ABSTRACT
Streptococcus pneumoniae, the main cause of community-acquired pneumonia (CAP), also leads to exacerbations, hospitalizations, and mortality in chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF). The risk of CAP is increased in patients with diabetes mellitus (DM), and the risk of invasive pneumococcal disease is increased in HIV-infected patients. Pneumococcal vaccination is recommended for these patients in France. The objective was a large survey of pneumococcal vaccination coverage (PVC) by general practitioners (GPs) in these patients in France.
Diagnosis and treatment forms were extracted from the database of 2000 GPs. The GPs and population panels were representative of the metropolitan populations. The primary endpoint was the comparison of PVC in the adult patients diagnosed with COPD, CHF, DM, or HIV infection during the study (April 2013–April 2017) and the control (March 2012–March 2013) periods.
Of the 17,865 and 4,690 patients identified, 756 (4%) and 267 (6%) were vaccinated, respectively. During the study period, the PVC was significantly higher (35/282, 12%) in HIV-infected patients and lower in patients with DM (95/5994, 2%) than in other patients. Even though French pneumococcal vaccine recommendations in adults were updated in 2013, the PVC did not increase according to the years of the study period and slightly increased according to time after diagnosis.
S. pneumoniae is responsible only for some CAP and meningitis, and incomplete protection by vaccine, hesitancy from practitioners and patients, and the moving schedule of vaccination could explain the results. New tools and/or strategies must be implemented to increase PVC in France.
Abbreviations: CAP: community-acquired pneumonia; COPD: chronic obstructive pulmonary diseases; CHF: congestive heart failure; DM: diabetes mellitus; IPD: invasive pneumococcal disease; HIV: human immunodeficiency virus; PVC: pneumococcal vaccination coverage; PCV7: 7-valent pneumococcal conjugate vaccine; PCV13: 13-valent pneumococcal conjugate vaccine; PPSV23: 23-valent pneumococcal polysaccharide vaccine; GPs: general practitioners; CLM: Cegedim Logiciels Médicaux; MLM: monLogicielMedical; ICD-10: International Classification of Diseases; CNIL: Commission nationale de l’informatique et des libertés; HPV: human papillomavirus; HBV: hepatitis B virus
Introduction
Streptococcus pneumoniae is the main cause of community-acquired pneumonia (CAP), with major morbidity and mortality in the elderly and in comorbid patients.Citation1–3 CAP occurs most frequently as a non-bacteremic diseaseCitation3 and is a major cause of exacerbations, hospitalizations, disease progression, and mortality in chronic obstructive pulmonary disease (COPD) patients.Citation4 Fifty percent of congestive heart failure (CHF) exacerbations result from respiratory infections,Citation5 and CAP is the main cause of hospitalization and in-hospital mortality in CHF.Citation6 Patients with diabetes mellitus (DM) have an increased risk of death compared with nondiabetic patients after hospitalization for CAP,Citation7 and the risk of CAP in diabetic patients is twice that of nondiabetic patients.Citation8 Invasive pneumococcal disease (IPD) (isolation of S. pneumoniae from a normally sterile site) occurs in ~25% of pneumococcal diseases.Citation3 An IPD incidence of 331/100,000 person-years was found in HIV-infected patients in the late-antiretroviral treatment era in non-African countries.Citation9
Pneumococcal vaccination is recommended for the abovementioned at-risk populations: the experts in the USA in 2015Citation10 and in Europe in 2016Citation11 recommended the 13-valent pneumococcal conjugate vaccine (PCV13) and 23-valent pneumococcal polysaccharide vaccine (PPSV23) in at-risk adults or ≥65 y old. French recommendations were published in 2010,Citation12 updated in 2013Citation13 and 2017.Citation14 The PPSV23 was recommended in 2010 in HIV-infected adults, in patients with chronic respiratory failure, asplenic patients, patients with sickle cell disease, patients with nephrotic syndrome, patients with chronic alcoholic hepatitis and CHF, or patients with a history of CAP or IPD.Citation12 PCV13 and 8 weeks later PPSV23 were recommended in 2013 in HIV-infected adults, asplenic patients, patients with sickle cell disease, patients with nephrotic syndrome, patients with hereditary immunodeficiency, patients receiving chemotherapy or immunosuppressive therapy, or solid organ or stem cell transplant patients; PPSV23 alone was recommended in patients with chronic respiratory failure, COPD, CHF, or DM requiring a treatment, patients with asthma, patients with chronic hepatitis, patients with chronic renal insufficiency, or patients with an osteomeningeal breach.Citation13 After the Capita clinical trialCitation15 and a meta-analysis,Citation16 the schedule PCV13 and PPSV23 was harmonized in 2017 to all the abovementioned at-risk adults.Citation14
The effectiveness of the pneumococcal vaccine has been demonstrated in reducing the hospitalization, disability, dependency, and death of seniors and savings in health care societal costs in Europe.Citation17 In a retrospective study from a center in Paris, the PPSV23 and the PCV13 would have theoretically covered 78% and 70% of IPD cases, respectively.Citation18
As general practitioners (GPs) play a key role in the vaccination coverage, the aim of the study was to perform a large national survey of the pneumococcal vaccination coverage (PVC) by GPs in the abovementioned at-risk adults.
Materials and methods
GERS Patient Data, Cegedim Logiciels Médicaux (CLM)®, is a large national database collecting data (including treatments) since 1994 from medical records of 2000 GPs and 1000 specialist practitioners in ambulatory care using Crossway® software and the full web MLM (monLogicielMedical.com) solution. The physicians are distributed all over the metropolitan area and are responsible for the care of 3.9 million “active patients” (patients with one or more visits in 2017). Of the 2000 GPs, 1700 are selected every month to obtain a panel representative of the GP population. The GPs () and population () panel representativeness as regards sex ratio, population pyramid, and geographical area compared with the GPs population (from the French Medical Association) and global population (from the National Institute for Statistics and Economic Studies), respectively, has been demonstrated previously. The representativeness was also verified by comparing the prevalence of DM (4.8% and 4.9% in the population panel and global population, respectively), COPD (6.4% and 6.7%, respectively), and CHF (4% and 3.7%, respectively). The practitioners transmit the anonymized data for all patients who consulted demographic characteristics (birth date, sex, residence department, occupation, and marital status), medical history, allergies, reason for consultation, symptoms, diagnosis with International Classification of Diseases (ICD-10), prescription and results of biological/radiologic exams, prescription of treatments (including vaccines), the reason for prescription, reimbursements of biological/radiologic exams/treatments, hospitalizations (dates and diagnosis), and sick leaves. The study focused on patients with a diagnosis of HIV infection and/or COPD, CHF, or DM, as the other patients in whom pneumococcal vaccination is recommended are in the minority and taken in care by hospitalists, rather than GPs.
Figure 1. Representativeness of the GPs panel using Crossway® software and the full web MLM (monLogicielMedical.com) solution, compared to the metropolitan GPs population
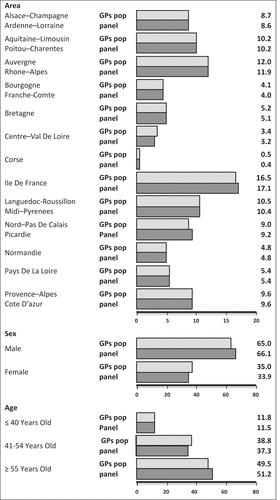
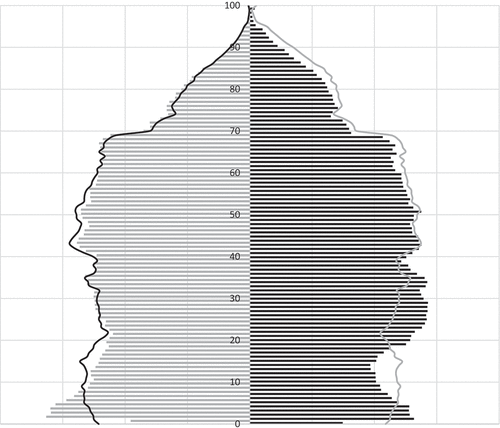
Figure 2. Population panel representativeness as regards sex ratio and population pyramid compared to the global population (from the National Institute for Statistics and Economic Studies)
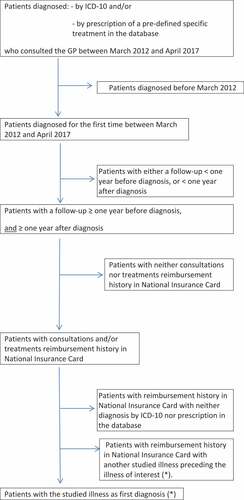
The study was registered with the “Commission nationale de l’informatique et des libertés” (CNIL), number 2211508 v 0. We compared two periods: the control period, from March 2012 to March 2013 (national recommendations update, with a new schedule of pneumococcal vaccination in adults and including new at-risk adult populations), and the study period, from April 2013 to April 2017 (new national recommendations update, with a uniform schedule of pneumococcal vaccination in adults). The selection process is described in . Schematically, inclusion criteria were (i) ≥18-y-old patients; (ii) with a diagnosis (by ICD-10 and/or by prescription of a predefined, specific of the studied illness, treatment) in the database during the control or the study period of HIV infection and/or COPD, CHF, or DM; (iii) with a consultation with the GP between March 2012 and April 2017; (iv) with a follow-up of ≥2 y (1 y before diagnosis and 1 y after diagnosis of the studied illness, so retaining only regularly followed patients); and (v) with consultations and/or treatment reimbursement history accessible from the National Insurance Card. The patients with accessible consultations and/or treatment reimbursement history from the National Insurance Card but without diagnosis and/or treatment in the database were then excluded. The patients vaccinated before diagnosis were excluded. To avoid double inclusions, the patients with several indications for pneumococcal vaccination were included in the group of the first diagnosed illness with the exception of HIV infection plus another illness, and these patients were included in the HIV-infected patient group.
Figure 3. Flowchart: selection of patients
COPD patients were diagnosed by ICD-10 (J41 or J42 or J43) or by prescription of long-acting beta-2 mimetics, long-acting anticholinergics, long-acting beta-2 mimetics or long-acting anticholinergics plus inhaled steroids, theophylline, or short-acting beta-2 mimetics. Patients diagnosed as asthmatics by ICD-10 were excluded. Patients with DM were diagnosed by ICD-10 (E10 to E14) or by prescription of insulins, metformin, sulfonylureas, thiazolidinediones, α-glucosidase inhibitors, dipeptidyl peptidase inhibitor agents (gliptines, GLP-1 analogs), or SGLT-2 inhibitors. Patients with DM treated by diet solely were excluded as there is no recommendation for them. Patients with CHF were diagnosed by ICD-10 I50; the prescriptions were not used to identify patients with CHF because they are not specific, being used for hypertension too. HIV-infected patients were diagnosed by ICD-10 (B20 to B24) or by prescription of nucleos(t)idic reverse-transcriptase inhibitors (except for monotherapies by lamivudine or tenofovir for hepatitis B virus infection [HBV]), protease inhibitors, non-nucleosidic reverse-transcriptase inhibitors, or integrase inhibitors.
The primary endpoint was the comparison of PVC (defined by a prescription and a reimbursement) of patients diagnosed during the study period with PVC of patients diagnosed during the control period. The secondary endpoints were (i) the comparison of PVC during the study period between the abovementioned populations; (ii) the comparison of PVC in the abovementioned populations during the first, (iii) second, (iv) third, and (v) fourth years of follow-up after the diagnosis during the study and the control periods, to assess if PVC increased or not over time; and (vi) the adherence to the recommended schedule. We used Khi 2 test with a p ≤ 0.05 significant.
Results
Of the 167,903 COPD patients who consulted the GPs from March 2012 to April 2017, 90,278 (53.8%) were diagnosed during the period; 44,875 (26.7%) were regularly followed up. Consultations or treatment reimbursement history was available in 33,100 (19.7%). After excluding the patients with consultations or treatment reimbursement history available without diagnosis or treatment in the database, 17,049 (10.2%) patients remained; COPD was the first diagnosed illness in 13,737 (8.2%) patients. Of these 13,737 patients, 10,916 were diagnosed during the study and 2,821 during the control periods, respectively.
Of the 124,853 patients with DM who consulted the GPs from March 2012 to April 2017, 58,147 (46.6%) were diagnosed during the period; 20,051 (16.1%) were regularly followed up. Consultations or treatment reimbursement history was available in 14,749 (11.8%). After excluding the patients with consultations or treatment reimbursement history available without diagnosis in the database and the patients treated by diet, 10,296 (8.2%) patients remained; DM was the first diagnosed illness in 7,622 (6.1%) patients. Of these 7,622 patients, 5,994 were diagnosed during the study and 1,628 during the control periods, respectively.
Of the 17,581 patients with CHF who consulted the GPs from March 2012 to April 2017, 6,666 (37.9%) were diagnosed during the period; 3,269 (18.6%) were regularly followed up. Consultations or treatment reimbursement history was available in 2,030 (11.5%); CHF was the first diagnosed illness in 816 (4.6%) patients. Of these 816 patients, 673 were diagnosed during the study and 143 during the control periods, respectively.
Of the 5,264 HIV-infected patients who consulted the GPs from March 2012 to April 2017, 3,225 (61.3%) were diagnosed during the period; 883 (16.8%) were regularly followed up. Consultations or treatment reimbursement history was available in 733 (13.9%). After excluding the patients with consultations or treatment reimbursement history available without diagnosis in the database, 380 (7.2%) patients remained. Of these 380 patients, 282 were diagnosed during the study and 98 during the control periods, respectively.
The demographic characteristics of patients are summarized in . Follow-up was 11.1 ± 5.0 and 12.4 ± 4.4 y for COPD patients, 11.5 ± 5.0 and 12.9 ± 4.4 y for patients with DM, 12.3 ± 5.3 and 12.9 ± 4.4 y for patients with CHF, and 10.6 ± 5.0 and 11.3 ± 4.3 y for HIV-infected patients in the study and the control groups, respectively. Of the 17,865 patients diagnosed with one or more of the abovementioned illnesses during the study period, 756 (4%) were vaccinated. Of the 4,690 patients diagnosed with one or more of the abovementioned illnesses during the control period, 267 (6%) were vaccinated.
Table 1. Demographic characteristics of patients in the study and control groups
Of the COPD patients diagnosed during the study and control periods, 598/10,916 (5%) and 213/2,821 (7%) were vaccinated, respectively (p = 3.8 10−5; 95% CI [0.01; 0.03]). Of the patients diagnosed with DM during the study and control periods, 95/5,994 (2%) and 28/1,628 (2%) were vaccinated, respectively (p = 0.78; 95% CI [−0.006; 0,009]). Of the patients diagnosed with CHF during the study and control periods, 28/673 (4%) and 10/143 (7%) were vaccinated, respectively (p = 0.21; 95% CI [−0.02; 0.077]). Of the patients diagnosed with HIV infection during the study and control periods, 35/282 (12%) and 16/98 (16%) were vaccinated, respectively (p = 0.42; 95% CI [−0.05; 0.12]). During the study period, PVC was significantly higher (p = 1.7 10−11; 95% CI [0.04–0.12]) in HIV-infected patients than in other patients and significantly lower in patients with DM (p < 2.2 10−16; 95% CI [−0.04; −0.03]) than in other patients. shows the number of patients according to the period and the diagnosis.
Table 2. Number of patients according to the period and the diagnosis
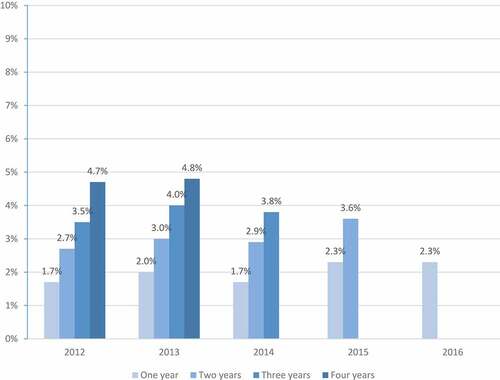
shows PVC according to the time from diagnosis. No statistically significant difference (p = 0.11) in PVC was observed during the first year: 80 (1.7%) of the 4,690 in 2012, 96 (2%) of the 4,709 in 2013, 80 (1.7%) of the 4,660 in 2014, 107 (2.3%) of the 4,689 in 2015, and 89 (2.3%) of the 3,807 in 2016. No statistically significant difference (p = 0.07) in PVC was observed over a 2-y period: 127 (2.7%), 142 (3%), 136 (2.9%), and 171 (3.6%) of patients diagnosed in 2012, 2013, 2014, and 2015, respectively. No statistically significant difference (p = 0.47) in PVC was observed over a 3-y period: 163 (3.5%), 187 (4%), and 179 (3.8%) of patients diagnosed in 2012, 2013, and 2014, respectively. No statistically significant difference (p = 0.28) in PVC was observed over a 4-y period: 221 (4.7%) and 228 (4.8%) of patients diagnosed in 2012 and 2013, respectively.
Figure 4. Pneumococcal vaccination coverage (PVC) according to the time of follow-up after diagnosis in the studied populations: patients diagnosed from 2013 to 2016 (study period) and patients diagnosed in 2012 (control period)
The majority of vaccinated COPD patients received the recommended vaccine (PPSV23): 339 (57%) in the study and 152 (71%) in the control periods, respectively. The majority of vaccinated patients with DM received the recommended vaccine (PPSV23): 53 (56%) in the study and 18 (64%) in the control periods, respectively. The majority of vaccinated patients with CHF received the recommended vaccine (PPSV23): 18 (64%) in the study and 8 (80%) in the control periods, respectively. Conversely, of the vaccinated HIV-infected patients, less than half, 16 (46%), received the recommended schedule (PCV13 and PPSV23 8 weeks later) in the study period and only three (19%) received the recommended vaccine (PPSV23) in the control period.
Discussion
Four and 6% of patients were vaccinated during the study and control periods, respectively, versus 0.9% of patients aged 18–65 y and 3.1% of patients aged ≥65 y without comorbidity vaccinated in France from March 2012 to April 2017 (data not shown). We observed a lower PVC during the study period compared to control period, but the trend was not statistically significant, except for COPD; for the latter, the large population contributes to give significance to this low (and possibly randomly) difference. PVC was lower in patients with DM and higher in HIV-infected patients, did not increase according to the years of the study period (no “time-effect”), and only slightly increased according to time after the diagnosis. The schedule was observed in slightly more than half of the patients with COPD, DM, or CHF and slightly less than half of the HIV-infected patients.
Our study has several strengths: first, we studied the largest populations of adults with one of the abovementioned illnesses; second, excluding the patients identified by reimbursement histories only prevented the inclusion of patients without diagnosis by the GP in the database. Low PVC in HIV-infected patients has already been reported in a study from a center in Paris, with <5% of the patients vaccinated.Citation18 PVC was low (44%) in France even in patients with a major risk of IPD (patients with asplenia).Citation19 There is clearly an overlap between the recommendations to use the influenza vaccination and the pneumococcal vaccine: The French government recommends the use of the influenza vaccination in all patients >65 y old and/or with comorbidities, but these patients require the influenza vaccination every year, which is not the case for the pneumococcal vaccine. Simultaneous use of the two vaccines is thus very difficult. The low vaccination coverage in France does not concern pneumococcal vaccination alone: from a survey of influenza and pneumococcal vaccination coverages in 2011, of patients >65 y old, 4.8% were aware of a previous pneumococcal vaccination; influenza vaccination coverage was 71% in patients >65 y with comorbidities, 58% in patients >65 y without comorbidities, 47% in patients <65 y with comorbidities, and 28% in health-care workers;Citation20 influenza vaccination coverage decreased in France in patients >65 y old from >60% in 2009 to 45% in 2016.Citation21
Low PVC could have several explanations: first, non-PCV13 serotypes emerged from IPD isolates after PCV13 implementation in France: 43% of isolates from 2008 to 2012 in patients >64 y.Citation22 Vaccine hesitancy could be related by several factors: an incomplete (not pan-serotypes) protection, and the responsibility of S. pneumoniae for only a part of CAP and meningitis.
A second reason could be knowledge, attitudes, and beliefs, determinants of vaccine hesitancy in practitioners and patients. In a survey in 2014, most GPs were not or slightly vaccine hesitant, 11% moderately hesitant, 15% highly susceptible to controversies and unsure, and 3% highly hesitant or opposed to vaccination.Citation23 GPs who considered information provided by mass media as reliable were more susceptible to controversies.Citation24 France is dealing with groups refusing recommended vaccinations. Europe is increasingly described as the region in the world with the least confidence in vaccination and particularly in the safety of vaccines but opposition focused in France on the human papillomavirus and the HBV vaccines. We could not distinguish between vaccine hesitancy from GPs and/or patients. The low PVC in at-risk adults contrasts with the high simultaneous PVC in children in France. Ninety-three percent of newborns 9 months old received two doses of PCV13 and 89% of children 24 months old received a complete schedule (with one dose of PCV13 at month 2 and one dose of PCV13 at months 4 and 11) in 2014 in France.Citation25 Childhood PCV can indirectly reduce illness in the unvaccinated population. In a meta-analysis,Citation26 Shiri projected that due to the additional six serotypes contained in PCV13 but not in PCV7, IPD would be nearly eradicated after about 9 y following the introduction of PCV13.
A third reason could be a rapidly moving schedule. High-risk individuals receiving both PCV13 and PPSV23 could be an economically favorable strategy, and vaccinating all 50 y old with both vaccines could be considered.Citation27 In France, pneumococcal vaccination in all patients >65 y with/without comorbidities is not recommended due to the high cost-effective ratio,Citation14 leading to a somewhat confusing setting. Some countries (England and WalesCitation28 and CanadaCitation29) recommend a simplified pneumococcal vaccination schedule in all patients >65 y with/without comorbidities and attain high coverages: 58% in CanadaCitation30 and 75% in England and Wales.Citation27
Some limitations of our study may be emphasized: a bias concerning the PVC could have resulted from different durations of follow-up between the study and the control groups; regarding the very low PVC in both groups, this was obviously not the case. Some patients could have been vaccinated at hospital, but only 20% of doses are used at hospital in France (GERS pharmacies, unpublished data). The most important limitation was that some patients could have been vaccinated by their specialists in ambulatory care and not taken into account. Nevertheless, this limitation was a priori in the study and the control groups.
Different tools and strategies should be used to increase the PVC in France: (i) recommendations mailed to practitioners and patients; (ii) administration of vaccines by pharmacists or nurses. In a systematic review, an increase in vaccine coverage was always found when pharmacists were involved;Citation31 (iii) medical prescription help software with alert messages of vaccine dates; (iv) financial bonus for GPs whose patients’ vaccine coverage is high; (v) a simple same schedule for all high-risk adult patients, as is the case since 2017 update; (vi) recommendations for all patients >65 y, with/without comorbidities; (vii) switching the vaccination from recommended to compulsory for all patients >65 y to overcome opposition groups.Citation32 The high vaccination coverage in England and Wales results from combined factors: administration of vaccines by pharmacists, free vaccine, and invitations sent with a date for vaccination.
Disclosure of potential conflicts of interest
All authors attest they meet the ICMJE criteria for authorship. There is no conflict of interest for all authors.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67:71–79. doi:10.1136/thx.2009.129502.
- Rozenbaum MH, Pechlivanoglou P, van der Werf TS, Lo-Ten-Foe JR, Postma MJ, Hak E. The role of Streptococcus pneumoniae in community-acquired pneumonia among adults in Europe: a meta-analysis. Eur J Clin Microbiol Infect Dis. 2013;32:305–16. doi:10.1007/s10096-012-1778-4.
- Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O’Brien KL, AGEDD. Adult Pneumococcal Burden Study Team. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8:e60273. doi:10.1371/journal.pone.0060273.
- Froes F, Roche N, Blasi F. Pneumococcal vaccination and chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2017;12:3457–68. doi:10.2147/COPD.S140378.
- Alon D, Stein GY, Korenfeld R, Fuchs S. Predictors and outcomes of infection-related hospital admissions of heart failure patients. PLoS One. 2013;8:e72476. doi:10.1371/journal.pone.0072476.
- OPTIMIZE-HF Investigators and Hospitals; Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O’Connor CM, Pieper K, Sun JL, Yancy CW, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847–54. doi:10.1001/archinte.168.8.847.
- Kornum JB, Thomsen RW, Riis A, Lervang HH, Schønheyder HC, Sørensen HT. Type 2 diabetes and pneumonia outcomes: a population-based cohort study. Diabetes Care. 2007;30:2251–57. doi:10.2337/dc06-2417.
- Ehrlich SF, Charles P, Quesenberry CP, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33:55–60. doi:10.2337/dc09-0880.
- van Aalst M, Lötsch F, Spijker R, van der Meer JTM, Langendam MW, Goorhuis A, Grobusch MP, de Bree GJ. Incidence of invasive pneumococcal disease in immuno-compromised patients: a systematic review and meta-analysis. Travel Med Infect Dis. 2018;24:89–100. doi:10.1016/j.tmaid.2018.05.016.
- Kim DK, Bridges CB, Harriman KH, Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP); ACIP Adult Immunization Work Group. Advisory committee on immunization practices recommended immunization schedule for adults aged 19 years or older–United States, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:91–92.
- Esposito S, Bonanni P, Maggi S, Tan L, Ansaldi F, Lopalco PL, Dagan R, Michel JP, van Damme P, Gaillat J, et al. Recommended immunization schedules for adults: clinical practice guidelines by the Escmid Vaccine Study Group (EVASG), European Geriatric Medicine Society (EUGMS) and the World Association for Infectious Diseases and Immunological Disorders (WAidid). Hum Vaccin Immunother. 2016;12:1777–94. doi:10.1080/21645515.2016.1150396.
- Le Calendrier des vaccinations et les recommandations vaccinales 2010 selon l’avis du Haut conseil de la santé publique. Bulletin Epidémiologique Hebdomadaire 22 avril 2010/n° 14-15. [accessed 2020 Apr 05]. santepubliquefrance.fr/determinants-de-sante/vaccination/documents/article/le-calendrier-des-vaccinations-et-les-recommandations-vaccinales-2013-selon-l-avis-du-haut-conseil-de-la-sante-publique/.
- Le Calendrier des vaccinations et les recommandations vaccinales 2013 selon l’avis du Haut conseil de la santé publique. Bulletin Epidémiologique Hebdomadaire 19 avril 2013/n° 14-15. [accessed 2020 Apr 05]. invs.santepubliquefrance.fr/beh/2013/14_15/.
- Infections à pneumocoque: recommandations vaccinales pour les adultes. Haut Conseil de la Santé Publique. Avis mis à jour le 27/11/2017. [accessed 2020 Apr 05]. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=636.
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25. doi:10.1056/NEJMoa1408544.
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422.
- Esposito S, Franco E, Gavazzi G, de Miguel AG, Hardt R, Kassianos G, Bertrand I, Levant MC, Soubeyrand B, Lopez Trigo JA. The public health value of vaccination for seniors in Europe. Vaccine. 2018;36:2523–28. doi:10.1016/j.vaccine.2018.03.053.
- Munier AL, de Lastours V, Varon E, Donay JL, Porcher R, Molina JM. Invasive pneumococcal disease in HIV-infected adults in France from 2000 to 2011: antimicrobial susceptibility and implication of serotypes for vaccination. Infection. 2013;41:663–68. doi:10.1007/s15010-013-0419-x.
- Coignard-Biehler H, Lanternier F, Hot A, Salmon D, Berger A, de Montalembert M, Suarez F, Launay O, Lecuit M, Lortholary O. Adherence to preventive measures after splenectomy in the hospital setting and in the community. J Infect Public Health. 2011;4:187–94. doi:10.1016/j.jiph.2011.06.004.
- Guthmann JP, Fonteneau L, Bonmarin I, Lévy-Bruhl D. Influenza vaccination coverage one year after the A(H1N1) influenza pandemic, France, 2010-2011. Vaccine. 2012;30:995–97. doi:10.1016/j.vaccine.2011.12.011.
- Vaux S, Van Cauteren D, Guthmann JP, Le Strat Y, Vaillant V, de Valk H, Levy-Bruhl D. Influenza vaccination coverage against seasonal and pandemic influenza and their determinants in France: a cross-sectional survey. BMC Public Health. 2011;11:30. doi:10.1186/1471-2458-11-30.
- Janoir C, Lepoutre A, Gutmann L, Varon E. Insight into resistance phenotypes of emergent non 13-valent pneumococcal conjugate vaccine type pneumococci isolated from invasive disease after 13-valent pneumococcal conjugate vaccine implementation in France. Open Forum Infect Dis. 2016;3:ofw020. doi:10.1093/ofid/ofw020.
- Verger P, Collange F, Fressard L, Bocquier A, Gautier A, Pulcini C, Raude J, Peretti-Watel P. Prevalence and correlates of vaccine hesitancy among general practitioners: a cross-sectional telephone survey in France, April to July 2014. Euro Surveill. 2016;21:pii:30406. doi:10.2807/1560-7917.ES.2016.21.47.30406.
- Le Marechal M, Fressard L, Agrinier N, Verger P, Pulcini C. General practitioners’ perceptions of vaccination controversies: a French nationwide cross-sectional study. Clin Microbiol Infect. 2018;24:858–64. doi:10.1016/j.cmi.2017.10.021.
- Données de couverture vaccinale. Pneumocoque par groupe d’âge. [accessed 2019 July 4]. https://santepubliquefrance.fr.
- Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, McCarthy ND, Petrou S. Indirect effect of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Global Health. 2016;5:e51–59. doi:10.1016/S2214-109X(16)30306-0.
- Wateska AR, Nowalk MP, Lin CJ, Harrison LH, Schaffner W, Zimmerman RK, Smith KJ. An intervention to improve pneumococcal vaccination uptake in high risk 50-64 year olds vs. expanded age-based recommendations: an exploratory cost-effectiveness analysis. Hum Vaccin Immunother. 2019 Jan 11;15(4):863–72. [Epub ahead of print]. doi:10.1080/21645515.2018.1564439.
- Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30:6802–08. doi:10.1016/j.vaccine.2012.09.019.
- Jensen C, Lerch R, National Advisory Committee on Immunization (NACI). Updates to the Canadian Immunization Guide: april 2015 to October 2016. Can Commun Dis Rep. 2016;42:256–59. doi:10.14745/ccdr.v42i12a04.
- Schneeberg A, Bettinger JA, McNeil S, Ward BJ, Dionne M, Cooper C, Coleman B, Loeb M, Rubinstein E, MacElhaney J, et al. Knowledge, attitudes, beliefs and behaviours of older adults about pneumococcal immunization, a Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) investigation. BMC Public Health. 2014;14:442. doi:10.1186/1471-2458-14-442.
- Isenor JE, Edwards NT, Alia TA, Slayter KL, MacDougall DM, McNeil SA, Bowles SK. Impact of pharmacists as immunizers on vaccination rates: a systematic review and meta-analysis. Vaccine. 2016;34:5708–23. doi:10.1016/j.vaccine.2016.08.085.
- Gualano MR, Olivero E, Voglino G, Corezzi M, Rossello P, Vicentini C, Bert F, Siliquini R. Knowledge, attitudes and beliefs towards compulsory vaccination: a systematic review. Hum Vaccin Immunother. 2019 Jan 11;15(4):918–31. [Epub ahead of print]. doi:10.1080/21645515.2018.1564437.
