ABSTRACT
Few studies in China focused on serotypes of Streptococcus pneumoniae in patients with invasive pneumococcal disease (IPD). We aimed at investigating the serotype distribution for IPD-causing S. pneumoniae and vaccine coverage among Chinese children and adults. This was a multicenter, observational study to collect S. pneumoniae isolates from normal sterile sites and IPD-related clinical information among children and adults. Serotyping was performed by a Capsule-Quellung reaction test using type-specific antisera. The study collected a total of 300 eligible isolates (pediatric = 148, adult = 152) were serotyped in a central laboratory. The most prevalent serotypes were 19A (20.9%) and 23 F (20.3%) in the pediatric group; 3 (21.7%) and 19 F (11.8%) in the adult group. PCV10 had low-to-moderate serotype coverage rates for children (60.8%) and adults (34.2%). PCV13 and PPV23 had high coverage rates for children (89.9%, 93.2%) and adults (70.4%, 82.9%), respectively, Investigational PCVs including PCV15 and PCV20 had high estimated coverage rates in children (89.9%, 93.9%). The study identified 269 subjects with IPD reported as the primary diagnosis in the medical records. Sepsis (48/136, 35.3%) and pneumonia (48/133, 36.1%) had the highest occurrence in the pediatric and adult groups, respectively. Study findings showed that non-PCV7 S. pneumoniae 19A and 3 were the most prevalent serotypes in Chinese children and adults, respectively. High-valent vaccines had similar coverage rates and may have a greater potential in preventing IPD.
Introduction
Streptococcus pneumoniae causes bacterial infections, namely pneumococcal disease, in children and the elderly worldwide.Citation1 Pneumococcal disease includes serious infections such as meningitis and pneumonia, as well as common illnesses such as sinusitis and otitis media. Pneumococcal disease can be invasive (IPD), defined as an infection confirmed by the isolation of S. pneumoniae from a normally sterile body site. The WHO estimated that pneumococcal disease caused approximately 5% of total deaths among children <5 y in 2008.Citation2 In the pre-vaccination era, incidence rates of IPD ranged from 15-49/100,000 in North America,Citation3,Citation4 11-27/100,000 in Europe,Citation4,Citation5 and up to 216/100,000 in specific Asian regions.Citation6 IPD is age-specific, in which 38%, 54% of all reported cases occurred in children <2 y and adults above 50 y of age, respectively.Citation7 The incidence of IPD in children <2 y was 44.4/100,000 in Europe,Citation8 167/100,000 in the US,Citation9 and 60-797/100 000 in African countries.Citation10,Citation11 In adults, mortality rates of IPD were 11-30% in the western worldCitation12-14 and 26–30% in Asia countries.Citation15,Citation16 Mortality is greatest in younger infants and elderly patients.
The capsular polysaccharides (CPS) on the surface of S pneumoniae are the primary bacterial virulence and the basis for serotyping, thereby serving the immunogenic targets as covered by S. pneumoniae vaccination. There are over 90 identified serotypes of S. pneumoniae,Citation17 among which IPD attributes to a relatively small subset. Serotypes differ in exhibiting invasive potential to cause IPD and that the prevalence of different serotypes can vary by geographic region. Studies suggested that serotypes 1, 5 and 7 were more likely to cause invasive disease, and 6, 9, 14, 19 and 23 were predominantly in pediatric populations.Citation18 In children <5 y, serotypes 1, 5, 6A, 6B, 14, 19 F, and 23 F are common causes of IPD globally whereas 18 C was common in some western countries.Citation19 Therefore, inclusion of these serotypes in different vaccine preparations is critical.
Heptavalent pneumococcal conjugate vaccines (PCV) significantly reduced the burden of IPD in young children.Citation20,Citation21 PCV7 (covers serotypes 4, 6B, 9 V, 14, 18 C, 19 F and 23 F) also reduced the disease incidence among non-vaccinated children and adult groups due to herd immunity.Citation22,Citation23 Relatively newer PCVs (PCV10, PCV13) have been available to cover more IPD-specific serotypes. Evidence has suggested that the coverage of IPD serotypes by PCV7, PCV10/PCV13 was ≥49%, ≥70%, respectively, across geographic regions.Citation19 Recently, 15- and 20-valent PCVs (PCV15, PCV20) with additional serotypes has been investigated in phase 2 and 3 clinical trials.Citation24 In China, PCV7 was on the market since 2008 for healthy children under 5 y old.Citation25 In addition, 23-valent pneumococcal polysaccharide vaccine (PPV23) is indicated for healthy children aged 2 y or older and the elderly.Citation26 These vaccines were not included in the China’s national immunization program (NIP) and the immunization coverages were generally low.Citation27 There were no PCVs available since 2015 until PCV13 received approval in China in late 2016Citation28,Citation29 and launched in the market in 2017.
Nevertheless, emergent non-vaccine serotypes increased as causes of IPD following the extensive use of PCVs. Serotype replacement by 19A was one of the primary causes of childhood IPD (>20.0%) in PCV7 accessed countries. Also, non-PCV13 serotypes such as 22 F, 12 F and 33 F contributed to 42.2% of childhood IPD cases in countries where higher valent vaccines were introduced.Citation30 Therefore, examining epidemiological effects of conjugate vaccines by investigating serotype distribution associated with pneumococcal diseases is of priority before universal immunization in new settings. Although studies collecting serotype data were conducted globally for children with IPD,Citation19,Citation30 relatively fewer studies focused on adults. Limited data in China documented IPD epidemiology and serotype distribution. Our objectives were to study the serotype distribution of IPD-causing S. pneumoniae and potential coverage by conjugate and polysaccharide vaccines among Chinese children and adults.
Methods and materials
Study design
This is a multicenter, observational study to collect S. pneumoniae isolates and corresponding IPD clinical information from both pediatric and adult subjects treated in 27 tertiary hospitals in China. Study sites were screened and selected individually by balancing the geographic location (north vs. south) and the evaluation of the investigator’s (clinical microbiologist) capability in collecting S. pneumoniae isolates, especially those from children, in the past three years at the time of the site initiation. The site’s routine clinical practice in diagnosing and treating IPD was also reviewed to meet the study needs. Between 2012 and 2015, the investigators obtained S. pneumoniae isolates once the subject had microbiologically confirmed IPD and clinical information was prospectively collected at the site. A retrospective study phase was conducted concurrently to collect cultured isolates from the microbiology laboratory and review the subject’s medical records for IPD primary diagnosis within the last three years upon the initiation of each site. Merck Sharp & Dohme (MSD) designed and sponsored the study and analyzed the data. The study was conducted following the guidelines of the International Conference on Harmonization and local regulatory guidance and was approved by independent ethics committees of all study sites.
Patients and S. pneumoniae isolates
The patient was defined as a study case (subject) once IPD was microbiologically confirmed by S. pneumoniae cultured from specimens obtained through a normally sterile site, including blood and cerebrospinal fluid (CSF) or, less commonly, joint, pleural, and pericardial fluid. IPD was defined as isolation of S. pneumoniae from a normally sterile site in an individual with clinical signs and symptoms of invasive disease.Citation1 Patients were not eligible as an IPD study case if they met the definition for non-IPD as proper S. pneumoniae cultures related to acute otitis media or pneumococcal pneumonia with an absence of bacteremia. Also excluded were patients whose S. pneumoniae cultures sampled from the nasopharynx or whose age information was missing. All patients were treated in the routine clinical practice where isolates were collected from both inpatient and outpatient departments at each site by the investigators with clinical microbiology specialty. Patients or their legal representatives gave written informed consent before any study procedure commenced. The ethics committee at each study site waived the informed consent process for IPD patients identified from the retrospective study phase in which acceptable conditions precluding the consent existed (e.g. death or discharge).
Study procedure and assessments
All isolates were transported to a central microbiology laboratory for S. pneumoniae identification and in vitro susceptibility tests (Department of Laboratory Medicine, Peking Union Medical College Hospital). In the study, one enrolled patient contributed one isolate to the central laboratory. An additional isolate was not required unless sample processing was not appropriate during the transportation or in the laboratory. The subject’s demographic and clinical data were collected via a prospective fashion or a review of medical records at the site. All isolates were then transported to a disease control central laboratory (National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention) for serogrouping and serotyping after all microbiology testings have been completed.
Serotyping
A simplified chessboard system processed by Pneumotest-kit (Demark, Serum Institute) was applied to determine the serotype grouping of all isolates. While S. pneumoniae serotyping was done by a Capsule-Quellung reaction test using type-specific antisera (Statens Serum Institut, Copenhagen, Denmark) against all 90+ serotypes.Citation31 The isolates identified as S. pneumoniae that reacted negatively were classified as ‘nontypable’.
Antimicrobial in vitro susceptibility
The Agar dilution method and E-test methods were used to determine the antibiotic susceptibility of isolates to 17 commonly prescribed antibiotics. The CLSI 2016 criteria for minimum inhibitory concentrations (MICs) were applied to classify the susceptible, intermediate, and resistant isolates.Citation32 Separate interpretive breakpoints for nonmeningeal versus meningeal isolates were used to define penicillin and parenteral cephalosporin resistance.
Demographics and clinical information
The demographic characteristics including age, gender, rural/urban, and geographic region were collected for both retrospective and prospective study phases. Clinical information including IPD primary and secondary diagnoses, site of culture specimen and antimicrobial regimen were also collected.
Study objectives
The study objectives were to determine the serotype distribution for IPD-causing S. pneumoniae and potential serotype coverage by conjugate vaccine (PCV10, PCV13, and PCV15) and polysaccharide vaccine (PPV23) in Chinese pediatric and adult populations. Post-hoc analysis was made to investigate serotype coverage by PCV20 which is under clinical investigation.
Statistical analysis
Sample size consideration
The study was descriptive and no priori hypothesis was made for study objectives. Sample size was dependent upon the precision of estimation (two-sided 95% confidence interval) on the proportions of combined serotypes 22 F/33 F which the investigational PCV15 serotype may potentially cover. A sample size of 140 from a studied population (pediatric or adult) yielded a two-sided precision of 8.0% for the estimation, assuming that the combined serotypes 22 F/33 F accounted for 5.0% of serotypes identified for all isolates. Meanwhile, this sample size was statistically sufficient if an estimated proportion of PCV15 coverage was 80% with a two-sided precision of 14%. The Clopper-Pearson Exact method was applied as the formula for the calculation.Citation33 This sample size was also statistically sufficient if an estimated proportion of PCV15 coverage was 80% with a two-sided precision of 14%. Considering a loss by an approximately 5-10% of all isolates during study execution, approximately 150 isolates was to be collected from in pediatric and adult populations, respectively. Therefore, a total sample size of 300 isolates was collected for the study. Due to operational difficulties, randomized sampling was not conducted for the study.
Analysis population
Subjects with proven S. pneumoniae isolates and evaluable clinical information were included for the analyses. The analyses were performed in pediatric (<18 y of age) versus adult study populations.
Analysis of study objectives and other variables
For serotype distribution, number and percentage of total isolates were provided by serotype. Vaccine coverage was summarized by number and percentage and corresponding 95%CI was given. Resistant isolates to antimicrobials were interpreted by MICs and were summarized by number and percentage. For demographics and clinical data, descriptive statistics were made to display the results. Chi-square or t-test was used to test the significance of difference in categorical or continuous variables wherever appropriate. Subgroup analysis by age intervals was performed for serotype distribution and vaccine coverage. There was no imputation for missing IPD primary diagnosis in the study. Statistical analyses were performed using SAS 9.1 and SAS JMP 14.3 (SAS Institute, Cary, NC, USA), and a p value of 0.05 was considered statistically significant whenever applicable.
Results
S. pneumoniae isolate collection
displays the study enrollment and Figure 1b shows the site geographic distribution. The investigators identified a total of 312 S. pneumoniae isolates cultured between 2010 and 2015, among which 300 (pediatric = 148, adult = 152) were included for serotyping and study analysis. The most frequent reason for the exclusion from serotyping was storage issue in the central microbiology laboratory (pediatric = 3, adult = 4). In the pediatric group, more isolates (102/148, 68.9%) were collected through a retrospective phase; whereas in the adult group, more isolates (93/152, 61.2%) were obtained in the prospective study phase.
Figure 1a. Study flowchart for enrollment.S. pneumoniae isolates were collected by both prospective and retrospective phases; 300 isolates were eligible [children: 148 (49.3%), adults: 152 (50.7%)] and included for analysis. In the pediatric group, 102 (68.9%) isolates were collected via retrospective phase and 46 (31.1%) via prospective phase; in the adult group, 93 (61.2%) were collected via retrospective phase and 59 (38.8%) via prospective phase;Laboratory issues included isolate death or loss in the central laboratory.Geographic distribution of study sites in China.The study collected S. pneumonia isolates from 27 sites located in 13 provinces in China. A total of 149 isolates were from the North region and 151 isolates were from the South region. Provinces in the North region: Beijing, Gansu, Hebei, Jiangsu (north), Liaoning, Shandong, Shanxi, and Tianjin; provinces in the South region: Anhui, Hainan, Hubei, Jiangsu (south), Shanghai, and Zhejiang
![Figure 1a. Study flowchart for enrollment.S. pneumoniae isolates were collected by both prospective and retrospective phases; 300 isolates were eligible [children: 148 (49.3%), adults: 152 (50.7%)] and included for analysis. In the pediatric group, 102 (68.9%) isolates were collected via retrospective phase and 46 (31.1%) via prospective phase; in the adult group, 93 (61.2%) were collected via retrospective phase and 59 (38.8%) via prospective phase;Laboratory issues included isolate death or loss in the central laboratory.Geographic distribution of study sites in China.The study collected S. pneumonia isolates from 27 sites located in 13 provinces in China. A total of 149 isolates were from the North region and 151 isolates were from the South region. Provinces in the North region: Beijing, Gansu, Hebei, Jiangsu (north), Liaoning, Shandong, Shanxi, and Tianjin; provinces in the South region: Anhui, Hainan, Hubei, Jiangsu (south), Shanghai, and Zhejiang](/cms/asset/68ab9675-bc6a-4e1f-bd47-9a34cda687c1/khvi_a_1757996_f0001_oc.jpg)
In the study, there were 39 distinct S. pneumoniae serotypes identified in the central laboratory. Fewer serotypes (21) were obtained from the pediatric group as compared with those (37) from the adult group.
Demographics and clinical characteristics
The subject’s demographic and clinical information are shown in . The subject’s mean age was 2.6 and 54.3 y in the pediatric and adult groups, respectively. Gender and rural residence did not differ significantly between the two groups; however, the North China region had more isolates for the adult group as compared to that for the pediatric group [94 (62.8%) versus 55 (37.2%)]. Most isolates were cultured from the blood (72.3%) and the cerebrospinal fluid (19.3%), among which there was no significant difference in the source of specimens between the two groups. Cephalosporins (58.8%) and carbepenems (29.6%) were more frequently prescribed in the pediatric and the adult groups, respectively.
Table 1. Demographic and clinical information*
Serotype distribution and vaccine coverage
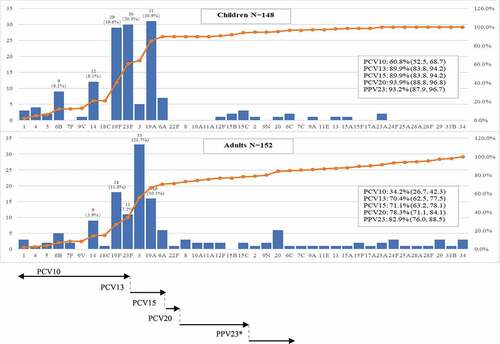
The most prevalent S. pneumoniae serotypes identified were 19A (15.7%), 19 F (15.7%), 23 F (13.7%), 3 (12.7%) and 14 (7.0%) among all subjects. exhibits numbers of S. pneumoniae isolates and cumulative percentages of serotype distribution and corresponding vaccine coverages by PCVs and PPV23 in the pediatric and adult groups. Full results of coverage rates and corresponding 95%CIs are shown in Supplemental Table 1. The most prevalent serotypes were 19A (20.9%), 23 F (20.3%), 19 F (19.6%), 14 (8.1%) and 6B (6.1%) in the pediatric group and 3 (21.7%), 19 F (11.8%), 19A (10.5%), 23 F (7.2%) and 14 (5.9%) in the adult group. There was one isolate serotyped 22 F (0.7%, 95% CI: 0.0%, 3.6%) in the adult group. Rates of serotype coverage were generally higher in the pediatric group as compared with those in the adult group. PCV10 had low-to-moderate serotype coverage rates for the adult group (34.2%, 95% CI: 26.7, 42.3) and the pediatric group (60.8%, 95% CI: 52.5, 68.7). PCV13, PPV23 and the investigational PCV15 and PCV20 had higher serotype coverage rates in the pediatric group (89.9%, 95% CI: 83.8, 94.2; 93.2%, 95% CI: 87.9, 96.7; 89.9%, 95% CI: 83.8, 94.2; 93.9%, 95%CI: 88.8, 96.8) as compared with those in the adult group.
Figure 2. Serotype distribution of S. pneumoniae isolates in children and adults
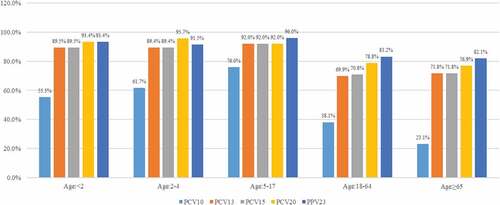
shows vaccine coverage by all subgroups (age <2, 2–4, 5–17, 18–64 and ≥65 y). PCV10 had relatively lower vaccine coverage rates for the infant group (<2 y) (55.3%) and the elderly group (≥65 y) (23.1%). PCV13 had high vaccine coverage rates for children subgroups (89.4–92.0%). PPV23 had high vaccine coverage rates across all age subgroups (82.1–96.0%). The investigational PCV20 had high vaccine coverage rates for children subgroups (92.0–95.7%). Full results of S. pneumoniae serotype distribution by age subgroups are shown in Supplemental Table 2. Serotype 3 most frequently appeared (38.5%) in the elderly group. Percentage of 19A was relatively higher in the infant group (25.0%) and the preschooler group (2–4 y of age: 23.9%). In children aged between 5 and 17 y, 19 F (28.0%) and 23 F (28.0%) were most prevalent. The percentages of serotype coverage in all age subgroups were similar with those in the adult and pediatric groups.
Invasive pneumococcal disease
The results of IPD overall and by serotype distribution are summarized in . Individual serotypes were presented if numbers of collected isolates >10. A total of 269 patients with a primary diagnosis of IPD were confirmed by the investigators (children = 136, adult = 133). Only the primary diagnosis for each IPD patient was identified at the time of isolate collection. Sepsis had the highest occurrence [35.3% (48/136)] in the pediatric group and pneumonia was the most prevalent [36.1% (48/133)] in the adult groups. Meningitis accounted for 21.3% (29/136), 17.3% (23/133) in the pediatric and the adult groups, respectively.
Table 2. Invasive pneumococcal disease overall and by serotype
In each IPD primary diagnosis, serotype 19A (37.8%, 14/37) and 3 (25.0%, 12/48) had the highest proportions in children and adults who had pneumonia, respectively. In children with sepsis, 19 F accounted for 22.9% (11/48) of all serotypes identified. Serotype 23 F infected 31.0% (9/29) of all IPD children diagnosed with meningitis.
Non-PCV7/PCV13 serotypes
Non-PCV7 and PCV13 serotypes and associated IPD and in vitro susceptibility results are presented in . Serotype 19A was the prevalent among all non-PCV7 isolates in the pediatric group [49.2% (31/63)]. Among 29 IPD children contracted 19A, 14 (48.3%) had pneumonia and 8 (27.6%) developed sepsis. Serotype 3 was most commonly seen in non-PCV7 isolates in the adult group [30.8% (33/107)]. Among 28 IPD adults contracted serotype 3, 12 (42.9%) had pneumonia and 6 (21.4%) had bacteremia. Serotype 19A had exhibited a relatively high non-susceptibility to commonly prescribed antibiotics against IPD including ceftriaxone and meropenem in both children [ceftriaxone: 48.4% (15/31)] and adults [meropenem: 87.5% (14/16)].
Table 3. IPD distribution and Resistance rate of Non-PCV7/13 serotypes
The number of individual non-PCV13 isolates (n = 15) was relatively fewer in the pediatric and the adult groups. Serotype 15 C had 3 (20.0%) isolates in the pediatric group and serotype 20 had 6 (13.3%) in the adult group, respectively.
Discussion
Findings from this multicenter, observational study suggested that the most prevalent S. pneumoniae serotypes were 19A, 19 F, 23 F, and 3 across different regions in China. Non-PCV7 serotype 19A and 3 was most prevalent in children and in adults, respectively, and 22 F/33 F were rare. New conjugate vaccines provided higher rates of serotype coverage in the pediatric and adult groups.
Relatively fewer epidemiological studies conducted in ChinaCitation34 and in AsiaCitation35 to study S. pneumoniae serotypes in IPD. Studies on IPD children reported data from the 1980sCitation36 or focused serotypes from invasive pneumonia or non IPDs although more recently conducted.Citation37,Citation38 The ANSORP Study collected 642 isolates from China but only obtained 33 IPD strains.Citation35 Other Asian studies were either geographically differentCitation39 or had no associated clinical informationCitation40 or limited to specimens from the respiratory tract.Citation41 This study, on the other hand, had obtained 300 serotyped isolates from normal sterile sites in both Chinese children and adults, and is the largest in China to study serotype distribution and associated IPD spectrum. The study design allowed a concurrent retrospective collection of isolates. Study data can thus cover serotype distribution in a 6-y interval between 2010 and 2015. In addition, this study emphasized the subject’s exact age information on isolate collection. An analysis on all age subgroups was performed, which provided descriptive results on a specific age group, including those who were younger than 2 or elder than 65 y of age.
Our study showed a high prevalence of non-PCV7 serotype 19A in children, especially in those who were less than 5 y of age. This finding was consistent with data previously reported from studies in China between 2005 and 2011.Citation42 Emergent 19A was an issue in western countries and in some Asian countries after the introduction of PCV7. But studies in Singapore,Citation43 South KoreaCitation44 and ChinaCitation42 showed an increase of serotype 19A among IPD children before PCV7 became available. PCV7 was marketed in China since 2008 for healthy children <5 y under private health care Results from this study, along with previous evidence, indicated that prevalence of 19A may be due to other factors instead of serotype replacement caused by PCV7 in China. In the adult group, a high proportion of non-PCV7 serotype 3 was observed. A recent study reported 14.0% of serotype 3 in Portuguese adults.Citation45 Similar results were seen in Japan where serotype 3 accounted for 14.7% among serotypes cultured from respiratory tract.Citation41 But a significantly lower distribution of serotype 3 was reported from recently cultured IPD isolates in China and India.Citation39,Citation42 Studies from 46 USD and GermanyCitation47 also reported a low prevalence of serotype 3 (7-8%) in the adult. A few studies demonstrated the lower efficacy of the serotype 3 compared with other serotypes in PCV13 for children,Citation48–50 but with conflicting results in different regions.Citation51,Citation52 A study in Hong Kong monitored PCV 13 immunization program on children between 2015 and 2017. An increase by 59% in the IPD caused by serotype 3 was seen.Citation53 This finding supported the lower efficacy of PCV13 against serotype 3 in an Asian pediatric population. However, studies identifying serotypes in adults were very limited in China, and PCV7 and PCV13 were not indicated for the adult. Results from our study on the prevalence of serotype 3 may warrant further investigations.
In the study, coverage rates of PCV10 in young children were relatively lower compared with that in western countries.Citation1 This can be partly explained by findings that non-PCV7 serotypes were more frequent in China and PCV10-specific serotypes were few. Nevertheless, rates of serotype coverage by higher valent vaccines (PCV13, PCV15 and PCV20) increased greatly as compared with PCV10 among children and adults. Considering high non-susceptibility to antibiotics and associated major IPDs caused by non-PCV7 strains, newer vaccination programs including PCV13, 15 and 20 are in need to substantially prevent IPD episodes. In addition, this study identified 21 and 37 serotypes in children and adults, respectively. Because more serotypes were identified from adults, rates of vaccine coverages are generally lower in the group. Due to the difference in serotype numbers and distribution between these two populations, observed herd effects toward adults from children among western countriesCitation22,Citation23 may not benefit to the similar extent in China when new vaccines are firstly indicated in children. In addition, an expected difference between PCV13 and PCV15 did not occur. Data from literature review of pediatric isolates and studies on IPD isolates have indicated 22 F/33 F serotypes were scarce (<1.0%) in China,Citation34,Citation37,Citation38,Citation42 as opposite to western countries, for example, the Netherlands.Citation54
Clinically findings presented from 269 study subjects when analyzing serotype distribution. Pneumonia had the highest occurrence in the adult group. Although the study was not able to distinguish if it was community or hospital acquired, the results were consistent with previously reported.Citation55 Sepsis, however, had been the most common in the pediatric group, followed by pneumonia and meningitis. A majority of isolates in the study were from the blood. Since clinical practice for blood culture may differ in study sites, sampling procedure might not be performed until primary infections clinically progress in pediatric patients. Several serotypes including 19A, 3, 23 F, and 19 F accounted for high proportions in individual IPDs in the pediatric and the adult group. Unlike some studies,Citation55,Citation56 serotypes categorized as invasive did not appear frequently. Because the adult group had a wide serotype spectrum, the role of conjugate and polysaccharide vaccines for individual IPDs must be monitored carefully if vaccinated.
This study has several limitations. Firstly, this study was not designed as a national surveillance program. Comparison in serotype distribution and monitoring particular serotypes over time was not feasible. In addition, the study was not conducted as a cross-sectional study to balance enrollment numbers across site and geographic region by age group. The study team did not perform additional tests including multilocus sequence typing (MLST) to determine specific strains from a certain serotype which caused multiple IPDs. Retrospective data collection was based on review of medical records. Incomplete data caused ascertainment bias in confirming a primary IPD diagnosis. Moreover, the sample size was not sufficient for PCV-targeted serotypes in a particular age subgroup. Last, the study was conducted in major tertiary hospitals in China and was not population-based. The results may not be generalizable to the entire population, especially that in the community and remote areas.
Conclusion
Study findings suggested that non-PCV7 covered S. pneumoniae 19A and 3 were the most prevalent serotypes in Chinese children and adults, respectively. Higher valent conjugate and polysaccharide vaccines had favorable rates of serotype coverage and may have a greater potential in preventing IPD caused by S. pneumoniae.
Disclosure of potential conflicts of interest
B.P., T.WM and W.J. are employees of MSD China, Shanghai, China. Other authors had none to declare.
Supplemental Material
Download MS Word (44.5 KB)Acknowledgments
The study team acknowledged the contributions of all study investigators in collecting sample isolates and study data.
Supplemental data
Supplemental data for this article can be accessed online at http://doi.org/10.1080/21645515.2020.1757996.
Additional information
Funding
References
- Pneumococcal vaccines WHO position paper. 2012. [accessed 2017 Oct]. (http://www.who.int/wer/2012/wer8714.pdf?ua=1
- Estimated Hib and pneumococcal deaths for children under 5 years of age. 2008.[accessed 2017 Oct]. (http://www.who.int/immunization_monitoring/burden/Pneumo_hib_estimates/en/index.html
- Kyaw MH, Christie P, Clarke SC, Mooney JD, Ahmed S, Jones IG, Campbell H. Invasive pneumococcal disease in Scotland, 1999–2001: use of record linkage to explore associations between patients and disease in relation to future vaccination policy. Clin Infect Dis. 2003;37(10):1283–91. doi:10.1086/379016.
- Lynch JP 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med. 2009;30(2):189–209. doi:10.1055/s-0029-1202938.
- Reinert RR, Haupts S, van der Linden M, Heeg C, Cil MY, Al-Lahham A, Fedson DS. Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001–2003. Clin Microbiol Infect. 2005;11(12):985–91. doi:10.1111/j.1469-0691.2005.01282.x.
- Hung IF, Tantawichien T, Tsai YH, Patil S, Zotomayor R. Regional epidemiology of invasive pneumococcal disease in Asian adults: epidemiology, disease burden, serotype distribution, and antimicrobial resistance patterns and prevention. Int J Infect Dis. 2013;17(6):e364–e373. doi:10.1016/j.ijid.2013.01.004.
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45–51. doi:10.1111/1469-0691.12461.
- Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis. 2010;14(3):e197–209. doi:10.1016/j.ijid.2009.05.010.
- Klugman KP, Black S, Dagan R, Malley R, Whitney CG. Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In: Plotkin SA, Orenstein WA, Offit P editors. Vaccines. 6th. Philadelphia (PA): WB Saunders Company; 2008. p. 504–42.
- Karstaedt AS, Khoosal M, Crewe-Brown HH. Pneumococcal bacteremia during a decade in children in Soweto, South Africa. Pediatr Infect Dis J. 2000;9(5):454–57. doi:10.1097/00006454-200005000-00012.
- Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, Oluwalana C, Vaughan A, Obaro SK, Leach A, et al. Gambian pneumococcal vaccine trial group. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in the Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365(9465):1139–46. doi:10.1016/S0140-6736(05)71876-6.
- Mufson MA, Stanek RJ. Bacteremic pneumococcal pneumonia in one American city: a 20-year longitudinal study, 1978–1997. Am J Med. 1999;107(1):34S–43S. doi:10.1016/S0002-9343(99)00098-4.
- Feikin DR, Schuchat A, Kolczak M, Barrett NL, Harrison LH, Lefkowitz L, McGeer A, Farley MM, Vugia DJ, Lexau C, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am J Public Health. 2000;90:223–29.
- Rock C, Sadlier C, Fitzgerald J, Kelleher M, Dowling C, Kelly S, Bergin C. Epidemiology of invasive pneumococcal disease and vaccine provision in a tertiary referral center. Eur J Clin Microbiol Infect Dis. 2013;32(9):1135–41. doi:10.1007/s10096-013-1859-z.
- Thomas K, Mukkai Kesavan L, Veeraraghavan B, Jasmine S, Jude J, Shubankar M, Kulkarni P, Steinhoff M. IBIS study group IndiaCLEN network. invasive pneumococcal disease associated with high case fatality in India. J Clin Epidemiol. 2013;66(1):36–43. doi:10.1016/j.jclinepi.2012.04.006.
- Song JY, Choi JY, Lee JS, Bae IG, Kim YK, Sohn JW, Jo YM, Choi WS, Lee J, Park KH, et al. Clinical and economic burden of invasive pneumococcal disease in adults: a multicenter hospital-based study. BMC Infect Dis. 2013;13(1):202. doi:10.1186/1471-2334-13-202.
- Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33(10):2759–62. doi:10.1128/JCM.33.10.2759-2762.1995.
- Alanee SR, McGee L, Jackson D, Chiou CC, Feldman C, Morris AJ, Ortqvist A, Rello J, Luna CM, Baddour LM, et al. International pneumococcal study group. association of serotypes of Streptococcus pneumoniae with disease severity and outcome in adults: an international study. Clin Infect Dis. 2007;45(1):46–51. doi:10.1086/518538.
- Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O’Brien KL. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7:pii: e1000348. doi:10.1371/journal.pmed.1000348.
- Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, Thomas AR, Harrison LH, Bennett NM, Farley MM, et al. Active bacterial core surveillance of the emerging infections program network. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–63. doi:10.1056/NEJMoa051642.
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Active bacterial core surveillance/emerging infections program network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi:10.1086/648593.
- van Werkhoven CH, Hollingsworth RC, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Sanders EA, Bonten MJ. Pneumococcal conjugate vaccine herd effects on non-invasive pneumococcal pneumonia in elderly. Vaccine. 2016;34(28):3275–82. doi:10.1016/j.vaccine.2016.05.002.
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–73. doi:10.1016/S0140-6736(10)62225-8.
- van der Linden M, Imöhl M, Perniciaro S. Limited indirect effects of an infant pneumococcal vaccination program in an aging population. PLoS One. 2019;14:e0220453. doi:10.1371/journal.pone.0220453.
- Yao KH, Wang LB, Zhao GM, Zheng YJ, Deng L, Huang JF, Wang JX, Zhao RZ, Deng QL, Hu YH, Yu SJ, Yang YH, Young M. Pneumococcal serotype distribution and antimicrobial resistance in Chinese children hospitalized for pneumonia. Vaccine. 2011;29(12):2296–301. doi:10.1016/j.vaccine.2011.01.027.
- Kong Y, Zhang W, Jiang Z, Wang L, Li C, Li Y, Xia J. Immunogenicity and safety of a 23-valent pneumococcal polysaccharide vaccine in Chinese healthy population aged>2 years: a randomized, double-blinded, active control, phase III trial. Hum Vaccin Immunother. 2015;11:2425–33. doi:10.1080/21645515.2015.1055429.
- Li Y, An Z, Yin D, Liu Y, Huang Z, Ma Y, Li H, Li Q, Wang H. Disease burden of community acquired pneumonia among children under 5 y old in China: A population based survey. Hum Vaccin Immunother. 2017;13(7):1681–87. doi:10.1080/21645515.2017.1304335.
- Mo X, Gai Tobe R, Liu X, Mori R. Cost-effectiveness and health benefits of pediatric 23-valent pneumococcal polysaccharide vaccine, 7-valent pneumococcal conjugate vaccine and forecasting 13-valent pneumococcal conjugate vaccine in China. Pediatr Infect Dis J. 2016;35(11):e353–e361. doi:10.1097/INF.0000000000001288.
- [accessed Feb 10 2020]. https://www.pfizer.com/news/press-release/press-release-detail/pfizer_s_prevenar_13_receives_approval_for_use_in_infants_and_children_in_china
- Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis. PLoS One. 2017;12(5):e0177113. doi:10.1371/journal.pone.0177113.
- Sørensen UB. Typing of pneumococci by using 12 pooled antisera. J Clin Microbiol. 1993;31:2097–100. doi:10.1128/JCM.31.8.2097-2100.1993.
- Clinical & Laboratory Standards Institute Wayne, PA 19087, USA
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–72. doi:10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E.
- Chen Y, Deng W, Wang SM, Mo QM, Jia H, Wang Q, Li SG, Li X, Yao BD, Liu CJ, et al. Burden of pneumonia and meningitis caused by streptococcus pneumoniae in China among children under 5 years of age: a systemic literature review. PLoS One. 2011;6(11):1–9. doi:10.1371/journal.pone.0027333.
- Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, Lu M, So TM, Hsueh PR, Yasin RM, et al., ANSORP Study Group. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56(3):1418–26. doi:10.1128/AAC.05658-11.
- Yao KH, YH Y. Streptococcus pneumoniae diseases in Chinese children: past, present and future. Vaccine. 2008;26(35):4425–33. doi:10.1016/j.vaccine.2008.06.052.
- Liu Y, Wang H, Chen M, Sun Z, Zhao R, Zhang L, Wang H, Zhang H, Wang L, Chu Y, et al. Serotype distribution and antimicrobial resistance patterns of Streptococcus pneumoniae isolated from children in China younger than 5 years. Diagn Microbiol Infect Dis. 2008;61(3):256–63. doi:10.1016/j.diagmicrobio.2008.02.004.
- Xue L, Yao K, Xie G, Zheng Y, Wang C, Shang Y, Wang H, Wan L, Liu L, Li C, et al. Serotype distribution and antimicrobial resistance of streptococcus pneumoniae isolates that cause invasive disease among Chinese children. Clin Infect Dis. 2010;50(5):741–44. doi:10.1086/650534.
- Molander V, Elisson C, Balaji V, Backhaus E, John J, Vargheese R, Jayaraman R, Andersson R. Invasive pneumococcal infections in Vellore, India: clinical characteristics and distribution of serotypes. BMC Infect Dis. 2013;13(1):532. doi:10.1186/1471-2334-13-532.
- Phongsamart W, Srifeungfung S, Chatsuwan T, Nunthapisud P, Treerauthaweeraphong V, Rungnobhakhun P, Sricharoenchai S, Chokephaibulkit K. Changing trends in serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae causing invasive diseases in central Thailand, 2009-2012. Hum Vaccin Immunother. 2014;10:1866–73. doi:10.4161/hv.28675.
- Shoji H, Maeda M, Takuma T, Niki Y. Serotype distribution of Streptococcus pneumoniae isolated from adult respiratory tract infections in nationwide Japanese surveillances from 2006 to 2014. J Infect Chemother. 2017;23(8):538–44. doi:10.1016/j.jiac.2017.05.003.
- Zhao C, Zhang F, Chu Y, Liu Y, Cao B, Chen M, Yu Y, Liao K, Zhang L, Sun Z, et al. Phenotypic and genotypic characteristic of invasive pneumococcal isolates from both children and adult patients from a multicenter surveillance in China 2005-2011. PLoS One. 2013;8(12):e82361. doi:10.1371/journal.pone.0082361.
- Thoon KC, Chong CY, Tee NW. Early impact of pneumococcal conjugate vaccine on invasive pneumococcal disease in Singapore children, 2005 through 2010. Int J Infect Dis. 2012;16(3):e209–15. doi:10.1016/j.ijid.2011.11.014.
- Choi EH, Kim SH, Eun BW, Kim SJ, Kim NH, Lee J, Lee HJ. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis. 2008;14(2):275–81. doi:10.3201/eid1402.070807.
- Horácio AN, Silva-Costa C, Lopes JP, Ramirez M, Melo-Cristino J. Portuguese group for the study of streptococcal infections. serotype 3 remains the leading cause of invasive pneumococcal disease in adults in Portugal (2012-2014) despite continued reductions in other 13-valent conjugate vaccine serotypes. Front Microbiol. 2016;7:1616. doi:10.3389/fmicb.2016.01616.
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325(21):1453–60. doi:10.1056/NEJM199111213252101.
- Imöhl M, Reinert RR, Ocklenburg C, van der Linden M. Association of serotypes of streptococcus pneumoniae with age in invasive pneumococcal disease. J Clin Microbiol. 2010;48(4):1291–96. doi:10.1128/JCM.01937-09.
- Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–46. doi:10.1016/S1473-3099(14)70822-9.
- Galanis I, Lindstrand A, Darenberg J, Browall S, Nannapaneni P, Sjöström K, Morfeldt E, Morfeldt E, Naucler P, Blennow M, et al. Effects of PCV7 and PCV13 on invasive pneumococcal disease and carriage in Stockholm, Sweden. Eur Respir J. 2016;47:1208–18. doi:10.1183/13993003.01451-2015.
- Silva-Costa C, Brito MJ, Pinho MD, Friães A, Aguiar SI, Ramirez M, Melo-Cristino J. Pediatric complicated pneumonia caused by streptococcus pneumoniae serotype 3 in 13-valent pneumococcal conjugate vaccinees, Portugal, 2010–2015. Emerg Infect Dis. 2018;24(7):1307–14. doi:10.3201/eid2407.180029.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, Zansky SM, Rosen JB, Reingold A, Scherzinger K, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: A matched case-control study. Lancet Respir Med. 2016;4(5):399–406. doi:10.1016/S2213-2600(16)00052-7.
- Van der Linden M, Falkenhorst G, Perniciaro S, Fitzner C, Imöhl M. Effectiveness of pneumococcal conjugate vaccines (PCV7 and PCV13) against invasive pneumococcal disease among children under two years of age in Germany. PLoS One. 2016;11(8):e0161257. doi:10.1371/journal.pone.0161257.
- Ho PL, Law PY, Chiu SS. Increase in incidence of invasive pneumococcal disease caused by serotype 3 in children eight years after the introduction of the pneumococcal conjugate vaccine in Hong Kong. Hum Vaccin Immunother. 2019;15(2):455–58. doi:10.1080/21645515.2018.1526555.
- Jansen AG, Rodenburg GD, de Greeff SC, Hak E, Veenhoven RH, Spanjaard L, Schouls LM, Sanders EA, van der Ende A. Invasive pneumococcal disease in the Netherlands: syndromes, outcome and potential vaccine benefits. Vaccine. 2009;27(17):2394–401. doi:10.1016/j.vaccine.2009.01.127.
- Jansen AG, Rodenburg GD, van der Ende A, van Alphen L, Veenhoven RH, Spanjaard L, Sanders EA, Hak E. Invasive pneumococcal disease among adults: associations among serotypes, disease characteristics, and outcome. Clin Infect Dis. 2009;49(2):e23–e29. doi:10.1086/600045.
- Berg S, Trollfors B, Persson E, Backhaus E, Larsson P, Ek E, Claesson BE, Jonsson L, Radberg G, Johansson S, et al. Serotypes of Streptococcus pneumoniae isolated from blood and cerebrospinal fluid related to vaccine serotypes and to clinical characteristics. Scand J Infect Dis. 2006;38(6–7):427–3. doi:10.1080/00365540500532852.