ABSTRACT
Background: Healthcare Workers (HCWs) constitute a major group exposed to influenza. Researchers herein try to determine the influenza vaccine effectiveness (VE). Influenza VE depends on the vaccinated personal characteristics and the closeness of matching between the vaccine and the prevalent strains of the virus. The aim of our research was to identify the 2018–2019 seasonal influenza VE in HCWs.
Methods: a record-based study was carried out using the test-negative design from October 2018 to September 2019 to calculate the influenza VE. HCWs with influenza like illness (ILI) were screened to detect the positive cases, and the vaccination status was determined based on vaccination database. VE was assessed from the ratio of the odds of vaccination among positive cases to the odds of vaccination among negative controls. Statistical analysis Multivariable logistic regression was used to estimate adjusted VE
Results: a total of 556 HCWs presented with ILI, 65.6% were females, and 54.1% were nurses, 152 HCWs (27.3%) had laboratory-confirmed influenza, shows two peaks in January and March 2019. VE for all types was 35.0% and rose to 42.0% after adjustment for HCWs age, gender, nationality, and job position, influenza A (H3N2) VE was 78.0%. H1N1 VE was 55.0% but no significant VE for type B was found.
Conclusion: Our VE estimates are in agreement with VE estimates published for that season. The use of quadrivalent vaccine with two stains of influenza B is recommended.
Introduction
Influenza disease is primarily caused by influenza A and B viruses that spread mainly by airborne droplets. The infection is repeatable and may have serious consequences.Citation1,Citation2 Although vaccination may provide some protection, annual revaccination is required.Citation3 Each influenza season researchers try to determine how well influenza vaccines work as a public health intervention. Influenza vaccine effectiveness depends on the personal characteristics of the vaccinated individual, specifically age and health status, as well as the closeness of matching between the vaccine and the prevalent strains of the virus. There is good consensus that VE is also determined by the infection history of an individual. Vaccination provides 40% to 60% protection during influenza seasons when the vaccine matches the season strains.Citation4
Influenza vaccines are produced at different times yearly; in the beginning of March in the southern hemisphere and of September in the northern hemisphere include influenza A (H3N2) and influenza A (H1N1), in addition to a strain of influenza B.Citation5 For influenza vaccines to be maximally effective, the vaccine viruses have to be antigenically matched to the influenza viruses circulating in humans.Citation6 The effectiveness of influenza vaccine is regularly assessed by the US, Centers for Disease Control and Prevention (CDC) during each season.
Health-Care Workers (HCWs) represent a varied mix of professional and assisting staff. They constitute one of the major groups exposed to airborne infections including influenza,Citation7 and as well could be a source for such infections.Citation8 Hence, they should be an important target of influenza vaccination.Citation9 However, despite the evidence of the positive impacts of influenza vaccination of HCWs and its cost-effectiveness,Citation10 still the vaccination coverage of this group is low.Citation11
Prince Sultan Medical Military City (PSMMC) is a Saudi military medical city, it is located in the Saudi capital, Riyadh founded in December 1978 with a capacity of 1134 beds for admission with 15903 staff of multiple nationalities (6375 physicians and nursing in addition to 9528 other HCWs). The services are provided to outpatients and inpatients in 23 medical and surgical specialties, the most prominent of which are cardiology, Oncology, Neurology, Urology and Hematology and Bone Marrow Transplant Unit. The aim of this study was to identify influenza VE in HCWs of the PSMMC.
Subjects and methods
Study design: This record-based study was carried out using the test-negative design, a variant of the case-control design. VE is estimated as (1-OR) x100%, where OR is the ratio of the odds of vaccination in HCWs with confirmed Influenza status (cases) with the odds of vaccination in those not confirmed as influenza positive (controls). Setting: The study was carried out at PSMMC in Riyadh. The Preventive Medicine Division (PMD) seasonal influenza vaccination database was used in addition to the clinical and laboratory data of HCWs attending the influenza and Contact Screening Clinic (FCSC)/or Emergency Room (ER) with ILI during the study period.
Study sample: The study included all the HCWs who attended the FCSC, or the ER in weekends, complaining of ILI and who had nasopharyngeal swabbing for detection of influenza-viral RNA by reverse transcriptase-polymerase chain reaction (RT-PCR) during the period from October 2018 up to September 2019.
The minimum sample size needed was 144 cases and 144 controls for the study based on an alpha error of 0.05, a power of 0.8, a detectable odds ratio of 0.6, and a vaccine coverage 50% among the source population.
Test-positive HCWs (n = 152) were identified as cases, while test-negative (n = 404) were identified as controls. Cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) were excluded. Also, all asymptomatic HCWs contact to confirmed positive influenza cases, who were screened for infection control purposes either positive or negative results, were excluded from the study.
Data collection: The necessary data were obtained from the PSMMC mainframe and PMD databases in the form of Excel file covered the relevant clinical and laboratory information of HCWs pertaining to vaccination status of participants included the date of influenza shot to ensured that HCWs developed protective antibodies and consider vaccinated and immune 2 weeks after receiving the influenza vaccine and nasopharyngeal swab results, in addition to basic personal characteristics as age, gender, job position, nationality.
Ethical considerations: The study protocol was approved by the PSMMC Ethics Review Board. The study was record-based and any identifying information were replaced with codes and the data were kept in secured files accessible only to the research team.
Statistical analysis: influenza VE in HCWs is calculated by the test-negative design as (1-OR) *100, and its 95% confidence interval was calculated according to Orenstein et al. (1985).Citation12 Analysis was carried out SPSS 21 software package. Descriptive statistics included means and SDs for continues variables, and frequencies and percentages for categorical ones. Chi-squared test was used for comparisons of categorical variables, with calculation of Odds Ratios (ORs) and their 95% confidence intervals. Logistic regression “Enter method” was used to calculate adjusted ORs and related VE for age, gender, nationality, and job with p=0.05.
Results
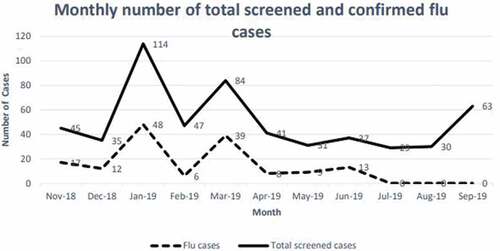
The study sample included 556 HCWs who attended to the FCSC complaining of ILI, 191 males (34.4%) and 365 females (65.6%), their mean age was = 34.3 years, SD = 7.86, Minimum age = 21, Maximum age = 63 and age range = 42. The professional job categories were nurses (54.1%), physicians (16.5%), technicians (15.8%) and admin workers (13.5%). The majority was Filipino (45.7%), Saudi (40.8%) and other nationalities (13.5%) (). Out of 556 HCWs who underwent nasopharyngeal PCR influenza screening swabs between October 2018 and September 2019, more than a half (58.3%) of these HCWs were vaccinated against influenza at that time, and a total of 152 cases had laboratory-confirmed influenza; influenza B (40.8%), influenza A H1N1 (38.2%) and influenza A H3N2 (21.1%)
Table 1. Relations between confirmed flu status and participants.’
There were two peaks in January and March 2019. Meanwhile, no confirmed influenza cases were reported from July to September 2019 ().
There were no statistically significant relations between confirmed influenza status and any of the HCWs’ personal characteristics. Nonetheless, there is a trend toward more positive cases among females (p = .099), and physicians in comparison with administrative jobs (p = .065) (). Results illustrated a statistical significant associations were revealed between HCWs vaccination and confirmed influenza status. This was noticed in type A (H3N2) (p < .001) and H1N1 (p = .034), as well as all-type influenza (p = .025).
In all these relations, higher vaccination coverage is shown among influenza negative HCWs ().VE was 78.0% (95% CI: 50.0–90.0%) for influenza A (H3N2), this VE was sustained after adjustment for HCWs age, gender, nationality, and job position. For all types, the VE was 35.0% and rose to 42.0% after adjustment. As regards influenza H1N1, the VE was 55.0% and statistically significant only after adjustment. Meanwhile, no significant VE could be revealed for type B ().
Table 2. Vaccine effectiveness
Table 3. Crude and adjusted OR and vaccine effectiveness
In stratified analysis, there was a tendency of increasing VE with increasing age until age group 40–49, and only in this age group it was statistically significant (p = .021), with a VE 73.19 (95% CI: 14.43–91.60). The VE did not show statistically significant differences by gender ().
Table 4. Vaccine effectiveness by age and gender
Discussion
Considering that the PSMMC recommends the annual mandatory seasonal influenza vaccination of all their HCWs, the study findings indicate a relatively low level of vaccination coverage (66.2%). All HCWs are requested by regulations to report to FCSC if they have ILI. As per PSMMC policy; the management of HCWs with ILI, screening and investigating the exposed HCWs to infectious diseases and the application of the preventive measures is the responsibility of FCSC. However, there is a good vaccine effectiveness for type A (H3N2) influenza vaccine, a lower effectiveness was observed regarding H1N1 type. Conversely, the vaccine has no significant effectiveness against type B influenza. The vaccination uptake in the current study is just above the mid-range of reported rates worldwide, which varies between <5% and >90%Citation13 in a study of the related challenges and proposed solutions.
In the present study sample of HCWs, around one-fourth had laboratory-confirmed influenza, with the highest peak during the month of March. This percentage is very close to that reported byCitation14 in a study in the United States during the 2018–19 season. The percentage of laboratory specimens testing positive for influenza virus ranged between 25.1% and 26.2%, compared to our rate of 27.3%. Moreover, these authors, and in agreement with our finding, noticed that the confirmed influenza cases peaked during the month of March.
Concerning the type of influenza, our results indicate higher rates of type B and H1N1, while type A was the lowest. The study findings differ than that reported by a study in the United StatesCitation15 that demonstrated that type B was the least detected among 3254 patients with ILI symptoms.
According to the present study results, the overall influenza VE for all types was 35.0%, and it increased to 42.0 after adjustment for age, gender, nationality, and job position. The figure is close to but slightly lower than the VE reported by Doyle et al. (2019)Citation16 where the overall VE turned to be 47% during the influenza season 2018–2019. However, their study had a number of limitations including being an interim report, in addition to influenza diagnosis based on self-reporting in some of the study sites. Nonetheless, their research design used is similar to the one used in the current study. In this respect, Mameli et al.Citation17 clarified that VE may vary by season and location, and is influenced by a number of factors such as age and vaccination history. Moreover, the surprisingly low VE of the influenza vaccines is often attributed to the lack of antigenic matching between the vaccine and the virus strains.Citation18 However, even in case of high matching, the influenza VE was reported to be unexpectedly low.Citation15
Concerning the effect of age and gender, the current study did not show any statistically significant differences in VE. However, the stratified analysis by age revealed a trend of better VE with increasing age with a statistical significance at the third age category (40–49 years). Meanwhile, the low VE with lack of statistical significance at the age group 50+ might be due to the very small number in this age group. Nonetheless, a similar trend of VE with age was reported by Flannery et al. Citation4 with a drop by age 50 years and older.
The current study design is more efficient than cohort studies in estimates influenza VE and it minimizes the confounding effects in health-care seeking between vaccinated and unvaccinated HCWs. The study limitation arises from self-reported of the ILI manifestations by HCWs and occasionally due to data limitations. Also results could not be generalized to the population.
Conclusion and recommendations
The results reveal a suboptimal influenza vaccination uptake among the HCWs in the study setting, but the vaccine effectiveness is close to universal figures. More endeavors are needed to boost influenza vaccination rates through integrative approaches and early September campaigns considering the related challenges that may underlie resistance or reluctance among HCWs. The use of quadrivalent vaccine with two types of influenza B vaccine is recommended to overcome the low response against influenza B.
Author contributions
Project Administration, A.A.A and M.S; Conceptualization, A.A.A and M.S; Methodology, Data curation M.S; N.H.H; S.F.A and A.L.A.; Supervision A.A.A and M.S; Validation a.L.a; N.H.H; Formal analysis, M.S.; A.L.A; K.H.S and C.M.; Original draft preparation, M.S., N.H.H and C.H.; Review and editing, M.S, and A.A.A.
Disclosure of potential conflicts of interest
The authors certify that there is no conflict of interest with any financial organization related to the research topic discussed in the current manuscript.
Acknowledgments
The authors are grateful to the Preventive Medicine Division staff and to the Family and Community Medicine Administration for their great support.
Additional information
Funding
References
- Gavigan P, McCullers JA. Influenza: annual seasonal severity. Curr Opin Pediatr. 2019;31(1):112–18. doi:10.1097/MOP.0000000000000712.
- Xie Z, Lin Y, Chen Y. Analysis of clinical characteristics of severe and critically ill influenza a (H1N1). Zhonghua wei zhong bing ji jiu yi xue. 2019;31(9):1154–57. doi:10.3760/cma.j..2095-4352.2019.09.019.
- Tomčíková K, Varečková E. Different mechanisms of the protection against influenza a infection mediated by broadly reactive HA2-specific antibodies. Acta Virol. 2019;63(4):347. doi:10.4149/av_2019_408.
- Flannery B, Kondor RJ, Chung JR, Gaglani M, Reis M, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, et al. Spread of antigenically drifted influenza a(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 Season. J Infect Dis. 2020 Jan 1;221(1):8–15. doi:10.1093/infdis/jiz543.
- Dwyer D, Barr I, Hurt A, Kelso A, Reading P, Sullivan S, Buchy P, Yu H, Zheng J, Shu Y. Seasonal influenza vaccine policies, recommendations and use in the World Health Organization’s Western Pacific Region. Western Pac Surveill Response J. 2013;4:51–59.
- Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev. 2013;26(3):476–92. doi:10.1128/CMR.00097-12.
- Dini G, Toletone a, Sticchi L, Orsi a, Bragazzi NL, Durando P. Influenza vaccination in healthcare workers: a comprehensive critical appraisal of the literature. Hum Vaccin Immunother. 2018;14(3):772–89. doi:10.1080/21645515.2017.1348442.
- Haviari S, Bénet T, Saadatian-Elahi M, André P, Loulergue P, Vanhems P. Vaccination of healthcare workers: a review. Hum Vaccin Immunother. 2015;11(11):2522–37. doi:10.1080/21645515.2015.1082014.
- Maltezou HC, Theodoridou K, Ledda C, Rapisarda V. Vaccination of healthcare personnel: time to rethink the current situation in Europe. Future Microbiol. 2019;14(9s):5–8. doi:10.2217/fmb-2018-0262.
- Imai C, Toizumi M, Hall L, Lambert S, Halton K, Merollini K. a systematic review and meta-analysis of the direct epidemiological and economic effects of seasonal influenza vaccination on healthcare workers. PLoS One. 2018;13(6):e0198685. doi:10.1371/journal.pone.0198685.
- Genovese C, Picerno I, Trimarchi G, Cannavò G, Egitto G, Cosenza B, Merlina V, Icardi G, Panatto D, Amicizia D. Vaccination coverage in healthcare workers: a multicenter cross-sectional study in Italy. J Prev Med Hyg. 2019 Mar 29;60(1):E12–E17. doi:10.15167/2421-4248/jpmh2019.60.1.1097. PMID: 31041405; PMCID: PMC6477557.
- Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, Bart KJ, Sirotkin B. Field evaluation of vaccine efficacy. Bull World Health Organ. 1985;63:1055–68.
- To K, Lai a, Lee K, Koh D, Lee S. Increasing the coverage of influenza vaccination in healthcare workers: review of challenges and solutions. J Hosp Infect. 2016;94(2):133–42. doi:10.1016/j.jhin.2016.07.003.
- Xu X, Blanton L, Elal AIA, Alabi N, Barnes J, Biggerstaff M, Brammer L, Budd AP, Burns E, Cummings CN, et al. Update: influenza activity in the United States during the 2018–19 season and composition of the 2019–20 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2019;68(24):544. doi:10.15585/mmwr.mm6824a3.
- CDC. Vaccine effectiveness: how well do the flu vaccines work? 2020 [accessed 2020 May 19]. https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm.
- Doyle JD, Chung JR, Kim SS, Gaglani M, Raiyani C, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, et al. Interim estimates of 2018–19 seasonal influenza vaccine effectiveness—United States, February 2019. MMWR Morb Mortal Wkly Rep. 2019;68(6):135. doi:10.15585/mmwr.mm6806a2.
- Mameli C, Cocchi I, Fumagalli M, Zuccotti G. Influenza vaccination: effectiveness, indications and limits in the pediatric population. Front Pediatr. 2019;7:317. doi:10.3389/fped.2019.00317.
- Lewnard JA, Cobey S. Immune history and influenza vaccine effectiveness. Vaccines. 2018;6(2):28. doi:10.3390/vaccines6020028.