ABSTRACT
Immunotherapy with a checkpoint inhibitor has revolutionized the treatment of advanced non-small cell lung cancer. Replacing cytotoxic chemotherapy in some settings, immunotherapy with checkpoint inhibitors enables many patients to live longer with much fewer side effects. Nonetheless, immunotherapy alone only works for about one-fifth of unselected patients and despite the durability of response, treatment will eventually fail. There are several important cofactors within the tumor microenvironment which can contribute to the efficacy of immunotherapy. These include T-cells, chemokines, and antigen presentations. Preliminary research has shown that these cofactors can be altered by epigenetic modulation. Specifically, hypomethylating agents or histone deacetylase inhibitors can lead to changes in the compositions and characteristics within the tumor microenvironment in a way that enhances the efficacy of checkpoint inhibitor. In recent clinical trials of combined immuno-epigenetic therapy, tumor responses were observed among patients who were previously resistant or refractory to immunotherapy. Furthermore, biological correlative studies also confirmed the mechanism of action of these agents, especially among patients who derived benefit. Nonetheless, at present, the efficacy in terms of tumor response seems modest and side effects, though mostly not serious, can result in treatment interruption or interfere with the quality of life.
Background
The advent of checkpoint inhibitor immunotherapy that targets cytotoxic T-lymphocyte associated protein 4 and programmed death protein 1 (PD-1) or its ligand (PD-L1) has revolutionized cancer care. Following the approval of ipilimumab, the first checkpoint inhibitor available in the United States for melanoma in 2011, several other checkpoint inhibitors have been added to the oncologic armamentarium. These agents act by disrupting the immune-escape mechanism exploited by cancer cells.Citation1 Normally, the defensive immune response to malignancy can occur only if several delicate steps occur in coordination, starting from presenting of cancer antigen by dendritic or antigen-presenting cells (APC), trafficking of T cell into the tumor, recognizing of cancer cells by T cells and finally killing of cancer cells.Citation2 Cancer cells can evade the destruction from the immune system by disabling any of these steps such as loss of antigenicity, loss of immunogenicity, or creating an immunosuppressive environment. The PD-1 or PD-L1 inhibitors, while helpful in the recognition of cancer cells by T cells,Citation3 may fall short and can lose efficacy over time. For instance, among unselected patients with advanced lung cancer, the tumor response rate of single-agent checkpoint inhibitors typically is 20%.Citation4 Among those who initially respond to treatment, the majority will develop the progression of disease with a median duration of response about 2 years. Immunotherapy failure is a serious problem and represents an active area of investigation.
Several lines of evidence indicate that tumor microenvironment plays a crucial role as a cofactor behind the success of checkpoint immunotherapy.Citation5 In a favorable microenvironment, tumor cells can be seen as highly expressive of PD-L1 and T cells will appear activated, actively infiltrating into the tumor bed. Whereas in an unfavorable microenvironment, T cells will be seen as not engaging, appearing to be stuck in a fibrotic nest of tumor-associated macrophages (TAM) in the periphery of tumors. It has been observed that in a favorable microenvironment, checkpoint immunotherapy will be more likely to work and the reverse is true. While it is still not entirely understood how and why different microenvironments come into place, it is likely that an interaction among multiple factors is at play.
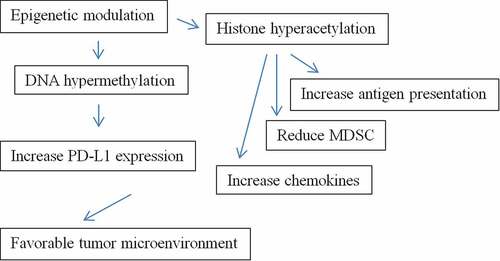
For instance, the level of PD-L1 expression on the tumor cells may be dependent on factors within the tumor microenvironment itself. Other possible mechanisms of resistance to checkpoint inhibitor therapy may include the abundance of TAM which can be seen in the unfavorable tumor microenvironment. TAM derives from myeloid-derived suppressor cells (MDSCs).Citation6 They are poor antigen presenters and can suppress T cell activation. Within the tumor microenvironment, if there is a way to induce PD-L1 expression or to reduce the presence of TAM or MDSC, such an approach may become useful to enhance the efficacy of immunotherapy. One approach under active investigation is by epigenetically altering the composition of myeloid cells.
Epigenetic modulation
In biology, epigenetic modification refers to a modification of genome that does not change the nucleotide sequence, yet still culminate in a change in the overall gene expression. The prefix epi- signifies an event that is “above” or “in addition to” genetic basis of inheritance. Hematopoiesis is regulated by both DNA make-up and gene expression. Alteration of DNA by genetic changes or alteration of gene expression by epigenetic events can result in a change of hematopoiesis. As oppose to genetic manipulation, epigenetic modulation has long been used as a therapeutic approach in medicine, especially for hematologic disorders including myelodysplasia and leukemia.Citation7 Epigenetic modulation can be carried out by externally modifying the DNA that switches genes on or off. In this way, gene expression will be altered without any change to the DNA sequence. There are two key basic mechanisms for epigenetic modulation: DNA methylation and histone modification.Citation8 DNA methylation will suppress gene transcription. For example, in myelodysplastic syndrome, hypermethylation of genes that control proliferation is responsible for the phenotype. Via epigenetic modulation by hypomethylating agents such as azacitadine or decitabine, the disease process can slowdown and the agents are now clinically used for this disease. On the other hand, histone modification by acetylation will promote gene transcription. For instance, in cutaneous T cell lymphoma where the histone-acetylation regulatory enzyme is defective, epigenetic modulation via histone deacetylase inhibitor (HDACi) vorinostat or romidepsin can restore normal acetylation and the treatment is now considered one of the treatment standards.Citation9
Unfortunately, hypomethylating agents or HDACi by themselves have little, if any, direct efficacy against solid tumors including NSCLC. Nevertheless, preclinical data indicate that these agents exhibit a unique property in converting tumor microenvironments into an immunotherapy-favorable state (). In preclinical studies, among lung cancer cell lines treated with azacitidine, there was an upregulation of gene expression related to immune evasion including increased PD-L1 expression.Citation10 It has been observed that azacitidine can up-regulate PD-L1 protein which is a key ligand-mediator of immune tolerance. Furthermore, when treated with HDACi, lung cancer cell lines appear to express a high level of T-cell chemokines which can promote T cell infiltrations into the tumors.Citation11 In melanoma, HDACi has been shown to reduce the number of MDSC within the tumor microenvironment.Citation12 HDACi has also been observed to increase chemokine production and antigen presentation.Citation13 It is relevant to note that there are several classes of HDACi with varied biological effects.Citation14 All these observations, however, support clinical investigations of epigenetic agents to be used in combination with checkpoint immunotherapy.
Clinical results of combined immune-epigenetic therapy
In three recent clinical trials, the preliminary efficacy of epigenetic modulation in combination with checkpoint immunotherapy for non-small cell lung cancer (NSCLC) were reported (). All of these trials utilized pembrolizumab, one of the current care standards for NSCLC, as an immunotherapy backbone. The first study investigated hypomythylating agent, azacitidine, while the other two studies examined HDACi, vorinostat and entinostat.
Table 1. Selected recent clinical trials of combined immune-epigenetic therapy in NSCLC
In the first study, Levy and colleagues reported on a phase-2 randomized trial of pembrolizumab plus placebo or pembrolizumab plus azacitidine.Citation15 All patients had received first line chemotherapy but were immunotherapy naïve. In the experimental arm, patients received oral azacitidine 300 mg on days 1 to14 and pembrolizumab on day 1 only, every 21 days. The investigators observed numerically worse progression-free survival among patients treated with azacitidine, though this was not statistically significant. Azacitidine plus pembrolizumab produced a slightly higher tumor response rate than pembrolizumab alone; however, the combination was more toxic, resulting in frequent treatment interruptions. It was quite possible that the increased treatment-related toxicity in this study explained the unfavorable overall outcomes with azacitidine.
In the second study, Gray and colleagues reported on a phase-1/1b trial of vorinostat plus pembrolizumab.Citation16 All patients had prior chemotherapy and the majority (79%) had previous immunotherapy. During phase-1b, vorinostat was administered at a dosage of 400 mg daily along with pembrolizumab every 21 days. The investigators found that among 24 immunotherapy-pretreated patients, 3 patients (12.5%) achieved tumor response. Considering that this group of patients was not expected to have tumor responses, this number was encouraging. Nevertheless, most patients required a dose reduction of vorinostat due to side effects. Interestingly, among those with tumor response, an increase in stromal, but not in the tumor bed, CD8 T-cells were observed.
Finally, in the third study, Hellman and colleagues reported on a phase-2 trial of entinostat plus pembrolizumab (also known as ENCORE-601 study).Citation17 All patients were pretreated with chemotherapy and immunotherapy. Entinostat was given 5 mg orally once a week along with pembrolizumab every 21 days. The investigators observed an overall response rate of 10%, comparable to the study by Gray and colleagues. Interestingly, although the number of patients for subgroup analysis was small, tumor response in this study can be observed regardless of PD-L1 status suggesting that there was a beneficial effect of HDACi among PD-L1-negative patients. Furthermore, among those with tumor response, a reduction in MDSC was observed.
Challenges and opportunities
Taken together these results suggest that epigenetic modulation via the use of HDACi in combination with a checkpoint inhibitor is clinically feasible. However, the use of azacitidine plus checkpoint inhibitor is probably not feasible as designed. Although these studies are still in early phases, some observations suggest that HDACi may optimize cofactors for immunotherapy efficacy as proposed in earlier studies. The correlative studies have also confirmed some, but not all, key proposed mechanisms. The presence of tumor response among patients who developed resistance to immunotherapy suggests that integrating epigenetic agents to the treatment regimen can help restore treatment efficacy. Given that resistance to immunotherapy is a common clinical problem, there is an opportunity to move these agents into the clinical arena. However, additional studies will be needed.
One clear signal from these trials, however, is the toxicity. Epigenetic agents are chemotherapy and they can cause unappealing toxicity profiles similar to other traditional chemotherapeutic agents. For instance, alopecia can occur to a varying degree. Nausea occurred in 73% of patients treated with azacitidine plus pembrolizumab compared with 27% of patients treated with placebo plus pembrolizumab. In the clinical trial of vorinostat plus pembrolizumab, the most common reasons for dose modification of vorinostat were nausea, anorexia, fatigue, or renal insufficiency. In the clinical trial of entinostat plus pembrolizumab, fatigue was experienced in 41% of patients. Although there is no clear increase in immune-related adverse events with combination therapy at this time, there is an increase in gastrointestinal side effects and fatigue. These side effects may not necessarily be serious; however, they can impair the quality of life and give a glaring contrast to treatment with a single-agent checkpoint inhibitor. Optimal dosage which yields biological effect but causes fewer side effects will be desirable.
In summary, clinical evidences now support that epigenetic therapy can modulate cofactors of immunotherapy efficacy by changing the characteristics within the tumor microenvironment. Based on available data from early-phase clinical trials in NSCLC to date, this approach may enhance tumor responses to immunotherapy or restore responsiveness to immunotherapy among those who develop resistance to immunotherapy. However, the effect appears to be modest. Nonetheless, even a small effect can be quite meaningful when there are no other viable alternatives. Side effects from these treatments, though not serious, can affect the quality of life. Additional research remains to be conducted to improve upon these important proof-of-concept studies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Granier C, De Guillebon E, Blanc C, Roussel H, Badoual C, Colin E, Saldmann A, Gey A, Oudard S, Tartour E, et al. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open. 2017;2:e000213. doi:10.1136/esmoopen-2017-000213.
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013 Jul 25;39(1):1–10. doi:10.1016/j.immuni.2013.07.012.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014 Nov 27;515(7528):568–71. doi:10.1038/nature13954.
- Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–41. doi:10.1200/JCO.2017.75.3384.
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018 May;24(5):541–50. doi:10.1038/s41591-018-0014-x.
- Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010 Jul 15;70(14):5728–39. doi:10.1158/0008-5472.CAN-09-4672.
- Uy GL, Duncavage EJ, Chang GS, Jacoby MA, Miller CA, Shao J, Heath S, Elliott K, Reineck T, Fulton RS, et al. Dynamic changes in the clonal structure of MDS and AML in response to epigenetic therapy. Leukemia. 2017 Apr;31(4):872–81. doi:10.1038/leu.2016.282.
- Schiffmann I, Greve G, Jung M, Lübbert M. Epigenetic therapy approaches in non-small cell lung cancer: update and perspectives. Epigenetics. 2016 Dec;11(12):858–70. doi:10.1080/15592294.2016.1237345.
- Zhang Q, Wang S, Chen J, Yu YZ. Histone Deacetylases (HDACs) guided novel therapies for T-cell lymphomas. Int J Med Sci. 2019 Jan 29;16(3):424–42. doi:10.7150/ijms.30154.
- Wrangle J, Wang W, Koch A, Easwaran H, Mohammad HP, Pan X, Vendetti F, VanCriekinge W, DeMeyer T, Du Z, et al. Alterations of immune response of non-small cell lung cancer with azacytidine. Oncotarget. 2013 Nov;4(11):2067–79. doi:10.18632/oncotarget.1542.
- Zheng H, Zhao W, Yan C, Watson CC, Massengill M, Xie M, Massengill C, Noyes DR, Martinez GV, Afzal R, et al. HDAC inhibitors enhance T-cell chemokine expression and augment response to PD-1 immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2016 Aug 15;22(16):4119–32. doi:10.1158/1078-0432.CCR-15-2584.
- Orillion A, Hashimoto A, Damayanti N, Shen L, Adelaiye-Ogala R, Arisa S, Chintala S, Ordentlich P, Kao C, Elzey B, et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res. 2017 Sep 1;23(17):5187–201. doi:10.1158/1078-0432.CCR-17-0741.
- Briere D, Sudhakar N, Woods DM, Hallin J, Engstrom LD, Aranda R, Chiang H, Sodré AL, Olson P, Weber JS, et al. The class I/IV HDAC inhibitor mocetinostat increases tumor antigen presentation, decreases immune suppressive cell types and augments checkpoint inhibitor therapy. Cancer Immunol Immunother. 2018 Mar;67(3):381–92. doi:10.1007/s00262-017-2091-y.
- Halili MA, Andrews MR, Labzin LI, Schroder K, Matthias G, Cao C, Lovelace E, Reid RC, Le GT, Hume DA, et al. Differential effects of selective HDAC inhibitors on macrophage inflammatory responses to the Toll-like receptor 4 agonist LPS. J Leukoc Biol. 2010;87(6):1103–14. doi:10.1189/jlb.0509363.
- Levy BP, Giaccone G, Besse B, Felip E, Garassino MC, Domine Gomez M, Garrido P, Piperdi B, Ponce-Aix S, Menezes D, et al. Randomised phase 2 study of pembrolizumab plus CC-486 versus pembrolizumab plus placebo in patients with previously treated advanced non-small cell lung cancer. Eur J Cancer. 2019 Feb;108:120–28. doi:10.1016/j.ejca.2018.11.028.
- Gray JE, Saltos A, Tanvetyanon T, Haura EB, Creelan B, Antonia SJ, Shafique M, Zheng H, Dai W, Saller JJ, et al. Phase I/Ib study of pembrolizumab plus vorinostat in advanced/metastatic Non–small cell lung cancer. Clin Cancer Res. 2019;25(22):6623–32. doi:10.1158/1078-0432.CCR-19-1305.
- Hellmann M, Jänne P, Opyrchal M, Hafez N, Raez L, Gabrilovich D, et al. Efficacy/safety of entinostat and pembrolizumab in NSCLC patients previously treated with anti-PD-(L)1 therapy. J Thorac Oncol. 2018;13(10):S330. doi:10.1016/j.jtho.2018.08.257.