ABSTRACT
Rotavirus (RV) is one of the leading causes of severe childhood gastroenteritis in children <5 years of age. Several countries have successfully implemented vaccination against RV disease; however, hesitancy to include RV vaccination in the national immunization program exists and relates, among other reasons, to the results of international post-licensure studies of RV vaccines that established an increased risk of intussusception (IS) in infants following immunization. IS is one of the major causes of bowel obstruction in infants between 4 and 10 months of age. Some studies have investigated the etiology of IS, including the role of natural RV infection and available evidence suggests that RV disease may be an independent risk factor for IS. In this regard, the benefit-risk profile of RV vaccination, which is recognized as positive, could potentially turn out to be even more favorable in preventing IS cases triggered by RV disease. However, further research is prompted to quantify the IS risk attributable to RV disease.
Intussusception (IS) is a common cause of bowel obstruction in children, with the peak age of onset between 4 and 10 months of age.Citation1 It occurs when a proximal segment of the gastrointestinal tract telescopes in the immediately adjacent segment.Citation2 While this acute, serious medical condition is relatively rare, it can result in blood vessel compression and intestinal ischemia, necrosis, and perforation. If not timely recognized or left untreated, IS can be fatal.Citation2
The incidence rates of IS hospital admission vary largely across geographical regions.Citation3 Between 2002 and 2018, the median (range) annual incidence of IS hospital admissions per 100 000 ranged from 34 (13–56) in Africa to 90 (9–380) in the Western Pacific region for children <1 year of age.Citation3 While pediatric IS is usually idiopathic, predisposing factors, such as infections (including adenoviruses and rotavirus [RV]) and anatomical variations, have been identified.Citation4
RV is one of the leading causes of severe childhood gastroenteritis in children <5 years of age, accounting for substantial morbidity globally and high mortality in resource-limited countries.Citation5 Several countries have successfully implemented vaccination against RV disease, demonstrating reduced cases of severe diarrhea and RV disease requiring hospitalizationCitation6,Citation7 but also reduced mortality rates.Citation6 However, vaccine uptake remains low in many regions, partially due to low priority ascribed to RV gastroenteritis prevention and cost constraints.Citation8 Furthermore, hesitancy to implement RV vaccination in the national immunization program relates to the evidence from post-licensure studies that established an increased risk of IS in infants shortly (during the 7-day period) following RV vaccine administration.Citation9–11
The first developed RV vaccine, tetravalent rhesus-human reassortant (Rotashield; Wyeth Laboratories) was withdrawn post-licensure in 1999, within a year following introduction, due to its association with IS (relative risk of IS was 58.9 [95% confidence interval: 31.7–109.6] post-dose 1 and 11.0 [4.1–29.5] post-dose 2).Citation10,Citation12 Following thorough pre-licensure safety evaluation with regard to IS,Citation13,Citation14 two second-generation RV vaccines (HRV; Rotarix, GSK, and HBRV; RotaTeq, Merck & Co., Inc.), have been recommended since 2009 by the World Health Organization (WHO) for worldwide use in routine RV vaccination programs in infants. Albeit lower than for Rotashield, accumulated evidence suggests an increased risk of IS during the 7-day period post-dose 1 (5.4 [3.9–7.4, 3 studies] for HRV and 5.5 [3.3–9.3, 3 studies] for HBRV) and to a lesser extent during the 7-day period post-dose 2 (1.8 [1.3–2.5, 4 studies] for HRV and 1.7 [1.1–2.6, 3 studies] for HBRV), in a meta-analysis of 5 post-licensure studies.Citation10 These data suggest a class effect for both RV vaccines in terms of IS risk after immunization. Nevertheless, the WHO Global Advisory Committee on Vaccine Safety emphasized that the benefit-risk profile of both licensed RV vaccines remains favorable, with the benefits outweighing the risk of IS.Citation15
Of note, the risk of IS attributable to RV vaccination depends on age, and recent evidence suggests that the absolute risk of IS is only slightly increased by vaccination (increased risk attributable to vaccination was 1.7 for HRV and HBRV) when the vaccines are administered within the recommended time window of <3 months.Citation16–18 An active surveillance study conducted in seven African countries reported a relative risk of 0.25 (<0.001–1.16) and 0.76 (0.16–1.87) during 1 week post-dose 1 and post-dose 2 of HRV, respectively, when administered in children <3 months of age.Citation17 More recently, WHO Global Advisory Committee on Vaccine Safety (GACVS) reviewed data on the safety of HBRV in five sub-Saharan African countries and of neonatal human rotavirus vaccine (nHRV; Rotavac) in parts of India, reporting a non-significant increase in IS risk post-dose 1 of HBRV in sub-Saharan Africa and of nHRV in India.Citation19 The risk of IS within 1 week after vaccination was 4.11 (0.79–11.52) post-dose 1, 0 post-dose 2, and 0.86 (0.28–1.92) post-dose 3 of HBRV.Citation19 For nHRV using self-controlled case-series (SCCS) method on smaller sample size, IRR for the first 7 days post-dose 1, 2 and 3 compared with the period from day 28 to 1 year of age was 0.83, 0.86, and 1.65, respectively. These data were not statistically significant, suggesting no association between nHRV vaccination and IS. Similarly, no significant difference was seen for the 8–21-day risk window after vaccination. Since the limited sample size comparison of products in the same risk window is not accurate, more data are needed to guide safety evaluation of new rotavirus vaccines and across their use in national immunization programs.Citation19 These data prompt pediatricians to counsel parents to monitor vaccinated children and to seek medical advice in case signs or symptoms suggestive of IS emerge.
Considering the real-world evidence accumulated since the launch of the two globally available RV vaccines (HRV and HBRV) and the high vaccine coverage in some regions, the increased risk of IS following RV vaccination does not seem to translate into an overall long-term increase of IS in countries with RV vaccination included in their national immunization programs. In Australia, where both Rotarix and RotaTeq are available, one of the first post-marketing surveillance studies for IS following vaccination revealed no overall increase in IS following receipt of RV vaccine although some evidence of an elevated risk following the first dose was observed. The authors suggest that any increased risk after the first dose is compensated by a reduced risk after later doses.Citation20 To unravel whether the observed association between RV vaccination and IS translates into a higher rate of IS-related hospitalizations, Tate et al.Citation21 examined retrospectively pre-(2000‒2005) and post-RV vaccination (2007‒2013) IS rates in the United States. No change in IS hospitalization rates was observed among children <12 months of age, but the rate in children 8 to 11 weeks was significantly elevated in all post-vaccination years, except in 2011 and 2013 ().
Table 1. Overview of calculated IS hospitalization rates recorded before and after the introduction of annotated rotavirus vaccinations into different immunization programs. All data are derived from Retrospective Analysis Studies
The observation of no sustained population-level change in overall IS hospitalization rates is in line with previous studies conducted in the US.Citation23,Citation27,Citation28 These studies reported no consistent change in IS hospitalization rates after vaccination when compared to pre-vaccination era levels despite increasing RV vaccine coverage in US infants over the period under evaluation ().Citation23,Citation27,Citation28 More specifically, Yen et al.Citation23 noted an increase in IS hospitalization rates among infants aged 8–11 weeks (to whom most first doses of RV are given) but also observed that IS hospitalization rates among older infants tended to be lower in post-vaccination years compared with pre-vaccination years, although these differences were not statistically significant (). As for the study in Australia, the authors expressed the need to confirm whether later in infancy IS hospitalization rates are decreased in vaccinated infants and whether this decline could offset, at population level, any short-term increase in IS after vaccination.
A retrospective study conducted in Canada showed no increase in the incidence of IS-related hospital admissions after the introduction of routine RV immunization programs.Citation24 Besides, despite a much higher risk of IS with Rotashield when compared to the currently available RV vaccines, ecological assessments of Rotashield did not reveal enhanced infant IS admissions during the Rotashield era.Citation22 The authors reported that the overall risk of Rotashield-related IS hospital admissions was considerably lower than previous estimates based on the immediate post-vaccine period.Citation22 Moreover, a recent longitudinal cohort study of commercially insured US children provides supporting evidence for an overall reduced risk of IS in RV-vaccinated children. In this study, a non-significant decrease in IS was found in fully RV-vaccinated children followed up to the age of 2 years.Citation29 Similar results were obtained in a study conducted in Taiwan, where mean IS hospitalization rates were lower during the post-vaccination period for children aged <12 months with the greatest decline among children aged 25–34 weeks, although the difference with pre-vaccination period did not reach statistical significance ().Citation25 This observation is in agreement with a recent report from South Korea documenting a decline in the incidence of IS in infants after the introduction of the RV vaccine ().Citation26
Considering the increased risk of IS following RV vaccination in birth cohorts, an impact on long-term IS incidence rates in vaccinated children would be expected over successive years. The reasons for the absence of such observations are likely to be multifactorial; however, the findings by Willame et al.Citation30 in the current edition of this journal may offer a partial explanation. Given the observed association between RV vaccination and IS, the authors explored the role of natural RV infection in the development of IS in infants below 1 year of age through a retrospective, self-controlled case-series analysis. They observed a positive association between RV gastroenteritis and IS using US insurance claims data. An increased risk of IS after RV gastroenteritis was observed in the main analysis, where the calculated risk factor was 79.6 (95% CI: 38.6–164.4) in the 7-day period and 25.5 (13.2–49.2) in the 21-day period. Notably, also the sensitivity analysis showed an increased risk of IS after fracture during the same periods (6.1 [3.0–12.7] and 2.8 [1.5–5.4]), which was an unrelated event used to evaluate the quality of the claims data to address this research question. To refine the data and to adjust for potential confounding detected in the main analysis, a post hoc analysis was performed, which still suggested an association between RV gastroenteritis and IS, but not between fracture and IS (see ref. Willame et al.Citation30). Due to limitations inherent to the study (e.g. diagnosis uncertainty, true disease onset possibly misclassified, potential unknown bias, and confounding), the risk could not be robustly quantified but is consistent with previous reports suggesting that RV gastroenteritis could constitute a risk factor for IS.Citation31–34
The question remains how to reconcile results from post-marketing studies (showing an increased risk of IS following RV vaccination, as reflected in the labels of the two RV vaccines) with long-term data on IS incidence rates in countries with implemented RV vaccination. It can be argued that while RV vaccination has been associated with an increased risk of IS, disease prevention through vaccination may have averted a substantial number of IS cases caused by natural RV infection that would have occurred later in childhood.
The study by Willame et al.Citation30 sheds new light on the risk of IS due to naturally occurring RV and on the implication of these findings for RV vaccination programs. Indeed, the pivotal question is centered around the quantification of the IS risk attributed to RV disease, and how to weigh it against the increase of IS following RV vaccination, in a situation of optimal vaccine coverage and early completion of the vaccination schedule. In this regard, the benefit-risk profile of RV vaccination, which is recognized as positive, could potentially turn out to be even more favorable in preventing IS cases triggered by RV disease. Area-specific factors, such as varying IS background incidence rates, could critically impact the benefit-risk assessment, with most likely tangible effects in areas experiencing the highest background rates of IS (). In this context, one may anticipate that this effect of RV vaccination would add to the multiple indirect effects attributed to RV vaccination, beyond reducing RV gastroenteritis-related mortality and morbidity (e.g. community protection through herd effect, reduction of RV nosocomial infections, reduced incidence of childhood seizures).Citation37
Figure 1. Schematic presentation of IS incidence rates occurring in the general pediatric population (in concordance with studies such as Tate et al.Citation35 and Tai et al.Citation36) and hypothetical IS incidence rates in children vaccinated against RV disease. IS, intussusception; RV, rotavirus; RV GE, rotavirus gastroenteritis
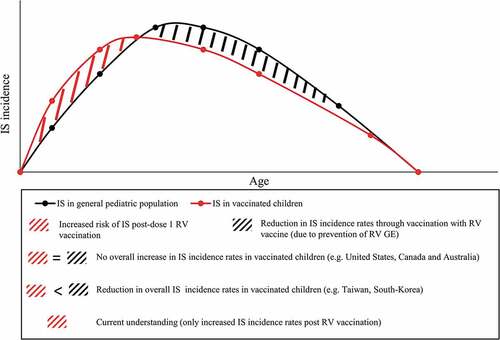
This recent new perspective on RV vaccination, which also includes its public health impact, might be critical in motivating health-care professionals and caregivers to encourage vaccination in general and in convincing policy-makers to further implement early RV vaccination into national immunization program. To support those assumptions, which should still be taken with caution, future research to quantitatively assess the IS risk attributed to RV disease is prompted, as well as generation of disease surveillance data, a fundamental aspect of the continuous evaluation of IS risk in vaccinated pediatric populations.
Disclosure of potential conflicts of interest
VV, PP, and BB are employed by and hold shares in the GSK group of companies.
Trademark statements
Rotarix is a trademark owned by the GSK group of companies. RotaTeq is a registered trademark of Merck&Co., Inc. Rotavac is a trademark of Bharat Biotech.
Acknowledgments
The Authors acknowledge Catherine Cohet, Corinne Willame, Emmanuel Aris, Leentje Moerman and Tina Singh for their critical review of the manuscript. The authors also thank the Modis platform for editorial assistance and manuscript coordination, on behalf of GSK. Anne-Theres Henze provided medical writing support and Emmanuelle Ghys coordinated the manuscript development and editorial support.
Additional information
Funding
References
- World Health Organization. Acute intussusception in infants and children. Incidence, clinical presentation and management: A global perspective. Geneva, Switzerland: World health organization. Document who/v & b/02.19.1–98; 2019. Accessed April. Available from https://vaccine-safety-training.org/tl_files/vs/pdf/acute-intussusception-infants-children.pdf.
- Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, Hansen J, Murphy TM, Music S, Niu M, Varricchio F, et al. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22;(5-6(5–6):569–74. doi:10.1016/j.vaccine.2003.09.016.
- Clark AD, Hasso-Agopsowicz M, Kraus MW, Stockdale LK, Sanderson CFB, Parashar UD, Tate JE. Update on the global epidemiology of intussusception: A systematic review of incidence rates, age distributions and case-fatality ratios among children aged <5 years, before the introduction of rotavirus vaccination. Int J Epidemiol. 2019. doi:10.1093/ije/dyz028.
- Marsicovetere P, Ivatury SJ, White B, Holubar SD. Intestinal intussusception: etiology, diagnosis, and treatment. Clin Colon Rectal Surg. 2017;30(1):30–39. doi:10.1055/s-0036-1593429.
- Tate JE, Burton AH, C B-P, Parashar UD. World health organization-coordinated global rotavirus surveillance N. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Clin Infect Dis. 2016;62(Suppl 2):S96–S105. doi:10.1093/cid/civ1013.
- Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, Flannery B, Esparza-Aguilar M, Montenegro Renoiner EI, Luna-Cruz ME, Sato HK, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364(24):2283–92. doi:10.1056/NEJMoa1012952.
- Molto Y, Cortes JE, De Oliveira LH, Mike A, Solis I, Suman O, Coronado L, Patel MM, Parashar UD, Cortese MM. Reduction of diarrhea-associated hospitalizations among children aged < 5 years in panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J. 2011;30(1 Suppl):S16–20. doi:10.1097/INF.0b013e3181fefc68.
- Poelaert D, Pereira P, Gardner R, Standaert B, Benninghoff B. A review of recommendations for rotavirus vaccination in europe: arguments for change. Vaccine. 2018;36(17):2243–53. doi:10.1016/j.vaccine.2018.02.080.
- Kassim P, Eslick GD. Risk of intussusception following rotavirus vaccination: an evidence based meta-analysis of cohort and case-control studies. Vaccine. 2017;35(33):4276–86. doi:10.1016/j.vaccine.2017.05.064.
- Rosillon D, Buyse H, Friedland LR, Ng SP, Velazquez FR, Breuer T. Risk of intussusception after rotavirus vaccination: meta-analysis of postlicensure studies. Pediatr Infect Dis J. 2015;34(7):763–68. doi:10.1097/INF.0000000000000715.
- Dong R, Yang YF, Chen G, Shen Z, Zheng S. Risk of intussusception after rotavirus vaccination: A meta-analysis. Int J Clin Exp Med. 2016;9:1306–13.
- Centers for Disease C, Prevention. Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48(43):1007.
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi:10.1056/NEJMoa052434.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a pentavalent human-bovine (wc3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi:10.1056/NEJMoa052664.
- Global advisory committee on vaccine safety. 11-12 december 2013. Releve Epidemiologique Hebdomadaire. 2014;89(7):53–60.
- Koch J, Harder T, von Kries R, Wichmann O. Risk of intussusception after rotavirus vaccination. Dtsch Arztebl Int. 2017;114(15):255–62. doi:10.3238/arztebl.2017.0255.
- Tate JE, Mwenda JM, Armah G, Jani B, Omore R, Ademe A, Mujuru H, Mpabalwani E, Ngwira B, Cortese MM, et al. Evaluation of intussusception after monovalent rotavirus vaccination in Africa. N Engl J Med. 2018;378(16):1521–28. doi:10.1056/NEJMoa1713909.
- Valcarcel Salamanca B, Hagerup-Jenssen ME, Flem E. Uptake and timeliness of rotavirus vaccination in Norway: the first year post-introduction. Vaccine. 2016;34(39):4684–89. doi:10.1016/j.vaccine.2016.08.017.
- Global Advisory Committee on Vaccine Safety. Weekly epidemiological record. World Health Organization;2020. 2020 January 24.
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, Booy R, Bines JE, Group PAS Intussusception following rotavirus vaccine administration: post-marketing surveillance in the national immunization program in australia. Vaccine. 2011;29(16):3061–66. doi:10.1016/j.vaccine.2011.01.088.
- Tate JE, Yen C, Steiner CA, Cortese MM, Parashar UD. Intussusception rates before and after the introduction of rotavirus vaccine. Pediatrics. 2016:138(3). doi:10.1542/peds.2016-1082.
- Simonsen L, Morens D, Elixhauser A, Gerber M, Van Raden M, Blackwelder W. Effect of rotavirus vaccination programme on trends in admission of infants to hospital for intussusception. Lancet. 2001;358(9289):1224–29. doi:10.1016/S0140-6736(01)06346-2.
- Yen C, Tate JE, Steiner CA, Cortese MM, Patel MM, Parashar UD. Trends in intussusception hospitalizations among us infants before and after implementation of the rotavirus vaccination program, 2000-2009. J Infect Dis. 2012;206(1):41–48. doi:10.1093/infdis/jis314.
- Hawken S, Ducharme R, Rosella LC, Benchimol EI, Langley JM, Wilson K, Crowcroft NS, Halperin SA, Desai S, Naus M, et al. Assessing the risk of intussusception and rotavirus vaccine safety in Canada. Hum Vaccin Immunother. 2017;13(3):703–10. doi:10.1080/21645515.2016.1240846.
- Yen C, Shih SM, Tate JE, Wu FT, Huang YC, Parashar UD, Hsiung CA. Intussusception-related hospitalizations among infants before and after private market licensure of rotavirus vaccines in Taiwan, 2001-2013. Pediatr Infect Dis J. 2017;36(10):e252–e257. doi:10.1097/INF.0000000000001644.
- Cho HK, Hwang S, Nam H Trends in the incidence of intussusception before and after the private market licensure of rotavirus vaccine in korea: A nationwide cross-sectional study. Poster presented on the 36th annual meeting of the european society for paediatric infectious diseases; may 28-june 2; 2018; malmö (sweden).
- Belongia EA, Irving SA, Shui IM, Kulldorff M, Lewis E, Yin R, Lieu TA, Weintraub E, Yih WK, Li R, et al. Real-time surveillance to assess risk of intussusception and other adverse events after pentavalent, bovine-derived rotavirus vaccine. Pediatr Infect Dis J. 2010;29(1):1–5. doi:10.1097/INF.0b013e3181af8605.
- Shui IM, Baggs J, Patel M, Parashar UD, Rett M, Belongia EA, Hambidge SJ, Glanz JM, Klein NP, Weintraub E. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. Jama. 2012;307(6):598–604. doi:10.1001/jama.2012.97.
- Burke RM, Tate JE, Dahl RM, Aliabadi N, Parashar UD. Does rotavirus vaccination affect longer-term intussusception risk in US infants? J Pediatric Infect Dis Soc. 2019. doi:10.1093/jpids/piz035.
- Willame C, Cheuvart B, Aris E, Vetter V, Cohet C. Association between rotavirus gastroenteritis and intussusception: suggested evidence from a retrospective study in claims databases in the United States. Submitted for publication. Hum Vaccin Immunother. 2020;17(1).
- Restivo V, Costantino C, Giorgianni G, Cuccia M, Tramuto F, Corsello G, Casuccio A, Vitale F. Case-control study on intestinal intussusception: implications for anti-rotavirus vaccination. Expert Rev Vaccines. 2018;17(12):1135–41. doi:10.1080/14760584.2018.1546122.
- Mansour AM, El Koutby M, El Barbary MM, Mohamed W, Shehata S, El Mohammady H, Mostafa M, Riddle MS, Sebeny PJ, Young SY, et al. Enteric viral infections as potential risk factors for intussusception. J Infect Dev Ctries. 2013;7(1):28–35. doi:10.3855/jidc.2321.
- Minney-Smith CA, Levy A, Hodge M, Jacoby P, Williams SH, Carcione D, Roczo-Farkas S, Kirkwood CD, Smith DW. Intussusception is associated with the detection of adenovirus c, enterovirus b and rotavirus in a rotavirus vaccinated population. J Clin Virol. 2014;61(4):579–84. doi:10.1016/j.jcv.2014.10.018.
- Robinson CG, Hernanz-Schulman M, Zhu Y, Griffin MR, Gruber W, Edwards KM. Evaluation of anatomic changes in young children with natural rotavirus infection: is intussusception biologically plausible? J Infect Dis. 2004;189(8):1382–87. doi:10.1086/382655.
- Tate JE, Simonsen L, Viboud C, Steiner C, Patel MM, Curns AT, Parashar UD. Trends in intussusception hospitalizations among us infants, 1993-2004: implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics. 2008;121(5):e1125–1132 doi:10.1542/peds.2007-1590.
- Tai JH, Curns AT, Parashar UD, Bresee JS, Glass RI. Rotavirus vaccination and intussusception: can we decrease temporally associated background cases of intussusception by restricting the vaccination schedule? Pediatrics. 2006;118(2):e258–264. doi:10.1542/peds.2005-2874.
- Standaert B, Alwan A, Strens D, Raes M, Postma MJ. Improvement in hospital quality of care (qoc) after the introduction of rotavirus vaccination: an evaluation study in Belgium. Hum Vaccin Immunother. 2015;11(9):2266–73. doi:10.1080/21645515.2015.1029212.
