 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A new Escherichia coli-produced human papillomavirus (HPV)-16/18 vaccine has been shown to be safe and highly efficacious and was recently licensed in China. As a post hoc analysis of the phase III trial, this study aimed to assess the impact of vaccination time deviations on the specific antibody response and guide the better usage of this vaccine in the real world. A total of 3689 healthy women aged 18–45 years old were randomly assigned to receive the bivalent HPV-16/18 vaccine according to a 0-, 1- and 6-month schedule with a wide vaccination interval. The first vaccination interval between the 1st and 2nd doses (the 1st interval) was divided into three groups: 28–40 d, 41–50 d and 51–60 d. The second vaccination interval between the 2nd and 3rd doses (the 2nd interval) was divided into three groups: 103–139 d, 140–160 d and 161–198 d. The reverse cumulative curves for the IgG of the three groups with different 1st vaccination intervals or with different 2nd vaccination intervals at month 7 almost overlapped for both HPV-16 and HPV-18. Compared with the standard vaccination schedule (a 1st interval of 28–40 d and a 2nd interval of 140–160 d) subgroup, all the subgroups had GMC ratios greater than 0.83, with the lower limit of 95% CIs higher than 0.64. In conclusion, a slight deviation in the vaccination time of the 2nd and 3rd doses has only a minor, insignificant impact on the immune response induced by the Escherichia coli-produced HPV-16/18 vaccine.
Introduction
Human papillomavirus (HPV) infection is the main cause of cervical cancer, and there is also evidence linking HPV infection with cancers of the anus, vulva, vagina, penis and oropharynx.Citation1,Citation2 HPV-16 and HPV-18 contribute to approximately 70.8% of cervical cancer cases.Citation3 Three current licensed eukaryotic expressed recombinant HPV vaccines, a bivalent vaccine (Cervarix®, HPV-16/18, by recombinant baculovirus-infected insect cells), a quadrivalent vaccine (Gardasil®, HPV-6/11/16/18, by Saccharomyces cerevisiae) and a nine-valent vaccine (Gardasil® 9, HPV-6/11/16/18/31/33/45/52/58, by S.cerevisiae) which is a successor to the quadrivalent vaccine (Gardasil®), are all administered in three doses (0/1/6 or 0/2/6 months).Citation4–Citation6 They have been widely introduced into the national immunization program of 104 countries. However, most people in low-income countries, who have a higher risk of cervical cancer, have reduced access to the vaccine due to the limited production capacity and high cost of these vaccines.Citation7,Citation8 Post-marketing data have suggested that these vaccines result in an effective reduction in the prevalence of HPV-16/18 and related precancerous lesions.Citation9–Citation11 The Director-General of the World Health Organization made a global call for action toward the elimination of cervical cancer in May 2018; however, the aforementioned high cost and limited access to vaccines have considerably slowed down the pace of implementation.Citation12,Citation13
A new Escherichia coli (E. coli)-produced HPV-16/18 human papillomavirus vaccine that is well tolerated and highly efficacious against high-grade genital lesions and persistent infection with HPV-16/18Citation14-Citation16 has just been licensed in China for usage in women aged 9–45 years old, with a 2-dose schedule for girls aged 9–14 years old and a 3-dose schedule for women aged 15–45 years old. Although the standard 3-dose schedule for the new HPV vaccine is to be administered at 0, 1 and 6 months, it is difficult to strictly follow this schedule in the real world, and women may delay receiving a vaccination for various reasons. Due to the high efficacy observed in clinical trials, the minimum antibody level threshold that is correlated with protection is still unclear for HPV vaccines;Citation17 however, specific antibody levels induced by HPV vaccines are a reasonable clinical indicator for the effect of vaccination. This study aimed to analyze the difference in the specific antibody levels induced by the vaccine administered at different intervals in the phase III clinical trial to assess the impact of vaccination time deviations and guide the better usage of this vaccine in the real world.
Methods
Study design and participants
This study was a post hoc analysis of a multicenter, randomized, double-blind and placebo-controlled phase III clinical trial, which was approved by independent ethics committees. The phase III clinical trial assessed the efficacy, immunogenicity and safety of the E. coli-produced bivalent human papillomavirus vaccine (HPV-16/18). It was conducted at five sites in China: Yangcheng County in Shanxi Province, Xinmi County in Henan Province, Fengning County in Hebei Province, Funing County in Jiangsu Province and Liuzhou City in Guangxi Province. A total of 7372 women aged 18–45 years old were enrolled and randomly assigned to receive three doses of the test vaccine (HPV-16/18) or control vaccine (hepatitis E vaccine, Hecolin®). Women who were healthy and nonpregnant, had an intact cervix, did not have acute cervicitis or acute lower genital tract infection, and had not been vaccinated with an HPV vaccine were eligible. Women who had a history of sexually transmitted diseases, condyloma and abnormal cervical cancer screening results, had previously suffered from cervical intraepithelial neoplasia (CIN); or had more than 4 sexual partners were excluded. More details about the inclusion and exclusion criteria have been described previously.Citation14
Vaccine and vaccination schedules
The HPV-16/18 bivalent vaccine, with the brand name Cecolin®, is a mixture of two aluminum hydroxide adjuvant-absorbed recombinant L1 virus-like particles (VLPs) of HPV-16 and HPV-18 expressed in E. coli. It contains 40 μg of HPV-16 L1 VLPs, 20 μg of HPV-18 L1 VLPs, and 208 μg of aluminum adjuvant, which are suspended in 0.5 mL of phosphate-buffered saline (PBS).Citation18 The vaccine was administered at day 0, month 1 (vaccination window of 28 to 60 d after the 1st dose) and month 6 (vaccination window of 150–240 d after the 1st dose).
Serum collection and antibody measurement
Serum samples were collected on day 0 before vaccination and month 7 (one month after the 3rd dose). According to the protocol, the visit window for month 7 was 21 to 89 d after the 3rd dose. Serum samples from day 0 and month 7 were tested by the National Institute for the Control of Pharmaceutical and Biological Products using the E. coli-expressed VLP-based enzyme-linked immunosorbent assay (ELISA) with the same assay kit that was used in previous papers for the test vaccine.Citation14,Citation15 The levels of IgG against HPV-16 and HPV-18 were measured with a cutoff value of 3.0 IU/mL for HPV-16 and 2.1 IU/mL for HPV-18,Citation14,Citation19 which were traceable to the World Health Organization international standards for antibodies against HPV-16 (NIBSC code 05/134) or HPV-18 (NIBSC code 10/140). Each well of the microtiter plate was coated with HPV-16 and HPV-18 VLPs, which were suspended in phosphate buffer at 4°C overnight. After blocking, diluted serum samples, diluted reference serum samples and controls were added to the plates separately and incubated for 45 minutes at 37°C. Then, after the plates were washed, horseradish peroxidase-conjugated goat anti-human IgG was added and incubated under the same conditions. After the second washing, tetramethylbenzidine was added, and the samples were incubated for 15 minutes at 37°C. Finally, 0.36 mol/L H2SO4 was added to stop the reaction, and the optical density (OD) was read at 450/620 nm. The antibody titers against HPV-16 and HPV-18 were calculated using the diluted sample with an OD that fell within the working range of the standard curve. The titers for negative serum samples were artificially set to half of the cutoff value.Citation19
Statistical analysis
The immunogenicity of HPV-16 and HPV-18 was analyzed in the per-protocol set (PPS) for immunogenicity (PPS-I 16 and PPS-I 18, respectively). PPS-I 16 or PPS-I 18 included participants who followed the immunization schedules to receive three doses of the HPV vaccine according to the protocol; were negative for HPV-16 IgG or HPV-18 IgG at day 0; were negative for the HPV-16 or HPV-18 type of HPV DNA at day 0 and month 7; had serum samples collected at day 0 and month 7; and had serum samples for month 7 collected between 21 and 40 d after the third dose. Modified intention- to -treat (mITT) for immunogenicity 16 and 18 (mITT-I 16 and mITT-I 18) included participants who received three doses of the vaccine and had available serum samples for day 0 and month 7.
Considering the reasonable sample size in each group, we divided the first vaccination interval between the 1st and 2nd doses (the 1st interval) into the following three groups: 28–40 d, 41–50 d and 51–60 d. In addition, the second vaccination interval between the 2nd and 3rd doses (the 2nd interval) was divided into the following three groups: 103–139 d, 140–160 d and 161–198 d. The standard vaccination schedule (a 1st interval of 28–40 d and a 2nd interval of 140–160 d) was set as the reference. The geometric mean concentrations (GMCs) of HPV-16- and HPV-18-specific IgG induced by the deviated schedules were compared to those of the reference group. The 95% confidence intervals (CIs) of the GMC ratios of IgG at month 7 were calculated by analysis of covariance (ANCOVA). As age had an important impact on the antibody response, it was analyzed as a covariate.Citation20 All analyses were conducted with SAS software (version 9.4).
Results
Baseline characteristics of the participants
A total of 3689 women aged 18–45 years old were randomly assigned to receive the bivalent HPV-16/18 vaccine, and among them, 2026 and 2444 women were included in PPS-I 16 and PPS-I 18, respectively. The seroconversion rates for anti-HPV-16 and anti-HPV-18 at month 7 were 100% (2026/2026) and 99.9% (2442/2444), respectively. The two women who did not seroconvert were 21 and 41 years old. The GMC of the HPV-16 antibody was 798.0 IU/mL (95% CI 770.9 to 882.4 IU/mL), and the GMC of HPV-18 was 271.0 IU/mL (95% CI 263.0 to 281.8 IU/mL). Among the subgroups with different vaccination intervals for both HPV-16 and HPV-18, the average ages were similar; however, younger women were prone to delaying the second and third doses ().
Table 1. Baseline characteristics of the immunogenicity subgroups (per-protocol set).
Antibody levels and GMC ratios compared with the standard vaccination schedule
Reverse cumulative curves for IgG in the three groups with different 1st dose vaccination intervals or the three groups with different 2nd dose vaccination intervals at month 7 almost overlapped, regardless of whether they were for HPV-16 or HPV-18 (), indicating that deviations from the standard interval only had a minor, insignificant impact on the immune response.
Figure 1. Reverse cumulative curves for IgG antibody levels of the different vaccination interval groups (per-protocol set) at month 7. Panel 1A: HPV-16 IgG antibody levels of the three groups with different 1st intervals; Panel 1B: HPV-16 IgG antibody levels of the three groups with different 2nd intervals; Panel 1 C: HPV-18 IgG antibody levels of the three groups with different 1st intervals; Panel 1D: HPV-18 IgG antibody levels of the three groups with different 2nd intervals.
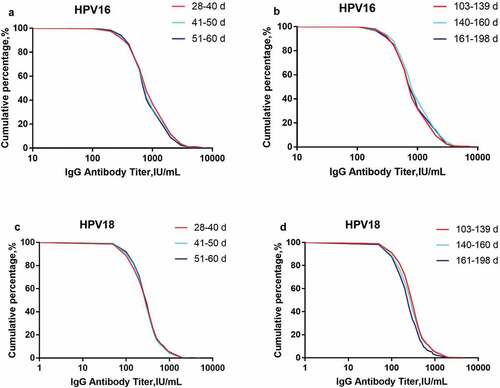
The GMCs from the different subgroups and their GMC ratios compared with the standard vaccination schedule (a 1st interval of 28–40 d and a 2nd interval of 140–160 d) are presented in . The data showed that compared with the values of the reference subgroup, the lowest GMC ratios of the other groups were 0.86 and 0.83 for HPV-16 and HPV-18, respectively. All the lower limits of the 95% CIs for the GMC ratios were greater than 0.64, indicating that receiving the second dose at 28 to 60 d after the first dose and receiving the third dose at 103 to 198 d after the second dose had minor, insignificant impacts on the immunogenicity of the vaccine for both HPV-16 and HPV-18. The subgroup with a 1st interval of 51–60 d and a 2nd interval of 161–198 d was not analyzed as it only included 2 people. Analyses of the mITT cohorts showed similar results ().
Table 2. Analysis of antibody levels and GMC ratios in the subgroups (per-protocol set).
Discussion
This study explored the immunogenicity of the new E. coli-produced bivalent human papillomavirus vaccine (Cecolin®) under different vaccination intervals. The data showed that for both anti-HPV-16 and anti-HPV-18, the GMC ratios for the subgroups with different vaccination intervals compared with the standard vaccination interval (a 1st interval of 28–40 d and a 2nd interval of 140–160 d) were higher than 0.83, and the lower limits of the 95% CIs were higher than 0.64, indicating that postponing the second dose to 60 d (2 months) after the first dose and receiving the third dose at 103 to 198 d after the second dose had little impact on the induced antibody levels. Although the protective antibody levels of HPV vaccines have not been determined, humoral immune response levels were used to extrapolate the protective effect of HPV vaccines when used in adolescents who cannot be enrolled in an efficacy clinical trial due to ethical constraints on gynecological examinations.Citation21,Citation22 The noninferiority criterion, such as a lower 95% confidence bound for the GMC ratio greater than 0.5, is accepted by regulatory authorities worldwide for HPV vaccines, and this criterion was also used for the registration of this E. coli-produced HPV vaccine in China.Citation16 Hence, the small differences in GMCs in this trial are considered to have no clinical significance, and flexible schedules for the second and third doses could maximize vaccination rates with little effect on the clinical performance of the vaccine.
There are three main assays widely used to measure HPV antibody levels: a pseudovirion-based neutralization assay (PBNA), a competitive Luminex immunoassay (cLIA) and an ELISA. Among them, the cLIA measures neutralizing antibodies that compete with one dominant neutralizing monoclonal antibody.Citation23 The PBNA measures the total functional neutralizing antibodies, which can block the entry of pseudovirions into cultured cells; therefore, the PBNA is regarded as the ‘gold standard’ for testing for the anti-HPV antibody.Citation24 However, it is difficult to use in large-scale studies because of the complicated, labor-intensive process involved. The ELISA detects IgG antibodies, including neutralizing and non-neutralizing antibodies, that bind to the HPV L1 VLP coating. Although the consistency of these two assays is poor when assessing natural infection-induced HPV antibodies due to nonspecific binding in the ELISA,Citation19 they are highly correlated when measuring HPV vaccine-induced antibodies.Citation19,Citation25–Citation27 Therefore, IgG antibodies were used instead to assess the immunogenicity of the test vaccine in this study.
There are some factors that impact the immune response: the nature of the vaccine, the immunization schedule, the use of adjuvants and immune memory, all of which can be influenced by the dose, intervals between doses and route of immunization.Citation28,Citation29 The antibody concentration and the number of memory B cells peaked one month after the first dose, and the secondary immune response was evoked when the memory B cells were reactivated after a second exposure to the same antigen. In the secondary response, the IgG class of antibodies appeared more quickly, and the concentrations were orders of magnitude higher than before. In addition, the binding affinity of the antibody for the antigen was significantly increased, and this result emerged when sufficient time had elapsed after the first immune response. Therefore, for most protein vaccines, the immunization schedules are 0, 1 or 2 and 6 months, which allow at least 4–6 months between the priming and the booster, with longer intervals generally associated with a greater response.Citation29,Citation30 There are some studies exploring the immunogenicity of the bivalent HPV vaccine or the quadrivalent HPV vaccine administered in three doses with longer time intervals.Citation31–Citation34 One of the studies showed that the antibody response was superior at one month after the third dose in the group with dose 2 taken on time and dose 3 delayed compared to the group with doses 2 and 3 both taken on time. Another study compared the immunogenicity of the HPV-16/18 vaccine when people were administered an alternative schedule (0, 1, and 12 months) or a standard schedule (0, 1, and 6 months), in which the seroconversion rates at one month after the third dose were 100% for HPV-16 in both groups and 99.7% (alternative schedule group) and 100% (standard schedule group) for HPV-18. The GMCs for HPV-16 and HPV-18 were comparable at one month after the third dose in the alternative and standard schedule groups (11884.7 EU/mL vs 10311.9 EU/mL for HPV-16; 4501.3 EU/mL vs 3963.6 EU/mL for HPV-18). The results of this study and the effects of longer vaccination intervals between the 3 doses, with longer than 60 d between the first 2 doses or 198 d between the last two doses in the vaccination schedule, deserve to be further studied in phase 4 large-sample and randomized clinical trials.
The major limitation of our study is that it is a post hoc analysis, and the participants in each subgroup were not well balanced. One subgroup only included 2 people which was insufficient for analysis. In addition, we lacked long-term data on the antibody level; therefore, whether the dose intervals influence the durability of antibody levels still needs further research.
In conclusion, slight deviations in the vaccination times of the 2nd and 3rd doses are acceptable, as such deviations has only a minor, insignificant impact on the immune response induced by the E. coli-produced HPV-16/18 vaccine.
Declaration of conflicts of interest
Z.-J.L., B.-Z.L., W.-D.H., H.-R.P. report being current employees of Xiamen Innovax. No other potential conflicts of interest relevant to this article were reported.
Supplemental Material
Download PDF (96.6 KB)Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Additional information
Funding
References
- Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S Human Papillomavirus and Related Diseases in the World. Summary Report 17 June 2019: ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). 2019 Jun 17 [accessed 2019 Aug 21].
- Word Health Organization, Human papillomavirus (HPV) and cervical cancer. Geneva: 2019. Jan 24. [accessed: 2019 Aug 21. https://www.who.int/en/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70. doi:10.1002/ijc.30716. PMID: 28369882
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, Chow S-N, Apter DL, Kitchener HC, Castellsague X, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled tria. Lancet. 2007;369(9580):2161–70. PMID: 17602732. doi:10.1016/s0140-6736(07)60946-5.
- The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27. PMID: 17494925. doi:10.1056/NEJMoa061741.
- Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, Moreira ED, Jr, Ngan Y, Petersen LK, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. Engl N, Med J. 2015; 3728: 711–23. 10.1056/NEJMoa1405044. PMID: 25693011.
- Word Health Organization. Vaccines in National Immunization Programme, Slides on introduction status of selected vaccines. Geneva: 2020. Jan 30 [accessed: 2020 Feb 15. https://www.who.int/immunization/monitoring_surveillance/data/en/
- The Global Alliance for Vaccines and Immunisation. Hpv Supply and Procurement Roadmap. Geneva: 2017. Dec 20. [accessed: 2019 Aug 21. https://www.gavi.org/sites/default/files/document/hpvsupplyandrocurementroadmappdf.pdf
- Garland SM, Kjaer SK, Munoz N, Block SL, Brown DR, DiNubile MJ, Lindsay BR, Kuter BJ, Perez G, Dominiak-Felden G, et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Clin Infect Dis. 2016;63(4):519–27. PMID: 27230391. doi:10.1093/cid/ciw354.
- Benard VB, Castle PE, Jenison SA, Hunt WC, Kim JJ, Cuzick J, Lee JH, Du R, Robertson M, Norville S, et al. Population-Based Incidence Rates of Cervical Intraepithelial Neoplasia in the Human Papillomavirus Vaccine Era. JAMA Oncol. 2017;3(6):833–37. PMID: 27685805. doi:10.1001/jamaoncol.2016.3609.
- Drolet M, Bénard É, Pérez N, Brisson M, Ali H, Boily M-C, Baldo V, Brassard P, Brotherton JML, Callander D, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497–509. PMID: 31255301. doi:10.1016/s0140-6736(19)30298-3.
- Word Health Organization. WHO Director-General calls for all countries to take action to help end the suffering caused by cervical cancer. Geneva: 2018. May 19 [accessed: 2019 Dec 19]. https://www.who.int/reproductivehealth/call-to-action-elimination-cervical-cancer/en/
- Word Health Organization. Immunisation Agenda 2030 A Global Strategy To Leave No One Behind. Geneva: [accessed: 2019 Dec 19]. https://www.who.int/immunization/immunization_agenda_2030/en/
- Qiao YL, Wu T, Li RC, Hu YM, Wei LH, Li CG, Chen W, Huang SJ, Zhao FH, Li MQ, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. 2019; Apr 23. [accessed 2019 Aug 21]. doi: 10.1093/jnci/djz074. PMID: 31086947.
- Su YY, Lin BZ, Zhao H, Li J, Lin ZJ, Qiao YL, Wei LH, Hu YM, Li RC, Zhuang SJ, et al. Lot-to-lot consistency study of an Escherichia coli-produced bivalent human papillomavirus vaccine in adult women: a randomized trial. Hum Vaccin Immunother. 2019;1–9. doi:10.1080/21645515.2019.1691413. PMID: 31770068.
- Hu Y, Guo M, Li C, Chu K, He W, Zhang J, Gu J, Li J, Zhao H, Wu X, et al. Immunogenicity noninferiority study of 2 doses and 3 doses of an Escherichia coli-produced HPV bivalent vaccine in girls vs. 3 doses in young women. Sci China Life Sci. 2019. PMID: 31231780. doi:10.1007/s11427-019-9547-7.
- Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011;85(24):13253–59. doi:10.1128/JVI.06093-11. PMID: 21976653
- Gu Y, Wei M, Wang D, Li Z, Xie M, Pan H, Wu T, Zhang J, Li S, Xia N. Characterization of an Escherichia coli-derived human papillomavirus type 16 and 18 bivalent vaccine. Vaccine. 2017;35(35 Pt B)):4637–45. doi:10.1016/j.vaccine.2017.06.084. PMID: 28736197
- Zhao H, Lin ZJ, Huang SJ, Li J, Liu XH, Guo M, Zhang J, Xia NS, Pan HR, Wu T, et al. Correlation between ELISA and pseudovirion-based neutralisation assay for detecting antibodies against human papillomavirus acquired by natural infection or by vaccination. Hum Vaccin Immunother. 2014;10(3):740–46. PMID: 24384608. doi:10.4161/hv.27619.
- Donken R, de Melker HE, Rots NY, Berbers G, Knol MJ. Comparing vaccines: a systematic review of the use of the non-inferiority margin in vaccine trials. Vaccine. 2015;33(12):1426–32. doi:10.1016/j.vaccine.2015.01.072. PMID: 25659273
- Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsague X, Rusche SA, Lukac S, Bryan JT, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118(5):2135–45. PMID: 17079588. doi:10.1542/peds.2006-0461.
- Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, Spiessens B, Descamps D, Hardt K, Lehtinen M, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health. 2007;40(6):564–71. PMID: 17531764. doi:10.1016/j.jadohealth.2007.02.015.
- Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J Infect Dis. 2009;200(2):166–71. doi:10.1086/599988. PMID: 19519255
- Ferguson M, Wilkinson DE, Zhou T. WHO meeting on the standardization of HPV assays and the role of the WHO HPV Laboratory Network in supporting vaccine introduction held on 24-25 January 2008, Geneva, Switzerland. Vaccine. 2009;27(3):337–47. doi:10.1016/j.vaccine.2008.10.062. PMID: 19007840
- Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, Pinto LA, Wettendorff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4(6):425–34. doi:10.4161/hv.4.6.6912. PMID: 18948732
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al. The HPV-010 Study Group. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5(10):15. doi:10.4161/hv.5.10.9518.
- Ferguson M, Heath A, Johnes S, Pagliusi S, Dillner J. Collaborative Study Participants. Results of the first WHO international collaborative study on the standardization of the detection of antibodies to human papillomaviruses. Int J Cancer. 2006;118(6):1508–14. doi:10.1002/ijc.21515. PMID: 16184553
- Stanley MA, Sudenga SL, Giuliano AR. Alternative dosage schedules with HPV virus-like particle vaccines. Expert Rev Vaccin. 2014;13(8):1027–38. doi:10.1586/14760584.2014.935767. PMID: 25001893
- Siegrist C-A, Vaccine Immunology. 2018; p. 16-34e7.
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381(27):751–58. doi:10.1038/381751a0. PMID: 8657279
- Widdice LE, Unger ER, Panicker G, Hoagland R, Callahan ST, Jackson LA, Berry AA, Kotloff K, Frey SE, Harrison CJ, et al. Antibody responses among adolescent females receiving two or three quadrivalent human papillomavirus vaccine doses at standard and prolonged intervals. Vaccine. 2018;36(6):881–89. PMID: 29306506. doi:10.1016/j.vaccine.2017.12.042.
- Esposito S, Birlutiu V, Jarcuska P, Perino A, Man SC, Vladareanu R, Meric D, Dobbelaere K, Thomas F, Descamps D. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine administered according to an alternative dosing schedule compared with the standard dosing schedule in healthy women aged 15 to 25 years: results from a randomized study. Pediatr Infect Dis J. 2011;30(3):e49–55. doi:10.1097/INF.0b013e318206c26e. PMID: 21273939
- Zimmerman RK, Nowalk MP, Lin CJ, Fox DE, Ko FS, Wettick E, Cost G, Hand L, Hayes J, Michaels M. Randomized trial of an alternate human papillomavirus vaccine administration schedule in college-aged women. J Womens Health (Larchmt). 2010;19(8):1441–47. doi:10.1089/jwh.2009.1753. PMID: 20629576
- Neuzil KM, Canh DG, Thiem VD, Janmohamed A, Huong VM, Tang Y, Diep NTN, Tsu V, LaMontagne DS. Immunogenicity and Reactogenicity of Alternative Schedules of HPV Vaccine in Vietnam. JAMA. 2011;305(14):1424–31. doi:10.1001/jama.2011.407. PMID: 21486975
