ABSTRACT
Diphtheria-tetanus-pertussis (DTP) vaccine may be associated with excess female deaths. There are few studies of possible nonspecific effects of the DTP-containing vaccine Penta (DTP-hepatitis B-Haemophilus influenzae type b). We therefore investigated whether Penta vaccinations were associated with excess female deaths in rural Bangladesh. Between June 29, 2011 and April 20, 2016, we examined the mortality rates of 7644 children followed between 6 weeks and 9 months of age. We analyzed mortality using crude mortality rate ratio (MRR) and age-adjusted MRR (aMRR) from a Cox proportional hazards model. Mortality was analyzed according to sex, number of doses of Penta, and the order in which BCG and Penta were administered. During follow-up, 43 children died. For children who were only BCG vaccinated (BCG-only), the adjusted F/M MRR was 0.47 (0.09–2.48). However, among children who had Penta as their most recent vaccination, the adjusted F/M MRR was 9.91 (1.16–84.44). Hence, the adjusted F/M MRR differed significantly for BCG-only and for Penta as the most recent administered vaccination. Although the mortality rate was low in rural Bangladesh, there was a marked difference between adjusted F/M MRR’s for children vaccinated with BCG-only compared with children where Penta was the most recent administered vaccination. Although usually ascribed to differential treatment and access to care, DTP-containing vaccines may be part of the explanation for the excessive female mortality reported in some regions.
Introduction
Numerous studies support that the Bacillus Calmette–Guérin (BCG) has beneficial nonspecific effects (NSEs) on child survivalCitation1–3 and that the beneficial effects are stronger for girls.Citation4 A reanalysis of BCG trials from the US and the UK in the 1940–1950s, in which prevention of tuberculosis (TB) was the original main outcome, showed that BCG vaccination was apparently associated with a 25% reduction in non-TB and non-accident deaths.Citation5 In contrast, the combined diphtheria, tetanus, and whole-cell pertussis (DTP) vaccine may have deleterious NSEs and has been associated with increased female mortality from infections other than diphtheria, tetanus and pertussis.Citation6–13 In Bangladesh, Penta (DTP, hepatitis B, Haemophilus influenzae type b) replaced DTP as the main vaccine in January 2009.
Potential detrimental effects of receiving BCG followed by DTP as recommended by the World Health Organization (WHO) have been observed in several countries including Gambia,Citation14 Senegal,Citation2 Malawi,Citation15 and Guinea-Bissau.Citation9 Three studies from Bangladesh,Citation16 India,Citation12 and SenegalCitation2 indicated that children who received BCG and DTP simultaneously as their first vaccines had a lower mortality rate than children who started the vaccination schedule with BCG-only and then DTP. Few studies of Penta have been reported but Penta was associated with increased female-to-male mortality rate ratio (F/M MRR) in one study from Guinea-Bissau.Citation17 Potential sex-differential effects of co-administration of BCG and Penta have yet to be examined.
The current study aimed to investigate NSEs of BCG and Penta in relation to mortality for boys and girls aged 6 weeks to 9 months in Chakaria, a rural sub-district of Bangladesh. This age interval was chosen to separate the follow-up from the measles vaccination (MV) recommended at 9 months of age.
Materials and methods
Settings and population
International Center for Diarrheal Diseases Research, Bangladesh (icddr,b) runs a Health and Demographic Surveillance System (HDSS) in Chakaria. The HDSS covers a population of 90,000 individuals living in 16,000 households in 49 villages. All households are visited every three months to enquire about marriages, pregnancies, births, migrations, and deaths. The expanded program on immunization (EPI) provides services in the HDSS areas through 95 EPI outreach centers. Vaccines are given as recommended by WHO: BCG at birth, Penta and Oral polio vaccine (OPV) in three doses at 6, 10, and 14 weeks of age, followed by MV at 9 months of age. Information on vital status and vaccinations of children below 3 years of age was collected during household visits every 3 months.
Childhood morbidity, mortality, and cause of death in study area
Lower respiratory infection (LRI) and diarrhea are the leading causes of morbidity and under-5 mortality for children in the Chakaria HDSS area.Citation18,Citation19 BCG is protective against disseminated TB in childhood and the single shot of Penta provides protection to children from five life-threatening diseases: diphtheria, pertussis (whooping cough), tetanus, hepatitis B, and haemophilus influenzae Type b (Hib). The incidence of these vaccine preventable infections is low among children below five years of age in Chakaria. During 2010–2012, only 2 pertussis, 8 meningitis, and 2 measles infection related deaths were reported among children less than five years of age. Hence, the mortality rates per 100,000 person-years (MR) were 7 for pertussis, 29 for meningitis, and 7 for measles infection among children less than five years of age.Citation19 It should be noted that studies have reported that females are neglected in seeking medical care among under-five children,Citation20,Citation21 even for free immunizationCitation22.
Assessment of exposure
The study was conducted between June 29, 2011 and April 20, 2016. Children were categorized according to the sequence in which they received BCG and Penta vaccines. We defined 11 mutually exclusive vaccine-sequence categories (Group I–XI) of children’s hitherto administered BCG and Penta vaccinations. Vaccination sequences could be initiated in three ways: (1) the WHO recommended schedule: BCG-first and then three doses of Penta denoted Penta1-3 (Group I–IV); (2) BCG+Penta1 administered simultaneously first and then Penta2-3 (Group V–VII); and (3) Penta1-first followed by Penta2-3 with BCG administered together with Penta2 or Penta3 (Group VIII–XI). In addition, we defined the two categories “documented no vaccination” (unvaccinated) and “have card but not seen” when the family reported possession of vaccination card but was unable to show card to the interviewer.
We furthermore focused on three broader, still mutually exclusive groups: BCG-only (Group I), Penta-vaccinated, i.e., Penta as the most recent administered vaccination (Group II–IV and VI–X), and “documented no vaccination.” Children who had only received “BCG and Penta1 simultaneously” (Group V) and the children who received “Penta1 followed by BCG” (Group-XI) were not included in the Penta-vaccinated group. This was because receiving BCG and DTP simultaneously has been associated with decreased mortality.Citation2,Citation12,Citation16
We also compared the mortality between children who started vaccination according to the WHO recommended schedule ((BCG-first-then-Penta) (Group I–IV)) and children who started their vaccination schedule with BCG and Penta administered simultaneously (Group V–VII).
The pneumococcal vaccine (PCV) and inactivated polio vaccine (IPV) were introduced on March 21, 2015 in Chakaria and the IPV was administered with PCV3. Therefore, we restricted our study to children who were born before February 9, 2015.
Epidemiological and statistical methods
Information on vaccinations and deaths was collected retrospectively through trimonthly visits to each household. Vaccinations of dead children are likely to be underreported because parents tend not to keep the vaccine card of a deceased child; even if they do keep the card, they may be reluctant to show the card to the interviewer. In such situations, using retrospectively updated information likely misclassifies some vaccinated deceased children as unvaccinated, resulting in excess deaths in the unvaccinated group.
Furthermore, vaccinated children who survive will contribute to a time-to-event analysis with risk-free survival time because they survived up to the next visit. Hence, using the retrospective updating approach to measure the effect of vaccination can introduce survival biasCitation23–25 which can distort the estimated effect of vaccinations. We therefore used a “landmark approach”Citation23–25 to measure the impact of vaccination status on mortality. Depending on the specific analysis reported, we define vaccination status as either the vaccination sequence administered hitherto or the most recent administered vaccination. In the landmark approach, the vaccination status is fixed from the household visit until the date of the next visit when the vaccination card is seen again. Vaccination status changes with visit; hence, children could, e.g., contribute with unvaccinated risk-time after the first visit and vaccinated risk-time after the second visit.
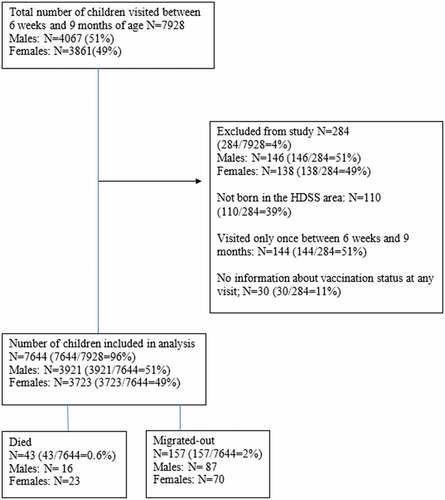
We limited the study to children aged 6 weeks (Penta1 is scheduled at 6 weeks) to 9 months of age (MV is scheduled at 9 months of age). Children who were visited only once were excluded from the present study (N = 144; ) because we could not follow these children for mortality between 6 weeks and 9 months of age. Children with no information about vaccination status at any visit were also excluded (N = 30). Furthermore, we excluded children who were not born in the HDSS area (N = 110) because mothers could not accurately report date of birth and date of vaccinations.
We used Cox proportional hazards models with age as the underlying time-scale to estimate adjusted MRRs (aMRR) of different hitherto administered vaccination sequences for both boys and girls. Children were followed from 6 weeks of age until migration, death, or 9 months of age, whichever occurred first. We included parity, maternal age, maternal education, season of birth, and village as potential confounders in the adjusted models. To assess the sex-differential effects of different sequences of BCG and Penta vaccinations on mortality, an interaction between sex and hitherto administered sequence of BCG and Penta were included in the adjusted Cox proportional hazards models. To test the sex-differential effects according to whether Penta1, Penta2, or Penta3 were the most recent administered vaccination, we conducted a log-rank test.
Results
Study children
Health, demographic, and vaccination information of 7928 children aged 6 weeks-9 months of age and born in the Chakaria HDSS area between January 1, 2011 and February 8, 2015, was collected by surveillance workers. A total of 284 children were excluded from the present study (). The remaining 7644 (Boys: 3921, Girls: 3723) were included in the survival analysis. Background characteristics for boys and girls are shown in . There was no difference in background characteristics between boys and girls. The mean number of visits was 2.39 (SD:0.49) for both sexes, respectively, and the median age in months was 4.57 (IQR:2.37–6.78) for females and 4.58 (IQR:2.34–6.88) for males.
Table 1. Background characteristics of study children according to sex
Vaccination coverage
Among the children who were visited after 6 months and before 9 months of age (N = 3414), the vaccination coverage at 6 months of age was 90% for BCG and 90%, 86%, and 75% for Penta1, Penta2, and Penta3, respectively. The proportion receiving BCG and Penta did not differ by sex (Penta1: p = .31; Penta2: p = .48; Penta3: p = .78). Among vaccinated children visited between 6 months and 9 months, 36% followed the WHO-recommended schedule of BCG-first-then- Penta1, 56% had received BCG-Penta1-simultaneously, and 8% had received Penta1 first followed by BCG. The proportion were similar for boys and girls (data not shown).
Vaccines and sex-differential mortality
Between 6 weeks and 9 months of age, 43 children died, the mortality rate being 13 per 1000 person-years (). Overall, the F/M MRRs was 1.78 (0.91–3.51) between 6 weeks and 9 months age.
Table 2. Deaths per 1000 person-years (pyrs) and female-male (F/M) mortality rate ratios (MRR) according to vaccination status in children aged 6 weeks-9 months
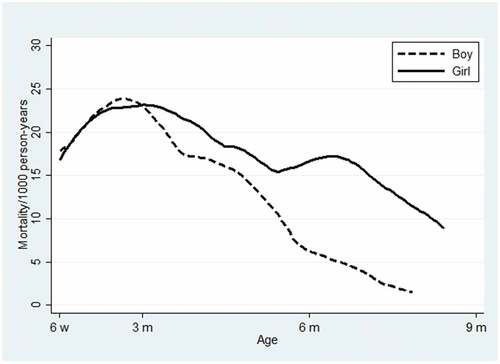
In general, the sex-difference in mortality increased as age increased. Around 5 months of age, the mortality gap between boys and girls seemed to increase more rapidly compared with the first period ().
Figure 2. Mortality rate per 1000 person-years according to sex for children ages 6 weeks to 9 months. The curves have been smoothed with a weighted kernel density utilizing the estimated hazard
In , crude F/M MRRs are reported according to various hitherto administered vaccination sequences. Among the children who received BCG first, the crude F/M MRR increased from 0.48 (0.04–3.38) for BCG-only vaccinated children (Group I) to 2.18 (0.11–128.67) for children vaccinated with Penta1 and Penta2 subsequent to BCG (Group III). For the children who received BCG and Penta1 simultaneously (Group V) the F/M MRR was 2.14 (0.11–126.57). Due to no death among boys no F/M MRRs were calculated for the sequences in which Penta2 or Penta3 were the most recent administered vaccination. However, a striking difference in mortality rate between girls (5 deaths/192 person-years) and boys (0 deaths/206 person-years) was observed after administration of the third dose of Penta (P = .03). Finally, the F/M MRR was 1.50 (0.60–4.00) among unvaccinated children (“documented no vaccination”) (P = .39).
As seen in , children with Penta as the most recent administered vaccination (irrespectively of number of doses) had an F/M aMRR of 9.91 (1.16–84.44); the sex-difference was particularly pronounced after three doses of Penta (p = .01) (Supplementary Table 1). Conversely, the F/M aMRR was 0.47 (0.09–2.48) among children only vaccinated with BCG and 1.51 (0.76–3.01) for unvaccinated children (“documented no vaccination”) resulting in a significant interaction between sex and BCG versus Penta vaccinated (p = .023 for interaction). Though no statistically significant interaction was observed for the unvaccinated children, they tended to have a lower F/M MRR than the Penta-vaccinated children (p = .10, test for interaction between sex and unvaccinated versus Penta vaccinated).
Table 3. Deaths per 1000 person-years (pyrs) and mortality rate ratios (MRR) according to sex and vaccination status
Co-administration of BCG with Penta versus the WHO schedule
We also compared the mortality rate when vaccinations were received following the WHO-recommended schedule compared with out-of-sequence. Children following the WHO schedule (BCG-first-then-Penta; Group I–IV) may have a slightly higher mortality than the children who receive BCG-Penta1 simultaneously (Group V–VII) (aMRR = 1.51 (0.58–3.98)). This increased mortality tendency when following the WHO schedule was more pronounced among males (aMMR = 5.69 (0.69–46.93)) than females (aMMR = 0.93(0.29–2.92)) (p = .12 for interaction between sex and vaccination status). Unvaccinated children had mortality rates similar to the children who followed the WHO-recommended schedule (Supplementary Table 2).
Discussion
Main findings
The mortality rate was low in Chakaria. Nonetheless, Penta as the most recent administered vaccination was associated with significant excess female mortality between 6 weeks and 9 months of age. This sex-differential pattern differed significantly from the BCG-only group where there was a tendency toward less female deaths compared with males. The WHO-recommended schedule with BCG-first-then-Penta was not associated with better survival compared with children who received BCG and Penta1 simultaneously.
Consistency with previous findings
Penta is the combination of DTP, HBV, and Hib vaccine. We found that girls had higher mortality than boys at the age of predominant Penta vaccination (2–9 months) consistent with several other studies of the DTP vaccine.Citation7,Citation9,Citation14,Citation15,Citation17,Citation26,Citation27 Several studies from Bangladesh have also shown that the F/M MRR may be strongly increased when DTP is the most recent vaccine.Citation16,Citation28 WHO’s Strategic Advisory group of Experts on Immunization (SAGE) recently sponsored a review on the NSEs of DTP. DTP was associated with a non-significant increase in all-cause mortality by 38% (95% CI: −8%-108%).Citation29 This SAGE review, however, included studies with survival bias. If only studies with prospective follow-up and no survival bias were included in the analysis, DTP was associated with 100% (50%-167%) higher mortality.Citation25
The pentavalent vaccine has only been tested for potential deleterious heterologous effects in one previous study.Citation17 This study from Guinea-Bissau showed that Penta was associated with increased female mortality and our results for Penta in the present study are clearly similar. Hence, Penta may have the same sex-differential negative NSEs as DTP. Though Chakaria has sex-differential access to health care it is unlikely that this is the main explanation for excess female mortality after Penta because there was no excess female mortality after BCG () or MVCitation30 .
Several studies have suggested that co-administration of BCG and DTP is associated with lower mortality compared with the WHO recommended vaccination schedule where BCG is administered first followed by DTP/Penta. In Bangladesh, children who received DTP1 after BCG had a two-fold higher mortality compared with children who received DTP1 simultaneously with BCG.Citation16 In rural India, children who received BCG and DTP1 simultaneously had a four-fold lower mortality up to 9 months of age compared with children who initiated their vaccination schedule with BCG first or DTP1 first.Citation12 Similarly, a study from Senegal found that children who received BCG and DTP1 simultaneously had lower mortality than those who started with either BCG first or DTP1 first.Citation2 Collating these three studies, the SAGE review estimated that simultaneous administration of BCG and DTP may be associated with 48% (20%-66%) lower mortality compared with BCG first followed by DTP (28). Though our study had few deaths, it was compatible with the idea that co-administration of BCG and Penta compared with BCG followed by Penta may reduce mortality between 6 weeks to 9 months of age.
Strengths and limitations
Most studies measuring the NSEs of BCG and DTP/Penta were conducted in the 1980s and 1990s in African and Asian countries when vaccination coverage was low and mortality was high. The present study was conducted in Bangladesh during 2011–2016 when vaccination coverage was high and mortality lowCitation19 and therefore provides important information about the effects of BCG and Penta in a low-mortality setting.
We excluded 4% of the children from the study mainly because the children were not born within the HDSS or received only one visit. There were no difference in exclusion among males and females. Furthermore, only 2% of the children migrated out of the study area between 6 weeks and 9 months.
It is evident that healthy children are more likely to be vaccinated first and one would therefore expect vaccinated children to be healthier than unvaccinated children and to have lower mortality (healthy vaccinée bias). In this study, we compared F/M MRR among vaccinated children and the sequence in which BCG and Penta vaccines were given. Thus, healthy vaccinée bias was not likely to influence the results.
Although we have included 7644 children aged 6 weeks to 9 months of age for this study and found a small number of deaths (n = 43 deaths). The results and conclusion would be more robust with a large number of children and deaths from studies in different settings.
Interpretation and implications: reasons for excess female mortality
In the present study, we found that Penta as the most recent administered vaccination was associated with increased mortality for females compared with males. In recent years, 10 countries including Bangladesh identified higher than expected under-5 female mortalityCitation31,Citation32 and explained these excess female deaths with differential treatment of girls compared with boys.Citation33–35 However, there may be at least two explanations for the sex-differential under-5 mortality: vaccinations and sex-differential treatment. We found that girls had lower mortality than boys when BCG was the most recent administered vaccine but increased to higher mortality than for boys when Penta was the most recent administered vaccine. This inversion of the F/M MRR from BCG as the most recent administered vaccine to Penta as the most recent administered vaccine could support the hypothesis that some excess female deaths are due to Penta vaccination. Future research should focus on examining whether excess female deaths are related to vaccinations,Citation7,Citation9,Citation14,Citation15,Citation17,Citation26,Citation27 sex-differential treatmentCitation31–33,Citation35,Citation36 or a combination of both.
In the present study, we found that BCG and Penta administered simultaneously was associated with slightly lower mortality compared with the WHO recommended schedule of BCG-first-then-Penta. If receiving BCG and Penta simultaneously lowers mortality then the recommended schedule of vaccines might be reexamined and a second dose of BCG with one of the subsequent Penta vaccinations could be considered. However, this needs to be examined further. Matlab HDSS, another field site of icddr,b in Bangladesh, that cover 234000 population, records around 5000 births annually with routine immunization information.Citation37 This study findings can be examined using the Matlab HDSS data with a large number of children and death cases. In addition, data for this manuscript come from the collaboration study of five countries including Bandim Health project (Guinea-Bissau), Navrongo and Kintampo (Ghana), Nouna (Burkina Faso), Nairobi (Kenya), and Chakaria (Bangladesh) under the INDEPTH Network. Therefore, the finding that Penta is associated with an increased F/M MRR can also be examined in African settings with the above-mentioned HDSS sites as efficiently as in Asia.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author contributions
MAH, PA, and AB conceived and designed the study and are the guarantors of the study. MAH, SBS, and AKGJ analyzed the data. PA and AB supervised the data analysis. MAH wrote the first draft of the manuscript. All authors contributed to the final version of the manuscript. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Data sharing
No additional data available
Transparency declaration
The first author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
Ethical approval
Ethical Review Committee of International Centre of Diarrhoeal Disease Research, Bangladesh provided approval for the study.
Acknowledgments
icddr,b acknowledges with gratitude the commitment of the INDEPTH Network to its research efforts. icddr,b is also grateful to the Government of Bangladesh, Canada, Sweden, and the UK for providing core/unrestricted support. The authors are grateful to the villagers for their cooperation in providing invaluable information. The untiring efforts of the team members of the Chakaria HDSS in maintaining the surveillance system are gratefully acknowledged.
Additional information
Funding
References
- Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, Stensballe L, Diness BR, Lausch KR, Lund N. Randomized trial of bcg vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204(2):245–52. doi:10.1093/infdis/jir240.
- Aaby P, Nielsen J, Benn CS, Trape JF. Sex-differential and non-specific effects of routine vaccinations in a rural area with low vaccination coverage: an observational study from senegal. Trans R Soc Trop Med Hyg. 2015;109(1):77–84. doi:10.1093/trstmh/tru186.
- Breiman RF, Streatfield PK, Phelan M, Shifa N, Rashid M, Yunus M. Effect of infant immunisation on childhood mortality in rural bangladesh: analysis of health and demographic surveillance data. Lancet. 2004;364(9452):2204–11. doi:10.1016/S0140-6736(04)17593-4.
- Roth A, Sodemann M, Jensen H, Poulsen A, Gustafson P, Weise C, Gomes J, Djana Q, Jakobsen M, Garly ML, et al. Tuberculin reaction, bcg scar, and lower female mortality. Epidemiology. 2006;17(5):562–68. doi:10.1097/01.ede.0000231546.14749.ab.
- Shann F. The non-specific effects of vaccines. Arch Dis Child. 2010;95(9):662–67. doi:10.1136/adc.2009.157537.
- Aaby P, Garly ML, Nielsen J, Ravn H, Martins C, Bale C, Rodrigues A, Benn CS, Lisse IM. Increased female-male mortality ratio associated with inactivated polio and diphtheria-tetanus-pertussis vaccines: observations from vaccination trials in guinea-bissau. Pediatr Infect Dis J. 2007;26(3):247–52. doi:10.1097/01.inf.0000256735.05098.01.
- Aaby P, Ravn H, Roth A, Rodrigues A, Lisse IM, Diness BR, Lausch KR, Lund N, Rasmussen J, Biering-Sorensen S, et al. Early diphtheria-tetanus-pertussis vaccination associated with higher female mortality and no difference in male mortality in a cohort of low birthweight children: an observational study within a randomised trial. Arch Dis Child. 2012;97(8):685–91. doi:10.1136/archdischild-2011-300646.
- Agergaard J, Nante E, Poulstrup G, Nielsen J, Flanagan KL, Ostergaard L, Benn CS, Aaby P. Diphtheria-tetanus-pertussis vaccine administered simultaneously with measles vaccine is associated with increased morbidity and poor growth in girls. A randomised trial from guinea-bissau. Vaccine. 2011;29(3):487–500. doi:10.1016/j.vaccine.2010.10.071.
- Aaby P, Benn C, Nielsen J, Lisse IM, Rodrigues A, Ravn H. Testing the hypothesis that diphtheria–tetanus–pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open. 2012;2:3.
- Flanagan KL, Plebanski M. Sex-differential heterologous (non-specific) effects of vaccines: an emerging public health issue that needs to be understood and exploited. Expert Rev Vaccines. 2016;1–9.
- Krishnan A, Srivastava R, Dwivedi P, Ng N, Byass P, Pandav CS. Non-specific sex-differential effect of dtp vaccination may partially explain the excess girl child mortality in ballabgarh, india. Trop Med Int Health. 2013;18(11):1329–37. doi:10.1111/tmi.12192.
- Hirve S, Bavdekar A, Juvekar S, Benn CS, Nielsen J, Aaby P. Non-specific and sex-differential effects of vaccinations on child survival in rural western india. Vaccine. 2012;30(50):7300–08. doi:10.1016/j.vaccine.2012.09.035.
- Mogensen SW, Andersen A, Rodrigues A, Benn CS, Aaby P. The introduction of diphtheria-tetanus-pertussis and oral polio vaccine among young infants in an urban african community: A natural experiment. EBioMedicine. 2017;17:192–98. doi:10.1016/j.ebiom.2017.01.041.
- Aaby P, Jensen H, Walraven G. Age-specific changes in the female-male mortality ratio related to the pattern of vaccinations: an observational study from rural gambia. Vaccine. 2006;24(22):4701–08. doi:10.1016/j.vaccine.2006.03.038.
- Aaby P, Vessari H, Nielsen J, Maleta K, Benn CS, Jensen H, Ashorn P. Sex differential effects of routine immunizations and childhood survival in rural malawi. Pediatr Infect Dis J. 2006;25(8):721–27. doi:10.1097/01.inf.0000227829.64686.ae.
- Aaby P, Andersen A, Ravn H, Zaman K. Co-administration of bcg and diphtheria-tetanus-pertussis (dtp) vaccinations may reduce infant mortality more than the who-schedule of bcg first and then dtp. A re-analysis of demographic surveillance data from rural bangladesh. EBioMedicine. 2017;22:173–80. doi:10.1016/j.ebiom.2017.07.012.
- Fisker AB, Biering-Sorensen S, Lund N, Djana Q, Rodrigues A, Martins CL, Benn CS. Contrasting female-male mortality ratios after routine vaccinations with pentavalent vaccine versus measles and yellow fever vaccine. A cohort study from urban guinea-bissau. Vaccine. 2016;34(38):4551–57. doi:10.1016/j.vaccine.2016.07.034.
- Bhuiya A Health knowledge and behaviour in five unions of chakaria. International Centre for Diarrhoeal Disease Research, Bangladesh; 1996. Report nr (ICDDR,B special publication number 52).
- Hanifi SM, Mahmood SS, Bhuiya A. Cause-specific mortality and socioeconomic status in chakaria, bangladesh. Glob Health Action. 2014;7(1):25473. doi:10.3402/gha.v7.25473.
- Hanifi SMA, Sultana A, Mia MN, Hoque S, Mahmood SS, Iqbal M, Bhuiya A. Chakaria health and demographic surveillance system: focusing on the sustainable development goals-2014. Dhaka; 2016. p. 52.
- Mahmud SS, Iqbal M, Hanifi SMA. Health seeking behavour. In: Bhuiya A, editor. Health for the rural massess: insights from chakaria. Dhaka (Bangladesh): International Centre for Diarrhoeal Diseases Research, Bangladesh. 2008.
- Hanifi SMA, Ravn H, Aaby P, Bhuiya A. Where girls are less likely to be fully vaccinated than boys: evidence from a rural area in bangladesh. Vaccine. 2018;36(23):3323–30. doi:10.1016/j.vaccine.2018.04.059.
- Jensen H, Benn CS, Lisse IM, Rodrigues A, Andersen PK, Aaby P. Survival bias in observational studies of the impact of routine immunizations on childhood survival. Trop Med Int Health. 2007;12(1):5–14. doi:10.1111/j.1365-3156.2006.01773.x.
- Fine PE, Smith PG. Editorial:‘Non‐specific effects of vaccines’–an important analytical insight, and call for a workshop. Trop Med Int Health. 2007;12(1):1–4. doi:10.1111/j.1365-3156.2006.01794.x.
- Aaby P, Ravn H, Benn CS. The who review of the possible nonspecific effects of diphtheria-tetanus-pertussis vaccine. Pediatr Infect Dis J. 2016;35(11):1247–57. doi:10.1097/INF.0000000000001269.
- Aaby P, Ibrahim SA, Libman MD, Jensen H. The sequence of vaccinations and increased female mortality after high-titre measles vaccine: trials from rural sudan and kinshasa. Vaccine. 2006;24(15):2764–71. doi:10.1016/j.vaccine.2006.01.004.
- Aaby P, Martins CL, Garly M-L, Balé C, Andersen A, Rodrigues A, Ravn H, Lisse IM, Benn CS, Whittle HC. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ. 2010;341(nov30 2):c6495. doi:10.1136/bmj.c6495.
- Clipet-Jensen C, Andersen A, Jensen A, Peter A, Khalequzzaman M. Out-of-sequence measles and dtp vaccinations. A re-analysis of demographic surveillance data from rural bangladesh. CID. 2020. (in press). doi:10.1093/cid/ciaa291.
- Higgins JP, Soares-Weiser K, López-López JA, Kakourou A, Chaplin K, Christensen H, Martin NK, Sterne JA, Reingold AL. Association of bcg, dtp, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi:10.1136/bmj.i5170.
- Hanifi SMA, Biering-Sørensen S, Jensen A, Aaby P, Bhuiya A The strategy of measles elimination: considering the non-targeted benefits of measles vaccine on child health. (submitted).
- Park JJ, Brondi L. Why are girls still dying unnecessarily? The need to address gender inequity in child health in the post–2015 development agenda. J Glob Health. 2015;5(2):2. doi:10.7189/jogh.05.020303.
- Alkema L, Chao F, You D, Pedersen J, Sawyer CC. National, regional, and global sex ratios of infant, child, and under-5 mortality and identification of countries with outlying ratios: A systematic assessment. Lancet Glob Health. 2014;2(9):e521–e530. doi:10.1016/S2214-109X(14)70280-3.
- Ram U, Jha P, Ram F, Kumar K, Awasthi S, Shet A, Pader J, Nansukusa S, Kumar R. Neonatal, 1–59 month, and under-5 mortality in 597 indian districts, 2001 to 2012: estimates from national demographic and mortality surveys. Lancet Glob Health. 2013;1(4):e219–e226. doi:10.1016/S2214-109X(13)70073-1.
- Pande RP. Selective gender differences in childhood nutrition and immunization in rural india: the role of siblings. Demography. 2003;40(3):395–418. doi:10.1353/dem.2003.0029.
- Krishnan A, Ng N, Kapoor SK, Pandav CS, Byass P. Temporal trends and gender differentials in causes of childhood deaths at ballabgarh, india-need for revisiting child survival strategies. BMC Public Health. 2012;12(1):1. doi:10.1186/1471-2458-12-555.
- Khera R, Jain S, Lodha R, Ramakrishnan S. Gender bias in child care and child health: global patterns. Arch Dis Child. 2014;99(4):369–74. doi:10.1136/archdischild-2013-303889.
- Health and demographic surveillance system-matlab: registration of health and demographic events 2015; volume −50, scientific report no. −135. Dhaka: International Centre for Diarrhoeal Disease Research, Bangladesh. 2017.