very similar with respect to study design and study population; DTaP-IPV-Hib-HepB vaccine or licensed control vaccines were administered to participants as an infant series at 2, 4, and 6 months of age, followed by the administration of a toddler dose of a DTaP-containing vaccine at 15 months of age. In addition, all participants were to have received a dose of monovalent hepatitis B vaccine at birth (outside of the context of the study). Protocol 007 and PRI01C were a 3 dose infant series at 2, 3, and 4 months and, for protocol 007, a toddler dose at 12 months. Protocol 008 was a 2 dose infant series given at 2 and 4 months with a toddler dose at 11–12 months.
The comparator vaccine (Control) was DTaP3-IPV-HepB/Hib (Infanrix™ hexa; GlaxoSmithKline Biologics S.A., Rixensart, Belgium) in European studies and DTaP5-IPV/Hib (PENTACEL™; Sanofi Pasteur, Swiftwater, PA, USA) in North American studies.Citation26,Citation27
For the assessment of safety in all studies, the participant’s parent/legal representative was provided a vaccination report card (VRC) at each vaccination visit. The primary investigators at each study site assessed causality (i.e., related or not related to vaccination) on all AEs. The solicited adverse events (AEs) were the same for all studies: solicited injection-site AE terms were redness, pain/tenderness, and swelling; solicited systemic AE terms were crying, decreased appetite, pyrexia, irritability, somnolence, and vomiting (Supplemental Table 2). The length of safety data collection differed slightly among the studies as described in Xu et al, PIDJ 2019.Citation25
Immunogenicity was assessed via serum samples obtained from study participants at 4 time points in the Phase III studies: prior to the first vaccination, 4 to 6 weeks after completion of the infant series (i.e., after the second [P008] or third [P004, P005, P006, and P007] dose), prior to the toddler vaccination, and 4 to 6 weeks after the toddler dose. Serologic testing for Hib and hepatitis B was performed by PPD Vaccines and Biologics, LLC, Wayne, PA, USA. Serologic testing for diphtheria, tetanus, pertussis, and polio was performed by Sanofi Pasteur Inc. GCI, Swiftwater, PA, USA. Antibody responses were defined based on accepted immune correlates of protection, or previously accepted definitions of vaccine response for licensed vaccines (Supplemental Table 3). The single premature infant in the Phase II study, protocol 004, was not included in the premature infant immunogenicity analysis for the purposes of this study.
As the number of participants identified as premature is small as compared to the whole study, safety and immunogenicity information is provided descriptively. No formal statistical comparisons were planned between DTaP-IPV-Hib-HepB vaccine and control within premature infants, or between the premature infant groups and the full study population.
Disclosure of potential conflicts of interest
MB Wilck, J Xu, JE Stek, and AW Lee are employees of the Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may hold stock and/or stock options in the company.
Supplemental Material
Download Zip (890.3 KB)Acknowledgments
We would like to thank Karyn Davis of Merck & Co., Inc., Kenilworth, NJ, USA for editorial support.
Supplementary material
Supplemental data for this article can be accessed online at online at http://dx.doi.org/10.1080/21645515.2020.1756668.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Recommended immunization schedule for children and adolescents aged 18 years or younger, United States; 2018 [accessed 2018 March 23]. https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html
- European Centre for Disease Prevention and Control. Vaccine scheduler: vaccine schedules in all countries of the European Union. [accessed 2018 March 23]. https://vaccine-schedule.ecdc.europa.eu/.
- Melville JM, Moss TJ. The immune consequences of preterm birth. Front Neurosci. 2013;7:79. doi:10.3389/fnins.2013.00079.
- Gagneur A, Pinquier D, Quach C. Immunization of preterm infants. Hum Vaccin Immunother. 2015;11(11):2556–63. doi:10.1080/21645515.2015.1074358.
- World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2019 global summary. [accessed 2019 December 6]. http://apps.who.int/immunization_monitoring/globalsummary/schedules.
- Wilson K, Hawken S, Holdt Henningsen K, Kwong JC, Deeks SL, Crowcroft NS, Law B, Manuel DG. On-time vaccination coverage in premature infants in Ontario, 2002–2009. Can J Public Health. 2012;103:e359–62. doi:10.1007/BF03404441.
- McCrossan P, McCafferty C, Murphy C, Murphy J. Retrospective review of administration of childhood primary vaccination schedule in an Irish tertiary neonatal intensive care unit. Public Health. 2015;129:896–98. doi:10.1016/j.puhe.2015.05.005.
- Sánchez PJ, Laptook AR, Fisher L, Sumner J, Risser RC, Perlman JM. Apnea after immunization of preterm infants. J Pediatr. 1997;130(5):746–51. doi:10.1016/S0022-3476(97)80017-0.
- Botham SJ, Isaacs D. Incidence of apnoea and bradycardia in preterm infants following triple antigen immunization. J Paediatr Child Health. 1994;30(6):533–35. doi:10.1111/j.1440-1754.1994.tb00728.x.
- Pfister RE, Aeschbach V, Niksic-Stuber V, Martin BC, Siegrist CA. Safety of DTaP-based combined immunization in very-low-birth-weight premature infants: frequent but mostly benign cardiorespiratory events. J Pediatr. 2004;145(1):58–66. doi:10.1016/j.jpeds.2004.04.006.
- Klein NP, Massolo ML, Greene J, Dekker CL, Black S, Escobar GJ, Datalink VS. Risk factors for developing apnea after immunization in the neonatal intensive care unit. Pediatrics. 2008;121(3):463–69. doi:10.1542/peds.2007-1462.
- Langkamp DL, Hoshaw-Woodard S, Boye ME, Lemeshow S. Delays in receipt of immunizations in low-birth-weight children: a nationally representative sample. Arch Pediatr Adolesc Med. 2001;155(2):167–72. doi:10.1001/archpedi.155.2.167.
- Vázquez L, Garcia F, Rüttimann R, Coconier G, Jacquet JM, Schuerman L. Immunogenicity and reactogenicity of DTPa-HBV-IPV/Hib vaccine as primary and booster vaccination in low-birth-weight premature infants. Acta Paediatr. 2008;97(9):1243–49. doi:10.1111/j.1651-2227.2008.00884.x.
- Munoz A, Salvador A, Brodsky NL, Arbeter AM, Porat R. Antibody response of low birth weight infants to Haemophilus influenzae type b polyribosylribitol phosphate-outer membrane protein conjugate vaccine. Pediatrics. 1995;96:216–19.
- Kristensen K, Gyhrs A, Lausen B, Barington T, Heilmann C. Antibody response to Haemophilus influenzae type b capsular polysaccharide conjugated to tetanus toxoid in preterm infants. Pediatr Infect Dis J. 1996;15(6):525–29. doi:10.1097/00006454-199606000-00010.
- Slack MH, Schapira D, Thwaites RJ, Burrage M, Southern J, Andrews N, Borrow R, Goldblatt D, Miller E. Immune response of premature infants to meningococcal serogroup C and combined diphtheria-tetanus toxoids-acellular pertussis-Haemophilus influenzae type b conjugate vaccines. J Infect Dis. 2001;184(12):1617–20. doi:10.1086/324666.
- Marshall GS, Adams GL, Leonardi ML, Petrecz M, Flores SA, Ngai AL, Xu J, Liu G, Stek JE, Foglia G, et al. Immunogenicity, safety, and tolerability of a hexavalent vaccine in infants. Pediatrics. 2015;136(2):e32332. doi:10.1542/peds.2014-4102.
- Lee AW, Jordanov E, Boisnard F, Marshall GS. DTaP5-IPV-Hib-HepB, a hexavalent vaccine for infants and toddlers. Expert Rev Vaccines. 2017;16(2):85–92. doi:10.1080/14760584.2017.1268920.
- Vaxelis [package insert]. Leiden (The Netherlands): MCM Vaccine B.V. [accessed 2018 Mar 23]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003982/WC500202435.pdf
- Tapiéro B, Halperin SA, Dionne M, Meekison W, Diaz-Mitoma F, Zickler P, Rubin E, Embree J, Bhuyan P, Lee AW, et al. Safety and immunogenicity of a hexavalent vaccine administered at 2, 4 and 6 months of age with or without a heptavalent pneumococcal conjugate vaccine: a randomized, open-label study. Pediatr Infect Dis J. 2013;32(1):54–61. doi:10.1097/INF.0b013e3182717edf.
- Halperin SA, Tapiéro B, Dionne M, Meekison W, Diaz-Mitoma F, Zickler P, Rubin E, Embree J, Bhuyan P, Lee A, et al. Safety and immunogenicity of a toddler dose following an infant series of a hexavalent diphtheria, tetanus, acellular pertussis, inactivated poliovirus, Haemophilus influenzae type b, hepatitis B vaccine administered concurrently or at separate visits with a heptavalent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2014;33(1):73–80. doi:10.1097/01.inf.0000437806.76221.20.
- Block SL, Klein NP, Sarpong K, Russell S, Fling J, Petrecz M, Flores S, Xu J, Liu G, Stek JE, et al. Lot-to-lot consistency, safety, tolerability and immunogenicity of an investigational hexavalent vaccine in US infants. Pediatr Infect Dis J. 2017;36(2):202–08. doi:10.1097/INF.0000000000001405.
- Vesikari T, Becker T, Vertruyen AF, Poschet K, Flores SA, Pagnoni MF, Xu J, Liu GF, Stek JE, Boisnard F, et al. A phase III randomized, double-blind, clinical trial of an investigational hexavalent vaccine given at two, three, four and twelve months. Pediatr Infect Dis J. 2017;36(2):209–15. doi:10.1097/INF.0000000000001406.
- Silfverdal SA, Icardi G, Vesikari T, Flores SA, Pagnoni MF, Xu J, Liu GF, Stek JE, Boisnard F, Thomas S, et al. A Phase III randomized, double-blind, clinical trial of an investigational hexavalent vaccine given at 2, 4, and 1112 months. Vaccine. 2016;34(33):3810–16. doi:10.1016/j.vaccine.2016.05.054.
- Xu J, Stek JE, Ziani E, Liu GF, Lee AW. Integrated safety profile of a new approved, fully liquid DTaP-IPV-Hib-HepB vaccine. Pediatr Infect Dis J. 2019;38(4):439–43. doi:10.1097/INF.0000000000002257.
- Infanrix hexa [package insert]. Rixensart (Belgium): GlaxoSmithKline Biologics s.a. [accessed 2018 Mar 23]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000296/WC500032505.pdf
- Pentacel [package insert]. Swiftwater (PA): Sanofi Pasteur Inc. [accessed 2018 Mar 23]. https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm109810.pdf.
- Marshall H, Clarke M, Rasiah K, Richmond P, Buttery J, Reynolds G, Andrews R, Nissen M, Wood N, McIntyre P. Predictors of disease severity in children hospitalized for pertussis during an epidemic. Pediatr Infect Dis J. 2015;34(4):339–45. doi:10.1097/INF.0000000000000577.
