ABSTRACT
Varicella is a potentially serious infectious disease caused by Varicella-Zoster Virus (VZV). In Italy childhood varicella vaccine have gradually introduced into national immunization program since 2003 and from 2017 a two-doses schedule has been stated nationally for all newborns and has become compulsory for school attendance. VZV exposures among healthcare workers (HCWs) and patients can be really dangerous and expensive. According to Centers of Disease Control and Italian national immunization plan health care, institutions should verify that all HCWs have clear evidence of immunity to VZV and should ensure that susceptible subjects will receive 2 doses of VZV vaccine. Currently, the vaccination of HCWs is not compulsory in Italy and the risk of varicella infection among these subjects is not well known. We evaluated the clinical records of 840 HCWs (256 male and 584 female) who underwent the annual occupational screening, from 1st January to 31st August 2018. HCWs were divided into three subgroups according to their age: 18–30, 31–40, and over 40 years old. We compared the mean values of IgG-specific antibodies between the age group through analysis of variance (ANOVA). A total of 784 (93.33%) HCWs were protected for VZV IgG antibodies level. There wasn’t a significant difference between male and female while was found between age group (P < 0.001). Protection levels for varicella are inadequate among HCWs. Despite the epidemiology of varicella in general population has changed with the implementation of the childhood varicella vaccination program transmission of VZV in hospitals is still a serious problem, so it is necessary to increase prevention activities in these settings, including vaccination.
Introduction
Varicella is an infectious disease caused by primary infection of Varicella-Zoster Virus (VZV), a member of the alpha herpesvirus family.
The virus spread from person to person by inhalation of contaminated aerosols or direct contact of vesicular fluid of skin lesions of acute varicella (primary infection) or herpes zoster (HZ), a painful vesicular rash known as “shingles”.Citation1
After the exposure, the infected subject remains asymptomatic for an average incubation period of 14–16 days (range: 10–21 days) before the typical rash appears.
Infected subjects can be contagious from 1 to 2 days before rash onset until all lesions are crusted (4–7 days after rash onset). Secondary attack rates for VZV among susceptible subjects for domiciliary contacts can reach 90%. Commonly, primary infection with VZV results in lifetime immunity, but VZV can remain dormant in nerve ganglia and can reactivate later in life, as a localized manifestation (HZ). The frequency of HZ cases in otherwise healthy persons is not well known; such illness is more likely to occur among immunocompromised persons.Citation1-3
In western countries before childhood vaccination program, 90% of varicella disease occurred among children aged <15 years.Citation1 In Europe, serological studies have shown that antibodies to varicella are generally acquired before 10–15 years of age, but substantial differences in VSV seroepidemiology across the European region have been found.Citation4-6
The onset of vaccination programs across Europe has led to dramatic declines of >85% in varicella incidence, hospitalizations, and deaths.Citation6,Citation7 Actually, the incidence of varicella among adults in western countries is considered low (<0.1/1,000 population), adult cases representing about 10% of all reported cases.Citation1,Citation6-9
Varicella infection, from a clinical point of view, is considered to be a benign infection, but complications (infectious disease as pneumonia, neurological, and hematological sequelae as encephalitis or purpura) can occur in 2–6% of cases.Citation1,Citation6,Citation10
It is well known that the severity of the infection tends to increase with the age of the subject; teenagers, adults, and immunocompromised patients usually develop the most severe clinical manifestations.Citation1,Citation11
The administration of two doses of live attenuated VZV is considered to be highly effective in inducing seroconversion in 90–99% of vaccinated children. Varicella vaccine effectiveness is expected to be lower in adults than in children: in published studies, 2 doses of varicella vaccine can lead to 80% reduction in the expected number of cases.Citation1,Citation2,Citation12
In Italy childhood varicella vaccine have gradually introduced into national immunization program since the year 2003, and from 2017, a two-dose schedule has been stated nationally for all newborns as one of the ten compulsory vaccines for school attendance.Citation13-15 Change in VZV epidemiology in Italy after the introduction of vaccine schedule has shown significant increase of seropositivity in age class 1–4 years, while comparison in other age groups (including working age) was not significantly different.Citation16,Citation17
Hospital transmission of contagious diseases is well known and exposures among healthcare workers (HCWs) and patients can be really dangerous, time-consuming, and expensive. Prevention activities, including vaccination of nonimmune HCWs and students, represent an important target.Citation18,Citation19
Studies regarding VZV exposure in healthcare facilities have proved that a single employee with unrecognized varicella can expose over 30 patients and 30 HCWs.Citation20,Citation21 According to CDC, healthcare institutions should verify that all HCWs have clear evidence of immunity to VZV and should ensure that susceptible subjects will receive 2 doses of varicella vaccine.Citation1
In 2017, the Italian government issued the national immunization plan:Citation14 according to this statement, the vaccination for varicella is strongly recommended but not compulsory for susceptible operators. The effectiveness of this strategy, in increasing the rate of vaccinated HCWs, has not been assessed and the risk to remain unprotected for those subjects is not well known.
The aim of our study is to evaluate the seroepidemiology of varicella among Italian HCWs and medical students after the introduction of the national vaccination plan.
Methods
We conducted a retrospective observational study, approved by the Ethical Committee for Research in Human Subjects of the Foundation PTV, Polyclinic Tor Vergata of Rome (PTV).
We reviewed the clinical records of HCWs who underwent the annual occupational screening, from 1st January to 31th August 2018. Values of VZV-specific IgG antibodies were collected in a Microsoft Excel worksheet. For each patient, the following data were recorded: age, gender, and IgG VZV antibody titer.
VZV-specific IgG serum antibodies value higher than 160 mlU/mL were considered protective against disease according to the literature.Citation1 In our hospital, the evaluation of the immunization against varicella is performed by means of the LIAISON® VZV IgG EIA assay.
HCWs were divided into three subgroups according to their age: 18–30 years old, 31–40 years old, and over 40 years old.
We excluded from the survey subjects with incomplete clinical and serological data, and 2 HCWs positive to VZV-specific IgM antibodies. These 2 workers had not previously been vaccinated, nor did the disease happen.
We compared the mean values of IgG-specific antibodies between the age group through analysis of variance (ANOVA). The SPSS analytic software performed statistical analysis. Only P values < .001 were considered in our study.
Results
We evaluated the clinical records of 840 HCWs (256 male and 584 female).
All the patients' data were included in our study: sex, date of birth, and levels of VZV antibodies at the first occupational health service’s visit. The mean age was 36.63 years old (range: 18–70); 292 HCWs were in the 18–30 years old group (100 male, 192 female), 253 were in the 31–40 years old group (71 male, 182 female), and 295 HCWs were older than 40 years old (85 male, 210 female) (). The survey analyzed was composed of 44 medical students, 463 doctors, 297 nurses, and 36 others (laboratory technicians, midwives, radiology technicians).
Table 1. HCWs (medical students, doctors, nurses, and others) distribution for gender and age
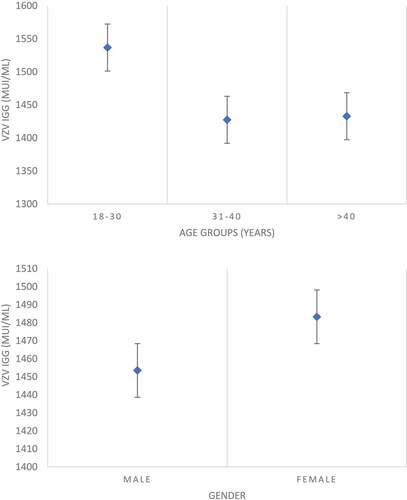
We found 784 HCWs with protective VZV IgG antibodies level (93.33%, CI: 91.4–94.9). Immune HCWs were 92.8% among those aged 18–30 years old, 88.9% in the age class 31–40 years, and 97.6% among those aged more than 40 years. The main findings are reported in .
Table 2. Number and percentage of immune (varicella antibody IgG >16.5 AU/ml)
The average antibody titer was 1474.2 mlU/mL and there was no significant difference between men and women, whereas a statistically significant association between age and varicella IgG antibody was detected (P < .001) ().
Discussion
Our study focused on the VZV serum analysis of HCWs and medical students after the introduction of the national immunization plan. We found a relatively high percentage of subjects not immune, especially in workers aged less than 40 years. Among those subjects, immunization rate is below the target of 95% coverage required for “herd immunity” ().
Due to the late introduction of VZV in Italy, vaccine coverage among subjects aged 30 years or more (birth cohort before 1999) are negligible, since the monovalent Varicella vaccine was locally introduced in 2001 and the MMRV formulation only in 2006.
We found the highest percentage of serologically immune subjects in the age class over 40 years: this finding in our opinion could be explained by the repeated contacts with infected children that could induce exogenous boosting of VZV immunityCitation1,Citation22 since the incidence of the infection among the Italian population in the last decades was higher than the actual.Citation8 For these reasons, in this age group, vaccination coverage evaluating only the physician’s anamnestic answers could lead to an overestimation of the rate of susceptible subjects and consequently the need for vaccination.Citation23
Among younger operators, the lack of humoral immunity could be explained by the natural decline in VZV antibody titer that was not balanced by exogenous booster due to the decrease of varicella prevalence among children.Citation22 Those data are consistent with the natural history of immune response to varicella: in fact even if typically, primary infection with VZV results in lifetime immunity due to cell mediated immunological response, detectable levels of specific antibodies trend to decline over time both in natural infection and after vaccination. Nevertheless, published studies suggest that VZV-specific cell-mediated immunity guarantees long-lasting protection to vaccinated subjects, even in the absence of a detectable antibody response.Citation1,Citation10,Citation22,Citation24
Varicella vaccination is still not mandatory for Italian HCWs and the low rate of coverage represents a major risk factor for the hospital transmission of the virus. It is reported that in cases of nosocomial outbreaks of varicella the unvaccinated or incompletely vaccinated subjects represent the main target of the infection.Citation21 In our hospital, the coverage rate was inadequate in younger operators and two cases of acute infection occurred among HCWs during the year 2018. In published studies, seroprevalence for varicella among HCWs varied from 92% to 99% and younger employees had a lower IgG titer than their older colleagues.Citation25
HCWs are at higher risk to be exposed to airborne pathogens and exanthematous illnesses due to higher probability to meet the virus in the workplace: despite national guidelines recommended to immunize all HCWs and medical students with varicella vaccine, the attitude to vaccination for this subjects is reported to be low, mainly due to vaccine hesitancy. In previous studies, we found inadequate immunization coverage for measles and mumps in the same hospital population, but the serological screening and direct workplace vaccination strategy resulted in highly cost-effective and reduced the risk of nosocomial transmission.Citation26-28 A pre-employment policy of mandatory serological screening and vaccination can increase the rates of immunity and can ensure that all HCWs are immune to varicella. This strategy is also crucial to eliminate the nosocomial transmission of the infection and to protect more vulnerable patients. Previous studies have demonstrated that even for varicella prevention strategies based on screening and vaccinating HCWs on workplace were shown to be cost-effective for healthcare facilities and are consistent with current international guidelines.Citation29,Citation30
Statistical analysis did not result in gender difference in varicella coverage, probably due to the higher rates of naturally immunized subjects compared to epidemiological data for measles that showed a higher percentage of MMR vaccinated females due to higher perception of risk to rubella infection in that gender.Citation31,Citation32
The study had some potential limitations: records of the previous vaccination were not evaluated and we did not consider the different exposure risks. Regarding vaccine anamnesis, as told, we can hypothesize that the percentage of vaccinated subject is negligible on the basis of the cohort of birth of subject included in the study, all born before the year 2000, one year before the introduction of the varicella vaccine in Italy.
Based on our serological screening, levels protection for varicella among HCWs is inadequate among HCWs and medical students and consequently those subjects are exposed to an unacceptable risk of contagion. Despite the epidemiology of varicella in general population has changed with the implementation of the childhood varicella vaccination program, VZV still represents a considerable health burden. In many countries, the incidence of varicella has declined following the National Immunization Program (NIP) implementation for varicella vaccine and vaccinated persons present with milder disease.Citation33 Otherwise, transmission of VZV in hospitals, where potentially susceptible immunocompromised patients are housed, is still a serious problem. Transmission of VZV in hospital settings can occur via airborne route or through contact with contaminated environment or hands of HCWs. A patient with either varicella or herpes zoster can be a source of transmission in healthcare settings.Citation34-36 Since healthcare setting represents a critical environment for the possible transmission of varicella, it is necessary to increase prevention activities, including compulsory vaccination of nonimmune HCWs and students. The promotion of an adequate vaccination program among HCWs is essential regardless of age.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and reports: morbidity and mortality weekly report. Recommendations Rep. 2007 Jun 22;56(RR–4):1–40.
- Arvin AM. Varicella-zoster virus: overview and clinical manifestations. Semin Dermatol. 1996 Jun;15(2 Suppl 1):4–7.
- Wharton M. The epidemiology of varicella-zoster virus infections. Infect Dis Clin North Am. 1996 Sep;10(3):571–81.
- Riera-Montes M, Bollaerts K, Heininger U, Hens N, Gabutti G, Gil A, Nozad B, Mirinaviciute G, Flem E, Souverain A, et al. Estimation of the burden of varicella in Europe before the introduction of universal childhood immunization. BMC Infect Dis. 2017 May 18;17(1):353.
- Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Hum Vaccin Immunother. 2019;15:645–57.
- EUVAC.NET. Surveillance of Varicella and Herpes Zoster in Europe2010.
- Eurostat. Statistical office of the European communities.
- Sauboin C, Holl K, Bonanni P, Gershon AA, Benninghoff, B, Carryn S, Burgess MA, Wutzler P. The impact of childhood varicella vaccination on the incidence of herpes zoster in the general population: modelling the effect of exogenous and endogenous varicella-zoster virus immunity boosting. BMC Infect Dis. 2019 Feb 6;19(1):126.
- Lo Presti C, Curti C, Montana M, Bornet C, Chickenpox: VP. An update. Med Mal Infect. 2019 Feb;49(1):1–8.
- CDC. Epidemiology and prevention of vaccine-preventable diseases. 2015.
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PG, Oxman MN, et al. Varicella zoster virus infection. Nature reviews. Dis Primers. Jul 2 2015. 1: 15016.
- Rieck T, Feig M, An der Heiden M, Siedler A, Wichmann O. Assessing varicella vaccine effectiveness and its influencing factors using health insurance claims data, Germany, 2006 to 2015. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin. 2017 Apr 27;22(17): 30521.
- Conferenza Stato-Regioni. Piano nazionale prevenzione vaccinale2017–2019. 2017 Feb 18.
- Decreto-legge 7 giugno 2017, n. 73. Disposizioni urgenti in materia di prevenzione vaccinale, di malattie infettive e di controversie relative alla somministrazione di farmaci. (17A05515) (GU SerieGenerale n.182 del 05- 08-2017)
- Ministero della Salute. Circolare n. 25233. 16 Agosto 2018.
- De Donno A, Kuhdari P, Guido M, De Donno A, Kuhdari P, Guido M, Rota MC, Bella A, Brignole G, Lupi S, et al. Study Group on seroepidemiology. Has VZV epidemiology changed in Italy? Results of a seroprevalence study. Hum Vaccin Immunother. 2017 Feb, 13(2): 385–90.
- Gabutti G, Rota MC, Guido M, De Donno A, Bella A, Ciofi degli Atti ML, Crovari P, Seroepidemiology Group. The epidemiology of Varicella Zoster Virus infection in Italy. BMC Public Health. Oct 27 2008. 8: 372.
- Koivisto K, Puhakka L, Lappalainen M, Blomqvist S, Saxen H, Nieminen T. Immunity against vaccine-preventable diseases in Finnish pediatric healthcare workers in 2015. Vaccine. 2017 Mar 14;35(12):1608–14.
- Ferraro M, Morucci L, Coppeta L, De Carolis G, Pietroiusti A, Franco E, Magrini A. Managing the risk of bacterial meningitis among healthcare workers. Occup Med (Chic Ill). 2019 Apr 13;69(2):113–17.
- Lopez AS, Burnett-Hartman A, Nambiar R, Ritz L, Owens P, Loparev VN, Guris D, Schmid DS. Transmission of a newly characterized strain of varicella-zoster virus from a patient with herpes zoster in a long-term-care facility, West Virginia, 2004. J Infect Dis. 2008 Mar 1;197(5):646–53.
- Haiduven-Griffiths D, Fecko H. Varicella in hospital personnel: a challenge for the infection control practitioner. Am J Infect Control. 1987 Oct;15(5):207–11.
- Ampofo K, Saiman L, LaRussa P, Steinberg S, Annunziato P, Gershon A. Persistence of immunity to live attenuated varicella vaccine in healthy adults. Clinical infectious diseases: an official publication of the infectious diseases society of America. 2002 Mar 15;34(6): 774–79.
- Squeri R, Genovese C, Trimarchi G, MAR P, La FV. An evaluation of attitude toward vaccines among healthcare workers of a university hospital in Southern Italy. Annali di igiene:medicina preventiva e di comunità, 2017 Nov-Dec; 29(6):595–606. doi:10.7416/ai.2017.2188.
- Saiman L, LaRussa P, Steinberg SP, Zhou J, Baron K, Whittier S, Della-Latta P, Gershon AA. Persistence of immunity to varicella-zoster virus after vaccination of healthcare workers. Infect Control Hosp Epidemiol. 2001 May, 22(5): 279–83.
- Gorny AW, Mittal C, Saw S, Venkatachalam I, Fisher DA, Tambyah PA. Varicella seroprevalence in healthcare workers in a tertiary hospital: an audit of cross-sectional data. BMC Res Notes. 2015;8:664. Published 2015 Nov 10. doi:10.1186/s13104-015-1656-0.
- Coppeta L, Pietroiusti A, Lieto P, Ferraro M, Grelli S, Stillo M, Magrini Al. Measles immunity in an Italian teaching hospital. Occup Med (Chic Ill). 2019 Apr 13;69(2):143–45.
- Coppeta L, Balbi O, Baldi S, Pietroiusti A, Magrini A. Pre-vaccination IgG screening for mumps is the most cost-effectiveness immunization strategy among health care workers. Hum Vaccin Immunother. 2019;15:1135–38.
- Coppeta L, Morucci L, Pietroiusti A, Magrini A. Cost-effectiveness of workplace vaccination against measles. Hum Vaccin Immunother. 2019;15:2847–50.
- Alp E, Cevahir F, Gokahmetoglu S, Demiraslan H, Doganay M. Prevaccination screening of health-care workers for immunity to measles, rubella, mumps, and varicella in a developing country: what do we save? J Infect Public Health. 2012 Apr;5(2):127–32.
- Baracco GJ, Eisert S, Saavedra S, Hirsch P, Marin M, Ortega-Sanchez IR. Clinical and economic impact of various strategies for varicella immunity screening and vaccination of health care personnel. Am J Infect Control. 2015 Oct 1;43(10):1053–60.
- Asari S, Deguchi M, Tahara K, Taniike M, Toyokawa M, Nishi I, Watanabe M, Iwatani Y, Makimoto K. Seroprevalence survey of measles, rubella, varicella, and mumps antibodies in health care workers and evaluation of a vaccination program in a tertiary care hospital in Japan. Am J Infect Control. 2003 May, 31(3): 157–62.
- Baer G, Bonhoeffer J, Schaad UB, Heininger U. Seroprevalence and immunization history of selected vaccine preventable diseases in medical students. Vaccine. 2005 Mar 14;23(16):2016–20.
- Wutzler P, Bonanni P, Burgess M, Gershon A, Sáfadi MA, Casabona G. Varicella vaccination - the global experience. Expert Rev Vaccines. 2017;16:833–43.
- Weber DJ, Rutala WA, Hamilton H. Prevention and control of varicella-zoster infections in healthcare facilities. Infect Control Hosp Epidemiol. 1996;17:694–705.
- Saidel-Odes L, Borer A, Riesenberg K, Frenkel A, Sherlis R, Bouhnick L, Schlaeffer F. An outbreak of varicella in staff nurses exposed to a patient with localized herpes zoster. Scand J Infect Dis. 2010;42(8):620–22.
- Bloch KC, Johnson JG. Varicella zoster virus transmission in the vaccine era: unmasking the role of herpes zoster. J Infect Dis. 2012;205:1331–33.