ABSTRACT
Annual influenza vaccination is recommended as a preventive measure for all patients with asthma since asthma exacerbations in children and adults are associated to viral infections including influenza. There is concern about the adequate immune response in asthmatics with ICS treatment. The production of antibodies to influenza in asthmatics has been demonstrated. However, cellular immunity is poorly understood. The aim of the study was to compare the humoral and cellular immune responses to influenza vaccine in asthmatic and healthy subjects.
Twenty-five asthmatic patients attending the Allergy and Clinical Immunology Service at the Hospital General de Mexico and 25 healthy adults were included. Blood samples were obtained before, 4 and 12 months after immunization with influenza vaccine, influenza-specific antibodies were determined by the hemagglutination inhibition test and influenza-specific memory B, TCD4+, and TCD8 + lymphocytes were determined by flow cytometry.
All the asthmatic patients received ICS treatment. The Geometric Mean titers for all influenza serotypes were similar in both groups; seropositivity and the cellular immune response increased in both groups over time and was comparable.
Influenza vaccination in asthmatic patients with immunotherapy and ICS achieved protective antibody levels and cellular immunity over time comparable to healthy subjects.
Introduction
The influenza virus causes respiratory disease in humans affecting the upper and lower respiratory airways, and has the ability to cause epidemics and pandemics.Citation1 Influenza in asthmatic patients is associated with an increase in health care services (hospital admissions, clinic visits, ancillary services, and emergency department visits).Citation2 Patients with asthma may have exacerbations, worst symptoms, risk of pneumonia, viral lower respiratory tract infection, bronchiolitis, secondary bacterial infection, sepsis and a prolonged decline in lung function after an influenza episode.Citation3 In addition, influenza remains one of the most common causes of hospitalization for asthma exacerbation in children.Citation4
Influenza vaccination is recommended by the World Health Organization (WHO) for all people older than 6 months who do not have contraindications and especially high-risk priority groups, including people with chronic illnesses such as asthma, and health care workers.Citation5,Citation6 However, the effectiveness of influenza vaccine depends primarily on the age and immunocompetence of the subjects, and the degree of similarity between the viruses in the vaccine and those in circulation.Citation7 The influenza vaccination is safe in the asthmatic population as has been demonstrated in multiple studies in children and adults, without worsening asthma symptoms, exacerbations or decrease in peak expiratory flow rates.Citation8,Citation9 Asthmatic patients should be vaccinated annually according to GINA recommendation since it prevents exacerbations, although there is concern about the immune response, especially in those receiving a high dose of inhaled steroids.Citation10 However, previous studies in patients with moderate to severe asthma showed that oral prednisone exposures did not decrease the immune response to influenza vaccination in children.Citation11 Although, this study only measured influenza-specific antibodies.
The immune response generated by pathogens including vaccines includes innate and adaptive immunity with a humoral and a cellular component. The humoral immunity is composed mainly of B cells that produce high-affinity and specific antibodies and the cellular immunity is divided into antigen-specific CD4 T-cells, who produce several cytokines and other stimulatory factors and the CD8T-cells with the ability to kill infected cells. Immune memory is generated within the adaptive immune response with development of antigen-high specificity and the capacity to rapidly generate large numbers of antigen-specific CD4, CD8 T cells and antibodies. The goal of a vaccine is to induce long-term immunological memory. However, many of the vaccines currently in use were developed with a little understanding of the cellular immune response.Citation12
The memory T cells differ from naïve and effector T cells in several ways.Citation13,Citation14 At least two types of memory T cells have been described within the CD4 and CD8 T cell populations based on their homing characteristics and their effector functions. In humans, T effector-memory (TEM) and T central-memory (TCM) cells differ both functionally and in their migratory properties and can be distinguished based on their CD62L and CCR7 surface expression. TCM cells express CCR7 and CD62L, whereas TEM cells do not express CCR7 or CD62L.Citation15
Asthmatic patients have been described with a Th2 and/or Th17 pattern that could predispose to a different immune response to vaccines.
The aim of this study was to characterize the cellular and humoral immune responses to influenza vaccination in asthmatic and healthy subjects.
Materials and methods
Study population and study procedures
A prospective, longitudinal, comparative, clinical trial was performed. Asthmatic patients who attend the Allergy and Clinical Immunology Service at the Hospital General de México, “Dr. Eduardo Liceaga” and healthy subjects were invited to participate in the study and signed a written informed consent. Inclusion criteria were mild to moderate asthma according to GINA (Global Initiative for Asthma) guidelines and age-matched healthy subjects. Exclusion criteria were egg allergy, previous allergic reaction to influenza vaccine, immunodeficiencies, and acute respiratory infection at the initial evaluation. Pregnancy and lack of a follow-up blood collection were elimination criteria. The subjects received the inactivated trivalent influenza vaccine. Blood samples were collected prior to vaccination and 4 and 12 months after vaccination for humoral and cellular assays. The immune response to influenza was measured 4 and 12 months postvaccination since we wanted to compare the long term or memory response to this vaccine in asthmatics and healthy subjects. It has been reported that antibody titers after vaccination present a peak at 1 month postvaccination and then decline slowly at 4 months and persists up to 12 months.Citation16 Clinical evaluation was made 7 days after vaccine administration, with a special interest in adverse events and asthma exacerbation.
Ethics
The study was approved by the Institutional Review Board of the Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (DI/12/309/04/044) and the Faculty of Medicine, Universidad Nacional Autonoma de Mexico, Mexico City, Mexico (055/2012). Written informed consent was obtained from all participants. The study was conducted following the Good Clinical Practice and the International Conference of Harmonization standards.
Influenza vaccine and vaccine administration
Inactivated trivalent vaccine (Vaxigrip-Sanofi Pasteur, Paris, France), containing 15 μg of hemagglutinin protein for each virus A/California/7/2009 H1N1pdm09-like virus, A/Victoria/361/2011 H3N2-like virus, B/Wisconsin/1/2010-like virus was used for the winter season of 2012–2013. The vaccine was administered intramuscularly over the deltoid area of the left arm.
Influenza antibody assay
IgG anti-influenza antibodies were detected by the hemagglutination inhibition (HI) technique. Briefly, the serum was separated by centrifugation and frozen at −70°C until tested in parallel. Sera treated with receptor-destroying enzyme overnight at several dilutions (1:10 to 1:10,240) were placed by duplicate in 96-well plates. A concentration of 8 hemagglutination units (HU) for each virus (A H1N1, A H3N2 and B) was added for 30 min. A 0.85% suspension of turkey erythrocytes was added for 20 min until HI was observed. A titer of ≥1:40 was considered positive and a four-fold rise in HI above levels before vaccination or seronegative to seropositive was considered as seroconversion.
Influenza specific cell proliferation
Fresh peripheral blood mononuclear cells (PBMCs) were separated from whole blood by Ficoll-Hypaque (GE Healthcare Life Sciences) gradient and stimulated with influenza A H1N1, A H3N2 and B antigen prepared from infected Madin-Darby Canine Kidney (MDCK) cell lysates in triplicate wells, the medium was used as negative control. Briefly, PBMCs were added to 96-well microtiter plates at a concentration of 3 × 105 cells/well in RPMI-1640 (Gibco) with 10% normal human serum (Sigma-Aldrich). Cell viabitily was measured with trypan blue and flow cytometry, the cells were processed the same day of the blood draw. The cells were stimulated with 1:32 inactivated influenza A H1N1, A H3N2, and B antigen, Concanavalin A (Sigma-Aldrich) was used as a mitogen control. After 5 days of incubation, the cells were pulse-labeled with EdU for 18 hrs, washed in 1% PBS/BSA, centrifuged, fixed in Click-iT® fixative and washed twice with saponin-based permeabilization-wash reagent. Detection of EdU incorporation into the DNA of proliferative lymphocytes was performed with the Click-iT® EdU reaction cocktail using a DNA staining solution with saponin-based permeabilization-wash buffer, RNase, Click-iT® EdU Cell Cycle Alexa Fluor 488-green (Invitrogen) stain and 1%PBS bovine serum albumin (BSA, Sigma-Aldrich). The cells were acquired with a BD FACSCanto II™ flow cytometer and analyzed with the FacsDiva software (Becton Dickinson-Biosciences).
Influenza specific T and B-cell memory populations
PBMCs were added to 96-well microtiter plates at a concentration of 3 × 105 cells/well in RPMI-1640 (Gibco) with 10% normal human serum (Sigma-Aldrich). The cells were stimulated with the influenza A H1N1, A H3N2 and B antigen at a 1:32 dilution or an uninfected cell control; Concanavalin A was used as a mitogen control. After 5 days, the cells were stained with monoclonal antibodies conjugated to CD4-PeCy5, CD8-PeCy7 and CD19-PE cells and with markers related to memory CCR7-APCy7, CD62L-APC and CD27-FITC cells (BD Pharmigen, La Jolla, CA, USA). Six colors per well were measured and compensation was performed with non-stimulated cells with simple staining for each color; 10,000 cells per patient were acquired with the FacsCanto II cytometer and analyzed with the FacsDiva software (Becton Dickinson-Biosciences). Cell viability was detected with trypane blue and with the FSC and SSC area of the cytometer with viable cells. All assays were performed side-by-side (asthmatics and controls) the same day of the blood draw.
Detection of Th1, Th2, and Th17 cytokines
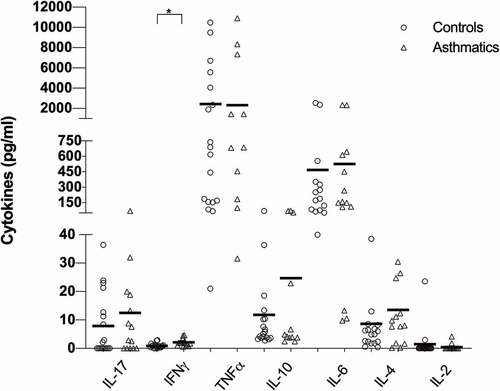
PBMCs were added to 96-well microtiter plates at a concentration of 3 × 105 cells/well in RPMI-1640 (Gibco). The PBMCs were stimulated with 3 μl of Cytostim (Miltenyi Biotec) or medium as a negative control and were incubated at 37°C with 5% CO2 for 4 hours. The supernatants were obtained, IL-17A, IFN-γ, IL-2, TNFα, IL-4, IL-6, and IL-10 cytokines were measured by flow cytometry, using a BD Cytometric Bead Array HumanTh1/Th2/Th17 kit (Becton Dickinson). Briefly, the supernatants were mixed with six bead populations with distinct fluorescence intensities coated with capture antibodies specific for each molecule, mixed with the PE-conjugated detection antibodies, incubated, acquired with a BD FacsCanto II flow cytometer and analyzed with the FCAP Array v3.0 software (BD Biosciences).
Statistical analysis
Univariate and bivariate analysis was used. Geometric mean titers (GMT), mean cpm values, the mean cell frequencies prior, 4 months and 12 months after vaccination and baseline cytokines were compared using the non-parametric Mann-Whitney U test. The percentages of seroconversion after vaccination were compared using Chi-square tests. Friedman test was used to determine differences in antibody means and compare memory T cells and cell proliferation at the beginning and at 4 and 12 months between the asthmatics and healthy subjects. A p-value less than 0.05 was considered significant.
Results
Study population, demographic and clinical characteristics
Twenty-nine asthmatics and 29 healthy subjects were included from October 2012 to March 2014, four asthmatic patients and four controls were lost to follow-up. There were no statistically significant differences between groups at baseline regarding gender, age and body mass index (BMI), except for a history of influenza vaccination where 68% of controls referred previous influenza vaccination compared to 36% of asthmatics. Twenty-four percent was receiving low dose intranasal corticosteroid (ICS) treatment and 76% a medium dose ().
Table 1. Demographic and clinical characteristics
Humoral immune response to influenza vaccine
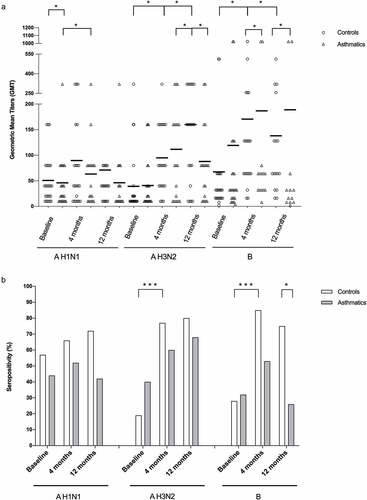
There was no difference in the Geometric Mean Titers (GMTs) between controls and asthmatics for Influenza A H1N1, A H3N2, and B at baseline. GMTs for influenza B were higher in asthmatics compared to controls at 12 months postimmunization (p = .028). (). There was no statistical difference between seroprotective titers between groups after vaccination, except for Influenza B at 12 months. Within groups, there was an increase in seropositivity in the control group compared to baseline for H1N1 and B at 4 months, which was not observed in the asthmatic group. The GMT increased through time in both groups, yet statistical significance was only achieved for Influenza H3N2 in controls (p < .0001) and asthmatics (p = .045) and Influenza B for the control group (p = .003). Baseline seropositivity was high in the control group for Influenza A H1N1 (57%), A H3N2 (19%) and B (28%) compared to asthmatics: A H1N1 (44%), A H3N2 (40%) and B (32%), without statistical significance. An increase in seropositivity through time for both groups for the three serotypes (A H1N1, A H3N2 and B) was observed, yet statistical significance was only achieved for Influenza A H3N2 in the control group ().
Figure 1. Humoral immune response to influenza vaccination. (A) Geometric mean titers (GMT) of hemagglutination-inhibition (HAI) antibodies and (B) percentage of seropositivity after vaccination with influenza A H1N1, A H3N2 and B at baseline, 4 and 12 months, in healthy controls and asthmatics. (HAI titers are expressed in geometric means, error bars represent the standard error of the mean)
Cell-mediated immune response to influenza vaccine
Influenza specific cell proliferation
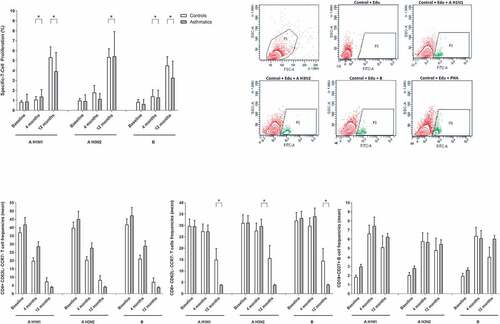
Prior to vaccination, proliferative responses for the three influenza serotypes were observed, which increased over time after immunization and was higher after 12 months for asthmatics and controls, with statistical significance for both groups (p < .0001). The cell proliferation between groups was comparable, except at 4 months for A H3N2 and 4 and 12 months for Influenza A H1N1 and B (p < .05) ().
Figure 2. Cellular immune response to influenza vaccination. (A) Influenza A H1N1, AH3N2 and B specific lymphoproliferation before, 4 and 12 months after vaccination in asthmatic and healthy individuals, representative image of cell proliferation with EdU incorporation. (B) Specific influenza AH1N1, AH3N2 and B memory TCD4+, TCD8+ and B lymphocytes in asthmatic and healthy individuals before, 4 and 12 months after vaccination
Influenza specific memory T and B cells
The frequency of specific influenza memory T CD4+ lymphocytes increased in the asthmatic group at 4 months postvaccination for the three serotypes (p < .05) but decreased at 12 months (p < .001). The control group had similar responses regarding CD4+ lymphocytes (p < .001). When we compared the CD4+ lymphocytes between groups, there was no statistical significance between asthmatics and controls. The CD8 + T cell frequencies was similar in both groups before and 4 months after vaccination, but at 12 months a decrease was observed for the 3 viruses in asthmatics (A H1N1 p < .002, A H3N2 p < .002 and B p < .0001). Specific influenza A H1N1, A H3N2 and Influenza B memory lymphocytes increased in asthmatics and controls at 4 months (A H1N1 p < .001, A H3N2 p < .001 and B p < .001) and persisted high at 12 months in controls (A H1N1 p < .0001, A H3N2 p < .083 and B p < .114) and asthmatics (A H1N1 p < .001, A H3N2 p < .001 and B p < .001). ().
Baseline cytokine patterns
The baseline cytokine patterns did not show differences among groups, only for IFN-γ that was higher in asthmatic patients (p < .001). Although not statistically significant, IL-10 had a tendency to be higher in asthmatic patients. No specific Th1, Th2 or Th17 pattern was observed in any group ().
Adverse effects
The vaccine was well tolerated; no serious adverse effects were reported and no hospitalizations due to influenza were identified. Most individuals presented local symptoms, including local pain (44% controls vs 36% asthmatics), erythema (32% controls vs 16% asthmatics) and fever (4% control vs 0% asthmatics). Local symptoms resolved within a few days following the administration of symptomatic treatment. Only one control subject had fever up to 39ºC and local reaction that lasted one week and was treated with analgesic and antipyretic.
None presented severe adverse reactions such as anaphylaxis or Guillain-Barré syndrome.
Discussion
Asthma is a chronic lung disease characterized by inflammation, with multiple endotypes, one of them Th2 and varying phenotypes, but all of them have in common a predisposition for exacerbations.Citation17 These exacerbations have been strongly related to viral infections such as influenza. There is a clear recommendation for influenza vaccination in at-risk populations, one of them being asthmatic patients.Citation18 There are a great number of studies related to influenza vaccine´s safety in these populations and it has been demonstrated its benefits with a reduced number of exacerbations, fewer hospitalizations, and asthma medication use.Citation19,Citation20 Even though, there is uncertainty regarding the level of protection that this vaccine provides to asthmatic patients,Citation21 especially if they receive steroids. The humoral immune response has been used as the main measure of vaccine immunogenicity but there are other immunologic parameters that can be measured to assess the immune response. The cellular immune response toward influenza vaccine is important since it has been recognized as an important mechanism of protection, especially in some populations such as older adults.Citation22
In this study, we found that despite all the asthmatic patients were using ICS, the humoral immune response to the influenza vaccine was effective for the 3 influenza serotypes increasing the GMTs and the percentage of seropositivity at 4 and 12 months after influenza immunization and was comparable to healthy subjects as has been reported by other authors where asthmatic patients with or without ICS treatment show an increase in HAI titers for the 3 viruses, although for high dose ICS treatment the B influenza virus response was lower.Citation10 The asthmatic patients achieved good levels of seroprotection that were similar to the healthy subjects.
All the asthmatic subjects were receiving immunotherapy, which could explain the lack of a Th2 or Th17 baseline pattern and a similar immune response to the influenza vaccination to healthy subjects. Also the IL-10, although not significant, showed a tendency to be higher in asthmatic patients, where allergen-specific immunotherapy produces an increase of IL10 an anti-inflammatory cytokine.Citation23 It is noteworthy that baseline seropositivity differs among groups; Influenza A H3N2 was lower in the healthy subjects and influenza B was lower in the controls, and comparable for influenza A H1N1. Although baseline GMTs were comparable for the 3 viruses among groups. These differences in the baseline specific humoral immune memory may be due to previous vaccination and previous influenza infection in both groups and depending on the previous infections the memory to each virus. It is also noteworthy that a higher percentage of healthy subjects had history of influenza vaccination compared to asthmatics even though the latter have an increased risk of complications related to influenza infection.Citation24 Regarding the assessment of the cellular immune response, memory T and B cells were present at baseline and increased over time in both populations, this also supports the fact that asthmatic and healthy subjects have been in contact to the virus previously either by vaccination or infection. And these specific memory cells decreased a year after vaccination, which is expected since the memory T and B cells have a rapid expansion when they become in contact with the antigen and produce not only memory but effector cells and then there is a contraction. The memory CD8 + T cells decrease over time in the asthmatic population for the 3 viruses, with significant differences with controls at 12 months, although lymphoproliferative responses are comparable to healthy subjects. CD8+T cells have been less studied in asthma in contrast to CD4+T cells which have been implicated primarily in its pathogenesis. Yet, both cellular groups are important as a measure of vaccine immunogenicity since CD4+T cells have been correlated with the antibody response to vaccination but decline 3–4 years after.Citation25 CD8+T cells have been found up to 10 years after infection,Citation26 but they may have a different pattern after vaccination since most influenza vaccines provide a weak stimulus for CD8+T cells.Citation22 These cells are critical against influenza infection in the virus clearance.Citation27 Another evaluation of the cellular immune response was the specific lymphoproliferation after stimulation with the 3 influenza antigens. This response was also similar between both groups and showed an increase at 4 months, but a dramatic increase at 12 months for the 3 influenza serotypes; this could suggest that the higher the exposure to the antigen, in this case with influenza vaccination, the memory cells might increase, and 12 months after another stimulus the proliferative response is even higher. The sample taken at 12 months after vaccination was drawn during the influenza season, it is possible that the priming with the previous vaccine and a mild or asypmptomatic influenza infection could have boosted the cellular response, which can explain the peak observed at that period of time.
There were no statistically significant differences in the cytokine levels between both groups despite what has been described extensively in the literature about a Th2 and/or Th17 cytokine pattern in asthmatics. Instead, we found increased IFN-γ levels in asthmatics compared to controls. This lack of difference in the cellular pattern could be a result of the immunotherapy, where the goal is to diminish the Th2 and Th17 response.Citation23 A limitation of the study is that we only measured cytokines before and not after immunization; it would be an interesting way to study protection against influenza since the IFN-γ: IL-10 ratio has been used as a predictor of such status.Citation28 Other studies have also found similar baseline serum cytokine patterns between asthmatics and healthy subjects, but found differences in chemokines between groups during a respiratory infection.Citation29 We could not find this difference since we only measure a baseline cytokine pattern to characterize the cellular response, and not a second measure after vaccination.
As has been extensively demonstrated, the influenza vaccine is safe in this population and only minor adverse reactions were observed without differences in both populations. No asthmatic patients presented exacerbations after vaccine administration, none had a diagnosis of influenza infection or were hospitalized for respiratory complications.
One limitation of the study is that the population included had a mean age of 30 years with SD of 7, we didn´t include asthmatics older than 60 years old because of the immunosenecense which can be a confounding variable when measuring the immune response, so it might not represent asmathics at younger or older ages.
This study provides evidence of the humoral immune response and a very strong cellular immune response especially at 12 months after influenza vaccination in asthmatic patients that is comparable to healthy subjects. It provides evidence that with immunotherapy in asthmatic patients a similar cytokine pattern to healthy subjects is observed which results in a comparable immune response to influenza vaccination. Some of the limitations include a small sample size but other translational studies where immune responses are measured are done with even fewer patients. Another limitation is that the cytokines were measured at baseline, they could develop a different pattern at 4 and 12 months after vaccination.
Conclusions
In conclusion, influenza vaccination in asthmatic patients with immunotherapy and ICS achieve protective antibody levels and cellular immunity over time comparable to healthy subjects with minor adverse events.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was part of the MSc dissertation submitted by Velasco-Medina AA. in partial fulfillment for the degree requirements of “Maestría en Ciencias Médicas de la Universidad Nacional Autónoma de Mexico”. We want to thank the subjects who agreed to participate in the study and finally to the Liver, Pancreas and Motility unit of the UNAM for the use of the FacsCanto II cytometer. The institutional Review Board of the School of Medicine, Universidad Nacional Autónoma de México and the Institutional Review Board of the Hospital General de México approved the study; written consent was obtained from all participants.
Additional information
Funding
References
- Wright PF, Webster R. Orthomyxoviruses. In: Virology Knipe DM, Howley PM editors. Fields. 4th. Philadelphia (PA): Lippincott Williams & Wilkins; 2001. p. 1534–79.
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2013-2014. MMWR Morb Mortal Wkly Rep 2013;62:1–43.
- Kondo S, Abe K. The effects of influenza virus infection on FEV1 in asthmatic children. The time-course study. Chest. 1991;100(5):1235–38. doi:10.1378/chest.100.5.1235.
- Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283(4):499–505. doi:10.1001/jama.283.4.499.
- Worl Health Organization. Vaccines against influenza WHO position paper-November 2012. Wkly Epidemiol Rec 2012;87::461–76.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Prac- tices (ACIP), United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61::613–18. doi:10.1016/s0002-9378(16)33297-5.
- Centers for Disease Control and Prevention. Immunization Practices Advisory Committee. Prevention and control of influenza: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep 2002;51:1–31.
- Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356(7):685–96. doi:10.1056/NEJMoa065368.
- Cates CJ, Rowe BH. Vaccines for preventing influenza in people with asthma. Cochrane Database Syst Rev. 2013;(2)CD000364. doi:10.1002/14651858.CD000364.pub4.
- Hanania NA, Sockrider M, Castro M, Holbrook JT, Tonascia J, Wise R, Atmar RL. Immune response to influenza vaccination in children and adults with asthma: effect of corticosteroid therapy. J Allergy Clin Immunol. 2004;113(4):717–24. doi:10.1016/j.jaci.2003.12.584.
- Fairchok MP, Trementozzi DP, Carter PS, Regnery HL, Carter ER. Effect of prednisone on response to influenza virus vaccine in asthmatic children. Arch Pediatr Adolesc Med. 1998;152(12):1191–95. doi:10.1001/archpedi.152.12.1191.
- Hilleman MR. Vaccines in historic evolution and perspective: a narrative of vaccine discoveries. Vaccine. 2000;18(15):1436–47. doi:10.1016/S0264-410X(99)00434-X.
- Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. doi:10.1016/s0065-2776(08)60022-x.
- Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12(3):336–41. doi:10.1016/s0952-7915(00)00096-0.
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi:10.1038/44385.
- Plotkin SA, Orenstein WA, Offit PA. Vaccines. Scotland: Elsevier; 2013.
- Kuruvilla ME, Lee FE, Lee GB, Phenotypes UA. Endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–33. doi:10.1007/s12016-018-8712-1.
- Veerapandian R, Snyder JD, Samarasinghe AE. Influenza in asthmatics: for better or for worse? Front Immunol. 2018;9:1843. doi:10.3389/fimmu.2018.01843.
- Vasileiou E, Sheikh A, Butler C, El Ferkh K, von Wissmann B, McMenamin J, Ritchie L, Schwarze J, Papadopoulos NG, Johnston SL, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis. 2017;65(8):1388–95. doi:10.1093/cid/cix524.
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2018-19 influenza season. MMWR Recommendations Rep. 2018;67(3):1–20. doi:10.15585/mmwr.rr6703a1.
- Schwarze J, Openshaw P, Jha A, Del Giacco SR, Firinu D, Tsilochristou O, Roberts G, Selby A, Akdis C, Agache I, et al. Influenza burden, prevention, and treatment in asthma-A scoping review by the EAACI Influenza in asthma task force. Allergy. 2018;73(6):1151–81. doi:10.1111/all.13333.
- Kumar A, McElhaney JE, Walrond L, Cyr TD, Merani S, Kollmann TR, Halperin SA, Scheifele DW. Cellular immune responses of older adults to four influenza vaccines: results of a randomized, controlled comparison. Hum Vaccin Immunother. 2017;13(9):2048–57. doi:10.1080/21645515.2017.1337615.
- Bohm L, Maxeiner J, Meyer-Martin H, Reuter S, Finotto S, Klein M, Schild H, Schmitt E, Bopp T, Taube C, et al. IL-10 and regulatory T cells cooperate in allergen-specific immunotherapy to ameliorate allergic asthma. J Immunol. 2015;194(3):887–97. doi:10.4049/jimmunol.1401612.
- Morbidity and Mortality Weekly Report. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices, United States. MMWR Recommendations Rep. 2019;68(3):1–21.
- Nayak JL, Fitzgerald TF, Richards KA, Yang H, Treanor JJ, Sant AJ. CD4+ T-cell expansion predicts neutralizing antibody responses to monovalent, inactivated 2009 pandemic influenza A(H1N1) virus subtype H1N1 vaccine. J Infect Dis. 2013;207(2):297–305. doi:10.1093/infdis/jis684.
- van de Sandt CE, Hillaire ML, Geelhoed-Mieras MM, Osterhaus AD, Fouchier RA, Rimmelzwaan GF. Human influenza A virus–specific CD8+ T-cell response is long-lived. J Infect Dis. 2015;212(1):81–85. doi:10.1093/infdis/jiv018.
- Grant EJ, Quinones-Parra SM, Clemens EB, Kedzierska K. Human influenza viruses and CD8(+) T cell responses. Curr Opin Virol. 2016;16:132–42. doi:10.1016/j.coviro.2016.01.016.
- McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176(10):6333–39. doi:10.4049/jimmunol.176.10.6333.
- Giuffrida MJ, Valero N, Mosquera J, Alvarez de Mon M, Chacin B, Espina LM, Gotera J, Bermudez J, Mavarez A. Increased cytokine/chemokines in serum from asthmatic and non-asthmatic patients with viral respiratory infection. Influenza Other Respir Viruses. 2014;8(1):116–22. doi:10.1111/irv.12155.