ABSTRACT
In recent years, the incidence of varicella cases is rising, and outbreaks of varicella are frequently being reported worldwide. Our study aims to analyze the association between the varicella incidence and serum antibody level in the post-vaccine era. We retrieved and analyzed the incidence and prevalence data for children age 1–14 years in Wenzhou, China during 2010–2018. A cross-sectional seroepidemiology analysis was carried out in a series of 168 general healthy children age 1–14 years as well as children at a varicella outbreak in Wenzhou. Our data showed a significant surge in the incidence and prevalence of varicella in children aged 10–14 years in 2017 and 2018 while they were kept relatively stable in 2010–2016. The seroepidemiological analysis revealed a 7.3-fold significantly higher level of serum varicella IgG in healthy control students who exposed at the outbreak than that in general healthy children (median 523.5 vs. 71.7 mIU/mL, p < .01). The children 10–14 years old had the lowest rate of second-dose vaccination among the three age classes (7%, 41%, and 65% in 10–14, 5–9, and 2–4 age class, respectively), and children 5–9 years old who received the second dose had a higher level of serum protective IgG than those who did not (254.7 vs 98 mIU/mL, p = .06). The findings from the present study warn a two-dose vaccine schedule to reduce the climbing incidence and prevalence observed in the older children and suggest a higher serum IgG threshold for effective protection of children from the varicella outbreak.
Introduction
Varicella (also known as chickenpox) is the primary infection caused by the varicella-zoster virus (VZV), and it is a common and highly contagious childhood disease.Citation1 Reports show the VZV seroprevalence in 80% varicella-unvaccinated children and adolescents in the European Union (most children in Germany get infected by 6 years of age)Citation2 and 90% unvaccinated adults 20 years or older in China before the universal vaccination era.Citation3 By 2014, varicella vaccines have been introduced into the universal vaccination programs at the national level in 33 countries; however, there are also concerns on the introduction in many other countries for reasons such as fears that vaccination may shift the disease to older individuals in whom the disease is more severe or may increase the incidence of herpes zoster, which implicates that the effective vaccine may result in changes in the epidemiology of varicella and zoster.Citation4,Citation5 Thus, vaccine effectiveness and safety are still topics in the post-vaccine era due to lack of ample evidence on associations between varicella incidence and immune status.
Wenzhou is the major city in the southeast of Zhejiang Province, with over 9.3 million of registered inhabitants, which is a typical population size for medium-sized cities in China.Citation6 In 1998, the Zhejiang Provincial Center for Disease Control and Prevention (ZJCDC) recommended the implementation of varicella vaccination (VarV) with a single dose for children at the age of 12 months and older.Citation7,Citation8 Since then, the first dose of VarV coverage is increasing rapidly from 35% in 2009 to 75% in 2013, leading to a substantial decrease in the incidence of varicella disease, varicella-related morbidity and mortality, and healthcare costs in the last decade.Citation8 While a great number of cohort studies with outbreak surveys have confirmed the efficacy in the overall prevention and control of varicella, these studies also found the insufficiency in the control of varicella outbreaks by the one-dose vaccination.Citation9–12 For instance, Fu et al. reported that breakthrough varicella accounted for 83.3% of cases (10/12) in a highly vaccinated preschool population in which 93.7% (135/144) had received single-dose varicella vaccine before the outbreak.Citation11 In 2014, ZJCDC updated the VarV recommendation to implement a two-dose of VarV schedule, with the first dose being given to children 12–15 months of age and the second dose for children at 3–4 years of age.Citation8 However, the VarV is a category II (parent-pay) vaccine in China that is recommended in the 1-dose schedule but optional in the two-dose schedule.
Presently, the second dose of VarV is optional and it has not been implemented in the Universal Immunization Program of Wenzhou City. In late 2018, an outbreak of varicella occurred in an elementary school in Wenzhou City. With increasing VarV coverage, varicella outbreak, particularly in school settings has become a major public health concern in China and around the world.Citation9,Citation13-15 To our knowledge, levels of serum immune antibodies and their association with the protection of school-age children from VZV infection after mass immunization are less reported. The epidemiology and seroepidemiology studies from Wenzhou are also very limited. To explore the possible causes for the recent occurrence of varicella outbreak, gain a more comprehensive understanding of the epidemiological characteristics of varicella in Wenzhou area, and determine the age-specific concentrations of immune antibodies against VZV among children, we conducted a seroepidemiological study and analyzed the incidence and prevalence of varicella in children from Wenzhou area. Our present study aims to define the association between the varicella incidence and serum protection antibodies in the post-vaccine era. It will provide evidence for the local administrations to develop and update the VZV immunization strategies and serve as references to other regions around the world.
Materials and methods
Epidemic data of cases with varicella
All cases included in this study were patients with varicella infection who visited the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University in Wenzhou, China between January 1, 2010, and December 31, 2018. This hospital is located at the metropolitan area of Wenzhou city and serves approximately 70% of children in the region. The data for the incidence of varicella in the hospital registry databases were retrieved as individual cases. In the total of 12408 non-duplicate entries from all ages including adults, 10612 (85.5%) were children age 0–14 years. Since there were no significant population migrations or major hospitals built in the area, we used the incidence number instead of rate ratio in the analysis. In addition, the data for the prevalence of varicella in the Wenzhou area for the same time period were requested for each age group from the Center for Disease Control and Prevention of Zhejiang Province. These prevalence data were based on total varicella cases reported for the area (Wenzhou) with the projected population size of specific age groups.
Case definition of varicella
An acute illness of varicella was defined for patients with diffuse macular/papular/vesicular rash AND with an epidemiologic linkage to another probable or confirmed case or with one of the following laboratory confirmations: (1) detection of VZV-specific IgM antibodies, (2) detection of VZV DNA, (3) isolation of chickenpox virus from a clinical specimen.Citation13 Generally, all clinically diagnosed cases were confirmed by laboratory tests.
Serum samples from varicella cases and controls
In the 2018 varicella outbreak at an elementary school in Wenzhou, there were a total of 11 patients in a single classroom, which is the only site in the region where the outbreak occurred. For the sake of comparability, serum samples were collected within 48 hours after the disease onset from these 11 patients and 9 healthy controls within the same classroom. These students (namely the outbreak cohort) should have had similar exposure to VZV and they were similarly aged at 10–11 years. With limited resources, the seroepidemiology survey was intended to provide a preliminary observation on the seroprotection in the general pediatric population. Thus, a reasonable double-digit sample size of 12 was chosen for each age group among children 1–14 years. A total of 168 serum samples were collected in January 2019 from healthy children who visited the Yuying Children’s Hospital of Wenzhou Medical University for physical examination (namely the general cohort). No children in the general cohort were from that specific school where the outbreak occurred. Consecutive children who visited the hospital and met the following inclusion criteria were enrolled: (1) 1–14 years old; and (2) physically healthy (i.e., ear temperature <38°C and no sign of acute or chronic diseases). The research protocol was approved by the Hospital Ethics Committee, and the written informed consent was obtained from all children in this study.
Determination of serum antibodies against varicella
Blood samples were drawn into BD vacutainer tubes without anti-coagulation agents and centrifuged at 3,000 rpm to obtain serum. Serums were then frozen at −80°C until assays were performed. Serum antibody concentrations including VZV-specific IgG and IgM were utilized as surrogate markers for the immunity against VZV. VZV-specific glycoprotein IgG has been recognized by the U.S. Food and Drug Administration as an alternative measure for vaccine efficacy and potential VZV immunity,Citation16 owing to the convenience and availability of this assay, compared with those for cell-mediated immunity. In this study, VZV-specific antibodies were measured with a previously validated quantitative ELISA method, which detected antibodies against VZV glycoproteins prepared from human fibroblasts infected by VZV.Citation17 Both VZV-specific IgG and IgM were determined using reagents from VIRION/SERION ELISA Classic (Würzburg, Germany). The manufacturer’s recommendations for interpretation of the test results specify that, for VZV-IgM, positive results (>15 U/mL) are considered the active phase of VZV infection, negative (<10 U/mL) and equivocal results (10–15 U/mL) indicate no active infection recently. For VZV-IgG, positive results (>100 mIU/mL) are considered immune, negative (<50 mIU/mL) and equivocal results (50–100 mIU/mL) are considered not immune. These recommendations are generally adopted in most of prior studies.Citation18,Citation19
Statistical analyses
Changes in the incidence and prevalence over the study years were tested by a time-series Spearman correlation analysis against years. VZV-specific antibody concentrations were presented as mean values from replicate assay wells. The difference in the antibody concentration between groups was analyzed with the nonparametric Mann–Whitney U test. An effective protective level of serum IgG against varicella was calculated with the confidence interval of the mean value. Data analyses were performed using the GraphPad software (version 8.0, San Diego, USA). A p value <.05 was considered statistically significant.
RESULTS
Annual distribution of chickenpox cases in the Wenzhou area
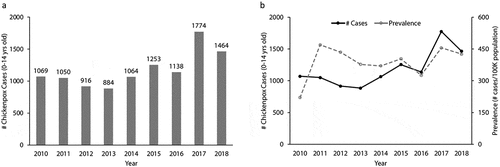
During the 9 years from January 1, 2010, to December 31, 2018, a total of 10612 cases at ages 0–14 years old were diagnosed with varicella in the Wenzhou area, of which 6241 (58.8%) were male and 4371 (41.2%) were female. Cases from urban, suburban, and rural areas accounted for 72.9%, 8.4%, and 18.8%, respectively. The annual case distribution showed a relatively stable number of incidence in 2010–2016 (r = 0.46, p > .05, ), with an average of 1053 cases per year, whereas a marked increase was seen in the years of 2017 and 2018, with 1774 and 1464 cases, respectively (r = 0.73, p < .05), which represents a 54% rise in the total number of cases from previous years (mean 1619 vs. 1053). Such a 2017–2018 uptrend in varicella incidence was in parallel with varicella prevalence for children 0–14 years old in the Wenzhou area for the same time period ().
Age-stratified case distribution over the years of 2010-2018
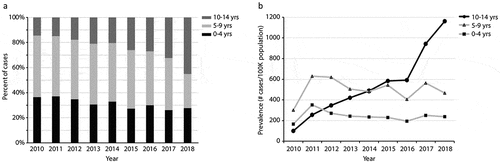
As shown in with age-stratified analysis, the percent of cases over the years of 2010–2018 in each of three age classes (0–4, 5–9, and 10–14 years) was different. Specifically, there was a significant uptrend in the percent of cases from 2010 to 2018 for the 10–14 age class (r = 0.98, p < .001), with a more remarkable increase in 2018 (45.1% for 2018 vs. 32.3% or less for previous years). As shown in , the prevalence of chickenpox in the 10–14 age class was apparently increasing over the years of 2010–2018 (r = 0.99, p < .001), with a significant surge (942–1163 cases per 100,000 population) in 2017–2018 when compared to 591 or less cases for the same population in previous years. This also confirmed that the 2017–2018 uptrend in varicella incidence observed in our hospital was not biased because the varicella prevalence for the same age class of the whole population in the area was also uptrend during the same time period.
Serum levels of VZV antibodies in general healthy children
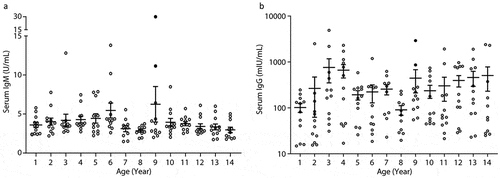
The seroepidemiological survey of 168 serum samples from general healthy children showed variable levels of both VZV IgG and IgM antibodies within each age group and across the 14 individual age groups ( -B). Two subjects in the age group of 9 years both had coincidently high levels of serum IgM and IgG concentrations, which may suggest a potentially recent varicella infection that could be at subclinical status thus they were excluded in the subsequent analyses. The median VZV-IgM concentration was trending down across the 1–4, 5–9 and 10–14 age classes (3.6, 3.2, and 3.1 U/mL, respectively), and none of these subjects had a positive IgM level except for excluded one at age 9 (). The median concentration of serum VZV-IgG was also down-trending across the three age classes (158, 152, and 76 mIU/mL for the 1–4, 5–9, and 10–14 age classes, respectively), and the median level of this antibody in the oldest age class (10–14 years) was only half of those values in the 2 younger age classes. Overall, there was no statistically significant difference in serum IgM and IgG levels across the three age classes (both p > .05).
Table 1. Summary of serum varicella antibody measurements in the healthy population
Figure 3. Serum varicella IgM and IgG levels of general healthy children aged 1–14 years old that were screened in January 2019. IgM (U/mL): >15 = positive, 10–15 = equivocal, <10 = negative. IgG (mIU/mL): >100 = positive, 50–100 = equivocal, <50 = negative. The individual data is presented with dot plots (empty circle) and the mean ± SEM is also shown for each age group (lines and bars). Solid black dots at age 9 subgroup indicate two subjects with potential “recent infection” of varicella at subclinical status. Each individual age group had 10–12 successfully assayed serum samples
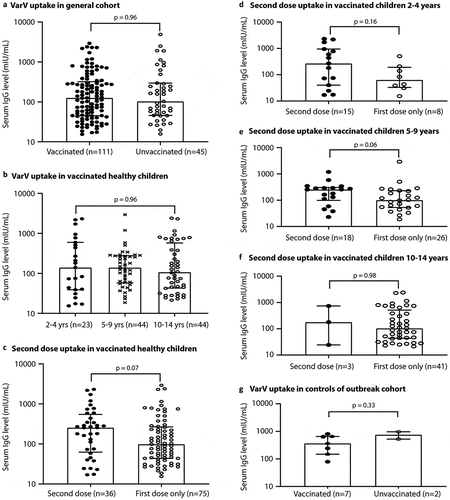
In 156 of 168 children who aged at 2–14 years (children aged 1 year were not counted in the vaccine history analysis as their first dose was too early to run through the full course of antibody production), 111 (71%) had received the first dose, of which 36 (32%) had the second dose of VZV vaccine (). Serum IgG levels between vaccinated and unvaccinated children overall were not significantly different (p > .05, ). In addition, serum IgG level in children who received the first dose of VZV was not significantly different across the three age classes (p > .05, ), albeit there was a downward trend (median IgM: 3.6, 3.5, and 3.25 U/mL, median IgG: 139.6, 139.2, and 106.9 mIU/mL for the 2–4, 5–9, and 10–14 age classes, respectively; both p > .05). The second-dose vaccination uptake was lowest for those aged 10–14 years among the three age classes: 65% (15/23) for 2–4 years, 41% (18/44) for 5–9 years, and 7% (3/44) for 10–14 years. Overall, children who received the second dose of vaccine had a higher level of serum protective IgG than those who did not receive a second dose (median 254.7 vs. 98 mIU/mL, borderline significance with p = .07, ). While there was no such a difference within each of the 2–4 and 10–14 age classes (p > .10, , F), there was a borderline difference in the serum IgG level between children received and not received the second dose of vaccine within the 5–9 age class (median 254.7 vs. 100.3 mIU/mL, p = .06, ).
Table 2. Characteristics of the general and outbreak cohorts with vaccination status
Figure 4. Association between the VZV vaccination and serum IgG level. (A) Difference in serum IgG level between children who received (vaccinated) and not received (unvaccinated) the first dose of VarV in the general cohort. (B) Difference in serum IgG level across the three age classes within the 111 vaccinated children from the general cohort (no matter a second dose was received or not). (C-F) Difference in serum IgG level between children who received and not received the second dose of VarV within all 111 vaccinated children (C) or within each of the three age classes (D-F). These “vaccinated” children were 1-dose vaccinated with or without the second dose of VarV. (G) Difference in serum IgG level between children who received (vaccinated) and not received (unvaccinated) the first dose of VarV in the outbreak cohort. Each dot (empty or solid) is an individual value for a sample. The empty frame indicates the median for each group. The horizontal short lines represent the interquartile range. The p-values shown are from non-parametric Mann–Whitney U tests
Serum levels of VZV antibodies at the varicella outbreak
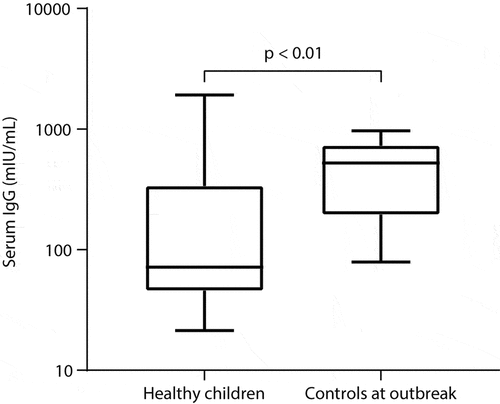
As shown in -B, the VZV-IgG and VZV-IgM concentrations both were significantly different between children from the case group and those from the control group (both p < .0001). The concentration of serum IgM in the patient group was 3.7-fold higher in median than that in the control group (32.4 vs. 9.1; 95% confidence interval 23.9 to 74.1 vs. 4.9 to 13.1 U/mL). The concentration of VZV-IgG in the patient group was also much higher than that in the control group (median 8237.9 vs. 523.5, i.e., 15.7-fold up in the patients; 95% confidence interval 5538 to 10415 vs. 257 to 725 mIU/mL). Notably, the serum IgG levels in the healthy controls from the outbreak cohort were 7.3-fold significantly higher than that in the healthy children from the general cohort (median 523.5 vs. 71.7 mIU/mL, p < .01, ), both cohorts of children were at the same age of 10–11 years, with similar VarV uptake rates (one-dose: 7/9 (78%) vs. 16/24 (68%); two-dose: 0/9 vs. 2/24).
Figure 5. Comparisons of serum varicella antibody concentrations between patients (n = 11) and controls (n = 9) whose blood samples were collected during the varicella outbreak occurred in November 2018 in a local elementary school in Wenzhou city. (A) IgM and (B) IgG levels. The data is presented with the Box and Whisker format indicating the minimum, 25%tile, median, 75%tile, and maximum from the bottom to the top
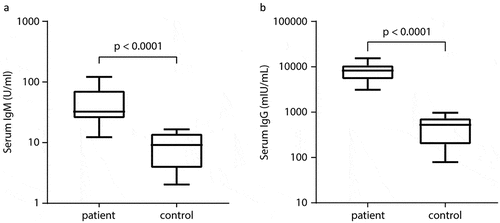
Figure 6. Comparison of serum IgG level between healthy children (n = 24) from the general cohort and healthy controls (n = 9) from the outbreak cohort (median 71.7 vs. 523.5 mIU/mL), both were of ages 10–11 years. The data is displayed with the Box and Whisker format indicating the minimum, 25%tile, median, 75%tile, and maximum from the bottom to the top
Among the 11 patients, 3 had not ever VZV-vaccinated (). Most patients experienced a similar clinical course with less than 3 mm small skin rash. One case without VZV vaccination had fever above 39°C and large herpes zoster (>5 mm) with skin infection. In the healthy control students, serum IgG level between vaccinated (n = 7, all 1-dose) and unvaccinated (n = 2) subjects were not significantly different (p > .05, ).
The susceptibility to varicella outbreak at effective serum protection IgG level
According to the manufacturer’s cutoff at 100 mIU/mL, there were 5.8 (collectively 23/48 from 4 individual age groups, 48%), 4.6 (23/57 in 5 age groups, 40%), and 5.6 (28/59 in 5 age groups, 47%) subjects on average who were susceptible to varicella, with serum IgG antibodies in the 1–4, 5–9 and 10–14 age classes, respectively (). In the present study, we sought to define a more reliable serum IgG level that effectively protects children from clinical disease at varicella outbreak. We calculated it using the lower limit of 95% confidence interval of the mean value for serum IgG in the control samples collected at the 2018 outbreak, which was 257.2 mIU/mL. Based on such an effective threshold, four of the 14 individual age groups (1, 2, 8, and 11 years) had more than 10 out of 11–12 (83% to 100%) tested children whose serum IgG levels were below the threshold, whereas remaining age groups had less than 9 out of 10–12 (42% to 75%) tested subjects with lower-than-effective-threshold serum IgG levels (). Collectively, the 1–4, 5–9, and 10–14 age classes had 9 (36/48, 67%), 8 (40/57, 70%), and 8 (40/59, 68%) children on average who had a lower-than-effective-threshold IgG level, which implicated that at least two-thirds of general healthy children might be at risk to develop varicella upon exposure.
Discussion
In recent years, the incidence of chickenpox cases is rising, and outbreaks of varicella are frequently being reported worldwide, including Asian countries and the United States, all implicate a critical public health problem in the post-vaccine era.Citation9,Citation13-15,Citation20,Citation21 Such a phenomenon is particularly stunning in modern days as the varicella vaccination coverage has expanded all over the world. Our current study has clearly demonstrated an upward trend in the incidence and prevalence of varicella in the Wenzhou area from 2010 to 2018, and that is more significant for children age 10–14 years in 2017–2018. This observation is in concert with the consensus on the epidemiology of varicella worldwideCitation21-23 in that the mass immunization has obviously changed the age distribution of varicella cases and the peak age has subsequently moved to older children. In general, the onset age has increased from 3–5 to 5–6 years, whereas the outbreak frequently occurred in older children and adolescents at school ages.Citation21–25 Beyond what is consistent with current consensus, the present study provides the new evidence for the association between the varicella incidence and serum protection IgG level and proposes that the effective serum protection IgG level could be significantly higher than the commonly adopted positive cutoff at the varicella outbreak.
The growing number of varicella cases in 2017 and 2018 and the outbreak in 2018 in the Wenzhou area may be attributed to several potential factors, such as the vaccine coverage, one-dose or two-dose schedule, and low levels of serum immune antibodies against the VZV. Several studies have tested the levels of VZV antibodies through seroepidemiological surveys in young children (1–9 years)Citation26 and school-age children.Citation27 These studies have focused either on the vaccine coverage rateCitation26 or on the seropositive rate of school grade-stratified children,Citation27 but they have not analyzed children from a wide range of ages in a single study to find age-specific levels that could be more useful to identify children at risk of contracting the disease when exposed. In the present study, the concentrations of VZV-IgM antibody for all 168 (except 1) serum samples were less than 15 U/mL (negative). This confirmed no active infection among these study children. The serum IgG levels represent the immunity for protecting children from varicella. Our results demonstrated that the majority of children at their early life (1–2 years) and at age 8 and 11 years had no sufficient protective antibodies against varicella. An age-matched comparison even found a 7.3-fold lower serum IgG level in general healthy children than that in healthy control students who exposed to the outbreak. These results suggest that the level of serum protective antibodies in general healthy children might not be sufficient to protect them from the varicella outbreak upon exposure.
Prior studies have assessed the ability of different serum IgG levels as the protection threshold.Citation18,Citation19,Citation28,Citation29 The most commonly adopted threshold for differentiating between immune and susceptible individuals was an IgG level at 100 mIU/ml,Citation18,Citation19 albeit some studies proposed 130 or 150 mIU/mL as the positive cutoff.Citation28,Citation29 In fact, the cutoff indicating immunity is thought to vary according to the ethnicity and age of the individual.Citation30 There is still no agreed standard that would allow a consistent screening test to be implemented. The measurements from children who exposed to VZV at the outbreak provide us a great opportunity to capture a practical protection threshold. In the current study, our results suggested an effective threshold at 257.2 mIU/mL. At this threshold, the number of at-the-risk children from the 10–14 age class was the greatest when compared to those numbers from the other two age classes (). Such a higher threshold was based on the 95% confidence interval of the mean value from those control students, which could be more sensitive for identifying at-risk children, especially during the varicella outbreak where higher viral loads may be encountered. Nonetheless, much larger cohorts of children who exposed but were immune to the varicella infection in different regions throughout the country will be required to establish a generalizable protection threshold.
Additional analyses with the children’s vaccination history implicate that the one-dose VZV vaccine might not always ensure a high level of serum antibody titter year after year. Several studies on school outbreaks have found that an extended time after the vaccination may be related to a risen chance for varicella outbreak.Citation31,Citation32 This suggests that the immunization effect of varicella vaccine could prevent the spread of varicella only to a certain extent.Citation33 A study from the United StatesCitation34 has yet found a significant decline in one-dose vaccination effectiveness from 94% at 5 years to 88% at 5 to 9 years and to 82% at 10 or more years after the vaccination. Recently, Marin and colleagues in their meta-analysis of varicella outbreaks came to the conclusion that waning immunity might be the reason for the decrease in vaccination effectiveness during the time being after the vaccination.Citation35
Notably, our study found that children at older age (10–14 age class) had the lowest rate of the second-dose vaccination when compared to younger children (5–9 and 2–4 age classes). This indicates in one way that more parents of older children in the Wenzhou area had lower awareness of the government’s recommendation for the voluntary second-dose vaccination. In addition, the low rate of the second dose of varicella vaccination might explain the reason, at least in part, for the increasing incidence and prevalence in the older children in the Wenzhou area, which is parallel with the finding that children who received the second dose of vaccine had a higher level of serum protective IgG antibodies than those who did not receive a second dose. In another way, as the awareness on the necessity of the second dose increases thus actual second-dose vaccination uptake would be increased, the prevalence of varicella can be anticipated to return to the previously low level. This will require the strengthened propaganda for the second dose, and optimally the implement and funding by the government for a two-dose varicella vaccine schedule. Such a two-dose schedule should be mandatory for children who would be enrolled in kindergartens and elementary schools. This approach could also be a remedy for the primary vaccine failure and may provide boosting to those experiencing secondary vaccine failure as well.Citation26
The current study has limitations. With limited resources, the seroepidemiological survey constituted only a preliminary observation as a small cohort of the regional population was sampled. The relatively small sample size might lead to a lack of power to detect the difference. The cohort of the varicella outbreak was only involved in a single classroom. In addition, due to a little lag at sampling time (48 hours), there was a possibility that the level of serum IgG for some of those healthy control children in the outbreak cohort might be boosted because there was a chance that varicella had circulated in that class for some time, which could induce exogenous boosting for healthy control children in the outbreak cohort. Therefore, timely sampling on a much larger cohort, especially more classroom-matched controls, will be required in future studies to help define a generalizable effective level of serum protection IgG antibodies against varicella outbreak.
Conclusions
Our current study clearly demonstrated an upward trend in the incidence and prevalence of varicella in the Wenzhou area from 2010 to 2018, particularly for children age 10–14 years in 2017–2018. Such an uptrend was parallel with a low level of serum antibodies and with a low rate of the second-dose vaccination in those older children. Our study discovered a significant higher serum level of varicella IgG antibodies in healthy control students who exposed to VZV at the outbreak than that in general healthy children. The findings from the present study warn a two-dose vaccine schedule to reduce the climbing incidence and prevalence observed in the older children and suggest a higher serum IgG threshold for effective protection of general healthy children from the varicella outbreak.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the funds from Wenzhou City to the Municipal Key Lab for Pediatrics Research at Department of Pediatric Medicine in the Second Affiliated Hospital of Wenzhou Medical University. We thank staffs at the Hospital Registry section and at Wenzhou Division of the Zhejiang Provincial Center for Disease Control and Prevention (ZJCDC) for their assistance in retrieving relevant data. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the ZJCDC.
Additional information
Funding
References
- Breuer J, Whitley R. Varicella zoster virus: natural history and current therapies of varicella and herpes zoster. Herpes. 2007;14(Suppl2):25–29. PMID:17939892.
- Wiese-Posselt M, Siedler A, Mankertz A, Sauerbrei A, Hengel H, Wichmann O, Poethko-Muller C. Varicella-zoster virus seroprevalence in children and adolescents in the pre-varicella vaccine era, Germany. BMC Infect Dis. 2017;17(1):356. doi:10.1186/s12879-017-2461-2. PMID:28525973
- Cao H, Du J, Jia Z. Prevalence of varicella-zoster virus infection in China. Chin J Vaccines Immunization. 1998;4:38–40.
- Wutzler P, Bonanni P, Burgess M, Gershon A, Safadi MA, Casabona G. Varicella vaccination - the global experience. Expert Rev Vaccines. 2017;16(8):833–43. doi:10.1080/14760584.2017.1343669. PMID:28644696
- Wu PY, Wu HD, Chou TC, Sung FC. Varicella vaccination alters the chronological trends of herpes zoster and varicella. PLoS One. 2013;8(10):e77709. doi:10.1371/journal.pone.0077709. PMID:24204928
- Population Bulletin of Wenzhou City. 2019. Statistical Bureau Wenzhou City in Zhejiang, China. [In Chinese]. [accessed 2020 Mar 15]. http://wztjj.wenzhou.gov.cn/art/2020/3/2/art_1243860_42058089.html
- Hu Y, Chen Y. Evaluating childhood vaccination coverage of NIP vaccines: coverage survey versus zhejiang provincial immunization information system. Int J Environ Res Public Health. 2017;14(7):E758. doi:10.3390/ijerph14070758. PMID:28696387
- Hu Y, Chen Y, Zhang B, Li Q. An evaluation of voluntary varicella vaccination coverage in Zhejiang Province, East China. Int J Environ Res Public Health. 2016;13(6):E560. doi:10.3390/ijerph13060560. PMID:27271649
- Suo L, Lu L, Wang Q, Yang F, Wang X, Pang X, Marin M, Wang C. Varicella outbreak in a highly-vaccinated school population in Beijing, China during the voluntary two-dose era. Vaccine. 2017;35(34):4368–73. doi:10.1016/j.vaccine.2017.06.065. PMID:28684165
- Zhu YF, Li YF, Du Y, Zeng M. Epidemiological characteristics of breakthrough varicella infection during varicella outbreaks in Shanghai, 2008-2014. Epidemiol Infect. 2017;145(10):2129–36. doi:10.1017/S0950268817000772. PMID:28446261
- Fu J, Wang J, Jiang C, Shi R, Ma T. Outbreak of varicella in a highly vaccinated preschool population. Int J Infect Dis. 2015;37:14–18. doi:10.1016/j.ijid.2015.06.003. PMID:26072038.
- Galil K, Lee B, Strine T, Carraher C, Baughman AL, Eaton M, Montero J, Seward J. Outbreak of varicella at a day-care center despite vaccination. N Engl J Med. 2002;347(24):1909–15. doi:10.1056/NEJMoa021662. PMID:12477940
- Vaidya SR, Tilavat SM, Kumbhar NS, Kamble MB. Chickenpox outbreak in a tribal and industrial zone from the Union Territory of Dadra and Nagar Haveli, India. Epidemiol Infect. 2018;146(4):476–80. doi:10.1017/S0950268818000201. PMID:29436318
- Vairo F, Di Bari V, Panella V, Quintavalle G, Torchia S, Serra MC, Sinopoli MT, Lopalco M, Ceccarelli G, Ferraro F, et al. An outbreak of chickenpox in an asylum seeker centre in Italy: outbreak investigation and validity of reported chickenpox history, December 2015-May 2016. Euro Surveill. 2017;22(46):1–9. doi:10.2807/1560-7917.ES.2017.22.46.17-00020. PMID:29162209.
- Yousaf MZ, Zia S, Anjum KM, Ashfaq UA, Imran M, Afzal S, Iqbal MS. Deadly outbreak of chickenpox at district Faisalabad, Pakistan: possible causes, and preventive way forward. Mol Biol Rep. 2018;45(6):2941–43. doi:10.1007/s11033-018-4347-9. PMID:30187309
- Gilbert PB, Gabriel EE, Miao X, Li X, Su SC, Parrino J, Chan IS. Fold rise in antibody titers by measured by glycoprotein-based enzyme-linked immunosorbent assay is an excellent correlate of protection for a herpes zoster vaccine, demonstrated via the vaccine efficacy curve. J Infect Dis. 2014;210(10):1573–81. doi:10.1093/infdis/jiu279. PMID:24823623
- Hammond O, Wang Y, Green T, Antonello J, Kuhn R, Motley C, Stump P, Rich B, Chirmule N, Marchese RD. The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J Med Virol. 2006;78(12):1679–87. doi:10.1002/jmv.20754. PMID:17063506
- Sauerbrei A, Wutzler P. Serological detection of varicella-zoster virus-specific immunoglobulin G by an enzyme-linked immunosorbent assay using glycoprotein antigen. J Clin Microbiol. 2006;44(9):3094–97. doi:10.1128/JCM.00719-06. PMID:16954232
- de Ory F, Echevarria JM, Kafatos G, Anastassopoulou C, Andrews N, Backhouse J, Berbers G, Bruckova B, Cohen DI, de Melker H, et al. European seroepidemiology network 2: standardisation of assays for seroepidemiology of varicella zoster virus. J Clin Virol. 2006;36(2):111–18. doi:10.1016/j.jcv.2006.01.017. PMID:16616612.
- Zhang X, Yu Y, Zhang J, Huang S, Wang Z, Zhang J, Yan Y, Liu F, Zhao J, He Y. The epidemiology of varicella cases among children in Beijing’s Fengtai District from 2008 to 2012. Vaccine. 2014;32(29):3569–72. doi:10.1016/j.vaccine.2014.04.069. PMID:24791731
- Guris D, Jumaan AO, Mascola L, Watson BM, Zhang JX, Chaves SS, Gargiullo P, Perella D, Civen R, Seward JF. Changing varicella epidemiology in active surveillance sites–United States, 1995-2005. J Infect Dis. 2008;197(Suppl2):S71–75. doi:10.1086/522156. PMID:18419413
- Ie B L, Chien YZ, Hsu PS, Chao DY. The changing epidemiology of varicella incidence after implementation of the one-dose varicella vaccination policy. Vaccine. 2011;29(7):1448–54. doi:10.1016/j.vaccine.2010.12.032. PMID:21185851
- Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, Reisinger K, Kim LL, Lupinacci L, Hartzel J, et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004;23(2):132–37. doi:10.1097/01.inf.0000109287.97518.67. PMID:14872179.
- Xu Y, Liu S, Che X, Liu Y, Zhang X, Du J, Zhang X, Wang J, Xu E. Seroepidemiology of varicella in Hangzhou, China in the vaccine era. Hum Vaccin Immunother. 2018;14(10):2464–71. doi:10.1080/21645515.2018.1477909. PMID:30019992
- Chang LY, Huang LM, Chang IS, Tsai FY. Epidemiological characteristics of varicella from 2000 to 2008 and the impact of nationwide immunization in Taiwan. BMC Infect Dis. 2011;11(1):352. doi:10.1186/1471-2334-11-352. PMID:22176638
- Zhang L, Ma W, Liu Y, Wang Y, Sun X, Hu Y, Deng X, Lu P, Tang F, Wang Z, et al. Analysis of sero-epidemiological characteristics of varicella in healthy children in Jiangsu Province, China. BMC Infect Dis. 2018;18(1):563. doi:10.1186/s12879-018-3496-8. PMID:30428851.
- Lin M-R, Kuo -C-C, Hsieh Y-C, Huang Y-L, Huang Y-C, Hung Y-T, Huang Y-C. Seroepidemiology of varicella among elementary school children in northern Taiwan. J Microbiol Immunol Infect. 2017;50(3):321–26. doi:10.1016/j.jmii.2015.07.007. PMID
- Chris Maple PA, Gunn A, Sellwood J, Brown DW, Gray JJ. Comparison of fifteen commercial assays for detecting Varicella Zoster virus IgG with reference to a time resolved fluorescence immunoassay (TRFIA) and the performance of two commercial assays for screening sera from immunocompromised individuals. J Virol Methods. 2009;155(2):143–49. doi:10.1016/j.jviromet.2008.09.032. PMID:18996415
- McDonald SL, Maple PA, Andrews N, Brown KE, Ayres KL, Scott FT, Al Bassam M, Gershon AA, Steinberg SP, Breuer J. Evaluation of the time resolved fluorescence immunoassay (TRFIA) for the detection of varicella zoster virus (VZV) antibodies following vaccination of healthcare workers. J Virol Methods. 2011;172(1–2):60–65. doi:10.1016/j.jviromet.2010.12.021. PMID:21192976
- De Paschale M, Clerici P. Microbiology laboratory and the management of mother-child varicella-zoster virus infection. World J Virol. 2016;5(3):97–124. doi:10.5501/wjv.v5.i3.97. PMID:27563537
- Miyachi M, Ihara H, Imafuku S. Incidence of serum antibody titers against varicella zoster virus in Japanese patients with herpes zoster. J Dermatol. 2017;44(6):656–59. doi:10.1111/1346-8138.13733. PMID:28012212
- Andrade AL, da Silva Vieira MA, Minamisava R, Toscano CM, de Lima Souza MB, Fiaccadori F, Figueiredo CA, Curti SP, Nerger M, Bierrenbach AL, et al. Single-dose varicella vaccine effectiveness in Brazil: A case-control study. Vaccine. 2018;36(4):479–83. doi:10.1016/j.vaccine.2017.12.011. PMID:29249544.
- Amjadi O, Rafiei A, Haghshenas M, Navaei RA, Valadan R, Hosseini-Khah Z, Omran AH, Arabi M, Shakib RJ, Mousavi T, et al. A systematic review and meta-analysis of seroprevalence of varicella zoster virus: A nationwide population-based study. J Clin Virol. 2017;87(1):49–59. doi:10.1016/j.jcv.2016.12.001. PMID:28011413.
- Thomas CA, Shwe T, Bixler D, Del Rosario M, Grytdal S, Wang C, Haddy LE, Bialek SR. Two-dose varicella vaccine effectiveness and rash severity in outbreaks of varicella among public school students. Pediatr Infect Dis J. 2014;33(11):1164–68. doi:10.1097/inf.0000000000000444. PMID:24911894
- Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global Varicella Vaccine Effectiveness: A Meta-analysis. Pediatrics. 2016;137(3):e20153741. doi:10.1542/peds.2015-3741. PMID:26908671