ABSTRACT
We report the clinical characteristics of two adult patients, presenting with a typical erythematous rash consistent with rubella disease after MMR vaccination. Both patients had an uncomplicated clinical course and recovered uneventfully.
One patient was confirmed to have vaccine-associated rubella via sequencing of virus isolated in viral culture. The other patient had a pharyngeal swab positive for rubella virus PCR, with sequencing matching the vaccine strain.
There are few reports of clinical disease from rubella vaccine-strains in the literature. Previous authors have reported severe disseminated vaccine-associated rubella in both immunodeficient and immunocompetent patients.
Further study is required to ascertain the incidence, risk factors, and clinical characteristics of this condition; as well as investigate the extent of horizontal transmission to guide infection control recommendations.
Background
Rubella results from infection by the rubella virus. Clinical disease is self-limiting, typically presenting with a generalized exanthem, fever, arthralgia, and lymphadenopathy. If it occurs early in pregnancy, congenital rubella syndrome may result with complications of deafness, mental retardation and ocular pathology.1 Despite the availability of an effective vaccine, often given in routine childhood immunization schedules as part of the measles, mumps, and rubella (MMR) vaccine, rubella still presents a significant health burden when there is suboptimal immunization.Citation2 Screening for immunity is recommended during pregnancy, and if nonimmune, women are advised to avoid infected persons and follow up with vaccination post-partum.Citation3
The use of rubella vaccine, a live-attenuated vaccine, has resulted in a dramatic reduction in the incidence of rubella worldwide, with complete eradication in countries (e.g. United States, Northern Europe) where widely used.Citation4 Large post-licensure studies have demonstrated safety and immunogenicity of MMR in multiple populations.Citation5 Adverse events include arthralgia and arthritis but reports of clinical disease from vaccine-strains are rare in the literature.Citation6 We describe the clinical and microbiologic characteristics of two patients with vaccine-associated rubella, adding to the body of knowledge on this entity.
Case A
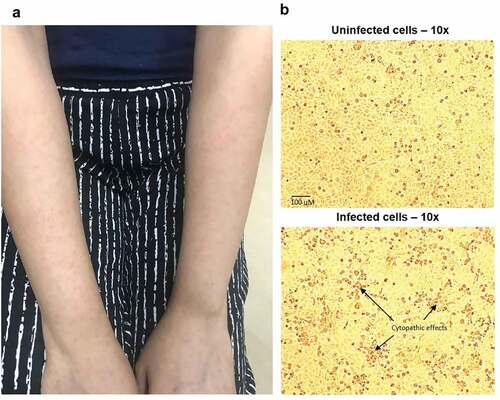
Patient A, a 31-year-old woman from the Philippines, had been working in Singapore for four years as a nurse in a dialysis center. She had no history of chronic illnesses or immunosuppression, and no previous receipt of rubella vaccination. She received the first MMR dose (Priorix®, GlaxoSmithKline, batch number AMJRD474AA) one-month post-partum as she was seronegative for rubella during antenatal screening. Six days after, she developed sore throat and myalgia, followed by a non-pruritic rash 13 days after vaccination (day seven of illness). The rash started over her upper limbs (), progressing to involve her face, trunk, and back. There was no fever or constitutional symptoms. Physical examination was unremarkable apart from the rash. She reported no sick contacts and had not yet returned to work. Her clinical course remained uncomplicated and rash resolved within one week.
Figure 1. A: Typical erythematous maculopapular rash from Case A; B: Top panel shows uninfected Vero/hSLAM cells;Citation7 bottom panel shows cytopathic effects (CPE) of rubella virus from Case A. Images at 10x magnification
Further investigations (on day nine of illness) ruled out syphilis (with rapid plasma reagin and enzyme-linked immunosorbent assay for syphilis IgG), Zika virus (with polymerase chain reaction (PCR) testing), measles (pharyngeal swab for PCR), dengue (with IgM, IgG, and NS-1 antigen assay), and human immunodeficiency virus (with 4th-generation antigen and antibody ELISA). Complete blood count and basic biochemistry were normal.
Rubella IgM was non-reactive and IgG equivocal (7.6 IU/mL; <5.0 IU/mL = negative, 5.0–9.9 IU/mL = equivocal, ≥10.0 IU/mL = positive). Pharyngeal swab for rubella PCR was negative. However, given the concern for vaccine-associated rubella, a pharyngeal swab was also sent for viral culture. Rubella virus was isolated from cell cultures after two passages (). In addition, another vial of the vaccine of the same batch that was administered to the patient was obtained from the clinic she visited.
The contents of the vaccine and the patient’s rubella virus isolate from cell culture were sequenced using an in-house method developed for the World Health Organization (WHO) by the Rubella Virus Team at US Centers for Disease Control and Prevention (US CDC). Briefly, the CDC Rubella Diagnostic RT-PCR Kit was used to detect rubella virus, followed by two further RT-PCR reactions using the CDC Rubella Virus Genotyping Kit to obtain overlapping PCR amplicons for sequence analyses and genotyping. The MEGA v5.1 programme was used to view, edit and align sequences, and generate a phylogenetic tree to determine the genotype.Citation8 The sequences obtained from both the vaccine strain and the patient’s rubella virus isolate were identical, matching the corresponding E1 gene region for the Wistar RA 27/3 strain (WHO name: RVi/Pennsylvania.USA/0.64/1a strain), which is the most commonly used rubella vaccine strain worldwide.Citation6,Citation9
Rubella IgG was repeated two months post-vaccination, demonstrating an adequate serologic response (IgG level 132.1 IU/mL). Her baseline measles IgG was reactive, consistent with previous immunity to measles. We thus opted not to give her further MMR boosters.
Case B
Patient B was a 61-year-old Singaporean man with no previous medical history. He had no prior documented MMR vaccine and was planning to travel to North America, and was offered MMR vaccination. He received his first MMR dose (Priorix®, GlaxoSmithKline, batch number A69CE757A) together with inactivated standard dose quadrivalent influenza vaccine. Thirteen days post-vaccination, he developed a maculopapular rash over his chest, which progressed to his trunk and back by the next day. There was no associated fever or respiratory symptoms. Throat swab was tested positive for rubella and negative for measles by PCR. He reported no sick contacts and was intending to board a flight on day six of his illness. As genotyping results would not have been available at the time of his intended travel, he was advised to defer travel until after day eight of illness, in the event this was wild-type rubella.Citation10
As he was seated in a crowded clinic area when he sought medical attention, we conducted contact tracing for 61 patients who were in the waiting area around the same time and who may have been exposed. No patient was pregnant; however, one was immunocompromised. Rubella serology for this contact showed that she was immune to rubella, hence no further action was taken.
Rubella genotype was determined according to WHO recommendations using a minimum of 739 nucleotides (genomic position 8731 to 9469) for routine molecular epidemiological analysis.Citation11 Genotyping found that the patient’s rubella virus was vaccine-associated strain. Virus culture was attempted from the throat swab but was not successful. His clinical course was uncomplicated, and his rash resolved completely within one week.
Discussion
Measles and rubella vaccinations were included in the national childhood immunization schedule in 1975 in Singapore. However, there remain susceptible segments of the population, such as foreign immigrants (e.g. case A) or individuals born before 1975 (e.g. case B). With outbreaks of measles and rubella occurring in many countries, individuals are screened and vaccinated when high-risk exposures are expected, including travel.
Nasopharyngeal secretion of live attenuated rubella virus is known to occur in many individuals in the immediate period (seven to 28 days) post-vaccination. Aoki et al. reported incidental isolation of vaccine-associated rubella and measles viruses in four patients as part of a surveillance study of acute respiratory infections in children, with the period of vaccination to specimen collection lasting 26 days in one case.Citation12 Rubella virus has also been demonstrated to be shed in breast milk, up to 34 days post-immunization, with subsequent transmission and nasopharyngeal isolation from breast-fed infants.Citation13
However, there are few reports of clinical disease from vaccine-associated rubella. Bayer, et al. reported a pediatric patient with disseminated vaccine-associated varicella and rubella infection as a first presentation of severe combined immunodeficiency.Citation14 Gualberto, et al. reported a case of rubella complicated by fatal encephalitis in a previously healthy Brazilian man, with successful virus isolation from cerebrospinal fluid, brain, and other clinical samples and subsequent sequencing confirming RA 27/3 vaccine strain.Citation15
An older case series described six patients with persistent arthritis and neurologic sequelae (carpal tunnel syndrome, paresthesias) after post-partum rubella vaccination. Interestingly, chronic rubella viremia was detected in peripheral blood mononuclear cells in five patients up to six years post-vaccination.Citation16 These patients did not have typical clinical presentations of rubella in terms of fever and exanthem, and it is unclear if these symptoms are related to direct viral invasion or a separate immune-mediated mechanism.
The two adult patients we presented had an uncomplicated clinical course, presenting only with rash and mild systemic symptoms. This differs from the various atypical presentations previously described.
Several unanswered questions remain regarding vaccine-associated rubella. Firstly, the reasons why certain individuals develop clinical disease with vaccine-associated rubella are unknown. The two patients we report were healthy with no history of immunosuppression, as was the Brazilian patient described by Gualberto, and were from sub-groups where vaccination is recommended (post-partum and pre-travel). Whether vaccine factors play a role in the development of vaccine-associated rubella disease is also not known. All the cases of vaccine-associated rubella described in the literature, as in our two patients, were vaccinated with the RA27/3 strain. However, we cannot conclude whether this strain has a greater risk of causing vaccine-associated disease, given the small number of reported cases.
Secondly, the incidence of vaccine-associated rubella is unknown. As the majority of clinical disease with rubella has a benign course, patients with mild symptoms after vaccination may not seek medical attention, and thus most cases of vaccine-associated rubella would be undiagnosed. Larger studies are needed to establish the incidence, risk factors, and clinical characteristics of this condition.
Lastly, the recommended infection control approach to vaccine-associated rubella is unclear. Horizontal transmission is possible given the presence of nasopharyngeal carriage and excretion of vaccine-strain viral particles. However, there are few reports in the literature, with only one previous study describing potential transmission of vaccine-associated virus to susceptible contacts, though this was prior to the advent of genomics and vaccine strain-typing could not be performed.Citation17 There is also a report of a 12-year-old stem cell transplant recipient who passed away from fulminant hepatic failure, with autopsy demonstrating disseminated vaccine-strain rubella (RA27/3).Citation18 This patient last received MMR vaccination eight years prior, and hence was deemed to have acquired vaccine-strain rubella via horizontal transmission from a newly-vaccinated visitor or healthcare worker, though no source patient was identified.
Further study is needed to investigate the extent of onward transmission in vaccine-associated rubella. This is especially important in this age of falling vaccination coverage globally with increasing numbers of susceptible individuals in the general population. This current report and previous reports notwithstanding, the risk to benefit ratio for rubella vaccination in all age groups is overwhelmingly positive, and universal childhood vaccination as well as catch-up vaccination in susceptible at-risk groups should continue to be recommended.
In conclusion, we report two interesting cases of vaccine-associated rubella post-vaccination, confirmed via genotyping of virus matching the vaccine strain. More studies are needed to understand the risk factors and management of this clinical entity.
Author contributions
SV, SPS, and KM managed the patients and provided the clinical data. LC, JCCW, KYP, and KPC conducted the laboratory methods and genetic sequencing. JCCW, KYP, and KPC conducted the viral culture and provided the colour figure showing cytopathic effect. PLL provided supervision and advice. SWXO and SV drafted the manuscript. All authors reviewed and approved the final manuscript.
Disclosure of potential conflicts of interest
No conflicts of interest declared.
Acknowledgments
Written informed consent from Patient A was obtained for photo publication.
Additional information
Funding
References
- Lee JY, Bowden DS. Rubella virus replication and links to teratogenicity. Clin Microbiol Rev. 2000 Oct;13(4):571–87. doi:10.1128/CMR.13.4.571.
- Banatvala JE, Brown DW. Rubella. Lancet. 2004 Apr 3;363(9415):1127–37. doi:10.1016/S0140-6736(04)15897-2.
- Bouthry E, Picone O, Hamdi G, Grangeot-Keros L, Ayoubi J-M, Vauloup-Fellous C. Rubella and pregnancy: diagnosis, management and outcomes. Prenat Diagn. 2014 Dec;34(13):1246–53. doi:10.1002/pd.4467.
- Plotkin SA. The history of rubella and rubella vaccination leading to elimination. Clin Infect Dis. 2006 Nov 1;43(Suppl 3):S164–8. doi:10.1086/505950.
- Lievano F, Galea SA, Thornton M, Wiedmann RT, Manoff SB, Tran TN, Amin MA, Seminack MM, Vagie KA, Dana A et al. Measles, mumps, and rubella virus vaccine (M-M-RII): a review of 32 years of clinical and postmarketing experience. Vaccine. 2012 Nov 6;30(48):6918–26. doi:10.1016/j.vaccine.2012.08.057.
- World Health Organization. Rubella vaccines: WHO position paper. Wkly Epidemiol Rec. 2011 Jul 15;86(29):301–16.
- Ono N, Tatsuo H, Hidaka Y, Aoki T, Minagawa H, Yanagi Y. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J Virol. 2001 May;75(9):4399–401. doi:10.1128/JVI.75.9.4399-4401.2001.
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011 Oct;28(10):2731–39. doi:10.1093/molbev/msr121.
- Rubella virus nomenclature update 2013. Wkly Epidemiol Rec. 2013 Aug 9;88(32):337–43.
- Rubella: Centers for Disease Control and Prevention; 2017 [updated 15th September 2017]. Available from: https://www.cdc.gov/rubella/hcp.html
- Manual for the laboratory diagnosis of measles and rubella virus infection: World Health Organization; 2007. [accessed 2020 Mar 10]. Available from: https://www.who.int/ihr/elibrary/manual_diagn_lab_mea_rub_en.pdf
- Aoki Y, Matoba Y, Tanaka S, Yahagi K, Ito S, Yoshida H, Itagaki T, Mizuta K. Chance isolation of non-pathogenic vaccine-derived measles and Rubella viruses from children with acute respiratory infections. Jpn J Infect Dis. 2016 Jul 22;69(4):350–51. doi:10.7883/yoken.JJID.2015.567.
- Losonsky GA, Fishaut JM, Strussenberg J, Ogra PL. Effect of immunization against rubella on lactation products. II. Maternal-neonatal interactions. J Infect Dis. 1982 May, 145(5): 661–66.
- Bayer DK, Martinez CA, Sorte HS, Forbes LR, Demmler-Harrison GJ, Hanson IC, Pearson NM, Noroski LM, Zaki SR, Bellini WJ, et al. Vaccine-associated varicella and rubella infections in severe combined immunodeficiency with isolated CD4 lymphocytopenia and mutations in IL7R detected by tandem whole exome sequencing and chromosomal microarray. Clin Exp Immunol. 2014 Dec;178(3):459–69. doi:10.1111/cei.12421.
- Gualberto FA, de Oliveira MI, Alves VA, Kanamura CT, Rosemberg S, Sato HK, Arantes BAF, Curti SP, Figueiredo CA. Fulminant encephalitis associated with a vaccine strain of rubella virus. J Clin Virol. 2013 Dec;58(4):737–40. doi:10.1016/j.jcv.2013.10.016.
- Tingle AJ, Chantler JK, Pot KH, Paty DW, Ford DK. Postpartum rubella immunization: association with development of prolonged arthritis, neurological sequelae, and chronic rubella viremia. J Infect Dis. 1985 Sep;152(3):606–12. doi:10.1093/infdis/152.3.606.
- Wilkins J, Leedom JM, Salvatore MA, Portnoy B. Transmission of rubella vaccine virus from vaccinees to contacts. Calif Med. 1971 Nov;115(5):16–22.
- Gonzalez Vicent M, Molina Angulo B, Echevarria Mayo JE, Diaz Perez MA. Vaccine Rubella: a rare cause of post-transplant hematopoietic death, but a major public health problem. Open Forum Infect Dis. 2018 Oct;5(10):ofy235. doi:10.1093/ofid/ofy235.
