ABSTRACT
Uzbekistan, the most populous country in central Asia, was the first in the region to introduce rotavirus vaccine into its national immunization program. Rotarix (GlaxoSmithKline Biologicals, RV1) was introduced in June 2014, with doses recommended at age 2 and 3 months. To evaluate vaccine impact, active surveillance for rotavirus diarrhea was reestablished in 2014 at 2 hospitals in Tashkent and Bukhara which had also performed surveillance during the pre-vaccine period 2005−2009. Children aged <5 y admitted with acute diarrhea had stool specimens collected and tested for rotavirus by enzyme immunoassay. Proportions testing rotavirus-positive in post-vaccine years were compared with the pre-vaccine period. Vaccine records were obtained and effectiveness of 2 RV1 doses vs 0 doses was estimated using rotavirus-case and test-negative design among children enrolled from Bukhara city. In 2015 and 2016, 8%−15% of infants and 10%−16% of children aged<5 y hospitalized with acute diarrhea at the sites tested rotavirus-positive, compared with 26% of infants and 27% of children aged<5 y in pre-vaccine period (reductions in proportion positive of 42%−68%, p <.001). Vaccine effectiveness of 2 RV1 doses vs 0 doses in protecting against hospitalization for rotavirus disease among those aged ≥6 months was 51% (95% CI 2–75) and is based on cases predominantly of genotype G2P[4]. Vaccine effectiveness point estimates tended to be higher against cases with higher illness severity (e.g., clinical severity based on modified Vesikari score ≥11). Our data demonstrate that the monovalent rotavirus vaccine is effective in reducing the likelihood of hospitalization for rotavirus disease in young children in Uzbekistan.
Introduction
On June 15, 2014, Uzbekistan became the first of the central Asian countries (Kazakhstan, Kyrgyzstan, Uzbekistan, Turkmenistan and Tajikistan) to introduce rotavirus vaccine into its Expanded Program on Immunizations (EPI). Tajikistan introduced rotavirus vaccine in January 2015 and Kyrgyzstan did so in December 2019. Uzbekistan is the most populous country in central Asia, and at the time of vaccine introduction had a birth cohort of 716,000, total population of 30.8 million and was classified as a lower middle-income county, with GNI per capita of 2,210 USD.Citation1 An estimated 754 (range 651−858) children died annually from rotavirus in the period just before vaccine introduction.Citation2 The health system has been largely government-owned, with the government being the principal payer and provider of health services.Citation3 In urban areas, primary health care and some secondary care services are provided by polyclinics, with catchment populations of 10,000–80,000. Throughout the country, primary care provided by public providers is free of charge as part of the state-guaranteed package of medical services (this does not include all pharmaceutical coverage). Primary care is also available in the private sector on a fee-for-service basis.
The EPI program in Uzbekistan introduced the human rotavirus vaccine, Rotarix (GlaxoSmithKline Biologicals, Rixensart, Belgium)(RV1), and vaccination was recommended at age 2 and 3 months, at the same time as the oral polio vaccine and pentavalent vaccine. The recommended maximum age for the first dose of rotavirus vaccine was 6 months. Vaccine introduction was supported financially by Gavi, the Vaccine Alliance. Based on largely administrative data that have important limitations, 2-dose rotavirus vaccine coverage for infants in Uzbekistan was reported to be ≥95% during 2015–2017.Citation4
Active sentinel-site surveillance for rotavirus disease among children aged <5 y hospitalized with acute diarrhea had been conducted in the country during 2005−2009.Citation5–7 During this period, the reported annual proportion of enrolled children who had rotavirus detected in their stool sample measured by enzyme immunoassay ranged between 22% and 33%.Citation5 Surveillance was reestablished in 2014 so that the impact and effectiveness of the vaccine could be measured and here we present the results of the evaluation.
Participants and methods
Sentinel-site surveillance for children hospitalized with rotavirus diarrhea
During 2005−2009, active sentinel-site rotavirus surveillance had been conducted at the 4th Tashkent City Infectious Diseases Hospital in the capital, Tashkent, and Bukhara Regional Infectious Diseases Hospital in Bukhara.Citation5,Citation6 Given the large number of diarrhea admissions at the Tashkent hospital, the goal was for every third child admitted with acute gastroenteritis to be approached for enrollment. At Bukhara, the goal was to enroll all eligible children.
Surveillance was reestablished on January 16, 2014, at the Tashkent hospital and August 1, 2014, at the Bukhara hospital. As in the earlier surveillance period, these two hospitals were the main hospitals designated to take care of children with diarrhea in the respective areas. Following the generic WHO protocol, children aged <5 y were eligible for enrollment in the surveillance if they presented with acute diarrhea (duration ≤7 d before admission) as the main reason or one of the main reasons for hospital admission, with diarrhea defined as ≥3 looser-than-normal stools within any 24-h period of the illness before admission.Citation8 Children for whom blood had been observed in the stool were not eligible. At Tashkent, because of the large number of diarrhea admissions, a sampling strategy for enrollment was used initially but this was revised during 2014−2015; it depended on a child’s date of birth (i.e., to maximize enrollment of children born in or after year of vaccine introduction, for a potential vaccine effectiveness evaluation). The enrollment strategy was: for children born < 01 January 2014, every other child was to be enrolled during January 2014−May 2015; for children born ≥01 January 2014, every other child was to be enrolled during January 2014−October 2014, and every child was to be enrolled during November 2014−May 2015; from June 2015 through December 2016, the Tashkent enrollment strategy was simplified to attempting to enroll every child aged <5 y who met the diarrhea criteria, regardless of the date of birth.
Among enrolled children, demographic information and information on the child’s illness before admission (number of days with diarrhea, number of days with vomiting, maximum number of episodes of diarrhea and vomiting in a 24-h period) and hospital course (i.e., maximum temperature during the first 24 h of admission, whether intravenous fluids were given for rehydration) were recorded on a form. A whole stool sample was to be obtained on each child as soon as possible after admission and ideally within 48 h of admission.
After vaccine introduction, the parents of enrolled children who were eligible to have received rotavirus vaccine before admission (i.e., born on or after April 15, 2014) were asked to provide the name of the polyclinic(s) where the child went to receive routine care, including immunizations. EPI managers of the regions then organized procedures to collect a copy of the vaccine records of the enrolled children from the respective polyclinics, including documenting if the child had received no vaccine doses or had moved. Dates of doses of OPV and rotavirus vaccine were abstracted from these copies.
Whole stool samples were stored at 4°C and delivered to the national Reference Laboratory of Research, Institute of Virology in Tashkent. Testing by enzyme immunoassay (EIA; ProSpecT Rotavirus Test; Oxoid) was performed there following the manufacturer’s instructions. For quality control, some EIA-positive and EIA-negative specimens were retested by ProSpecT EIA at WHO Regional Reference Laboratory in Minsk, Belarus. For the 2-y period with results available, a random selection of 200 EIA-positive and 100 EIA negative samples had been retested at the Regional Reference Laboratory; the overall concordance between EIA results at the Tashkent laboratory and the Minsk laboratory was 94% (281/300). Samples available on children in the Bukhara vaccine effectiveness analysis were transported for genotyping to the Global Rotavirus Reference Laboratory at the Centers for Disease Control and Prevention, Atlanta, GA. These samples had been stored at −20°C and were transported frozen. Genotyping was performed at CDC following published methods.Citation9,Citation10
The project was approved by the National Committee on Bioethics of Uzbekistan and was deemed to be public health evaluation by the Human Subjects Committee at CDC.
Trends in the proportion of hospitalized children testing rotavirus EIA-positive
For two age groups (age <1 y, age <5 y), we compared the annual proportion of children enrolled who were rotavirus EIA-positive during the post-introduction years to the earlier data from the pre-vaccine period. For the pre-vaccine period, we used data from the most complete years: 2006 and 2009 because children from each hospital were enrolled during each of the calendar months, and used adjusted data from 2005 (for January 2005, we used the data from February 2005 because enrollment started at the end of January, and for October 2005, we used data from the last 2 weeks of the month to also represent the data from the first 2 weeks because a strike had occurred at the hospitals that prevented enrollment in the first 2 weeks). For post-introduction data from Tashkent during January 2014–May 2015, the results were adjusted for the enrollment strategy used during that particular month (e.g., numbers of children testing positive vs negative were doubled for months when every second child in that age group was to be enrolled) so that the annual results appropriately represented children in each age group (age <1 y and age <5 y). During June 2015 through December 2016, the goal was for every eligible child at Tashkent to be enrolled so no adjustment was needed. No adjustment was needed for Bukhara data in the post-introduction period.
Vaccine effectiveness of RV1
Vaccine effectiveness was estimated among vaccine-eligible children (those born ≥15 April 2014) using case and test-negative control design, classifying the children based on the rotavirus EIA result. This evaluation was performed among those enrolled at Bukhara Hospital and who lived in Bukhara city, the location in which a high proportion of vaccine records of enrolled children was able to be retrieved from the polyclinics.
An RV1 dose was counted for analysis if it had been received ≥14 d before the admission date. RV1 VE was calculated as (1-odds ratio [OR] X 100%). ORs for receipt of 2 RV1 doses vs 0 doses for rotavirus case-subjects compared with test-negative controls were determined by unconditional logistic regression, controlling for birth year, birth month, admission year and admission quarter. In 90% of the control children with an analyzable vaccine record, rotavirus vaccine status did not change after age 6.0 months. Overall VE was calculated for children aged ≥6 months, which eliminated the need to control for confounding by age; children aged <6 months were not included in VE analyses. Sub-analyses to assess VE by age group (i.e., 6−11 months, ≥12 months) and against more severe rotavirus disease (i.e., receipt of intravenous fluids, higher severity score using a modified scoring system [Supplementary Table 1]) were planned a priori. A post hoc analysis using the duration of hospitalization as a marker of severity was also conducted. If a child was enrolled in any calendar year with >1 gastroenteritis episode, only the rotavirus-positive episode was used in the analysis if one occurred, and only the latest episode was used for that calendar year if all the child’s episodes were rotavirus-negative. Once a child was enrolled with a rotavirus-positive episode, any subsequent gastroenteritis episodes were not used in analyses. Analyses were performed with Stata software version 13.0 (StataCorp, College Station, TX, USA).
Results
Trends in the proportion of hospitalized children testing rotavirus EIA-positive
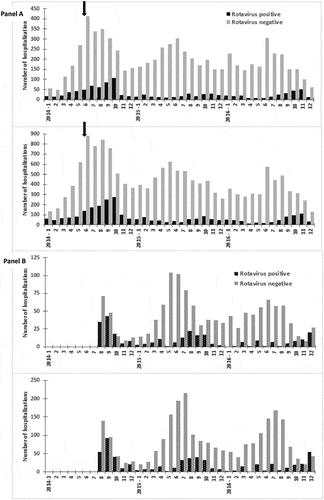
During the 2014–2016 enrollment, 13,220 samples from Tashkent and 2,594 from Bukhara were collected within 48 h of admission and tested. Compared with the data collected during 2005−2009, a reduction in the proportion of children testing rotavirus-positive was observed at both hospitals following the introduction of rotavirus vaccine in 2014 (, ). During the prevaccine years combined, 26% of infants enrolled at each hospital (513/1945 at Tashkent and 294/1129 at Bukhara) tested rotavirus-positive. In Tashkent, the proportion rotavirus-positive was reduced to 8% (231/2721) and 11% (257/2360) in 2015 and 2016, respectively, and at Bukhara, the proportion rotavirus-positive was 15% (105/716) and 13% (70/551) in those years, respectively. These corresponded to 68% and 59% reduction in positivity rate at Tashkent, and 43% and 51% at Bukhara. Among children aged <5 y, the proportion rotavirus-positive was reduced from 27% in both locations in pre-vaccine period (1153/4216 in Tashkent; 535/4216 in Bukhara), to 10% (624/5981) and 13% (590/4677) in Tashkent in 2015 and 2016, respectively, and to 15% (210/1359) and 16% (182/1162) at Bukhara in those years. These corresponded to reductions in proportion positive of 42%−68%. From the pre-vaccine data, peak numbers of rotavirus cases often occurred in October. Based on the timing of vaccine introduction, children who were eligible to have received the vaccine on the routine EPI schedule beginning at age 2 months would have been aged 2−6 months in October 2014, 2−18 months in October 2015, and 2−30 months in October 2016.
Table 1. Proportion of children who tested rotavirus EIA-positive by hospital, calendar year and age group, and percentage reduction after vaccine introduction
Figure 1. Number of children hospitalized for diarrhea who tested positive or negative for rotavirus by EIA, by hospital and age group, 2014−2016. Panel A. Tashkent (top: age <1 y; bottom: age <5 y). Panel B. Bukhara (top: age <1 y; bottom: age <5 y). Arrow indicates vaccine introduction, 6/15/2014. Note the differences in scale of y-axes. See footnote 2 of
Vaccine effectiveness of RV1
Among the Bukhara city vaccine-eligible children aged ≥6 months who were enrolled in surveillance and for whom a stool specimen was tested for rotavirus, vaccine records able to be used in analyses were obtained on 80% (102/128) of rotavirus cases and 85% (499/590) of rotavirus-test negative controls. Among the controls, the median age at the first RV1 dose was 2.4 months (IQR 2.1, 3.2) and the median age at the second dose was 3.7 months (IQR 3.3, 4.8).
Among the 102 cases aged ≥6 months, 75% (76) had received 2 RV1 doses, 10% (10) had received 1 RV1 dose, and 16% (11) had received no RV1 doses. Among the 499 test-negative controls, 80% (400) had received 2 doses, 9% (47) had received 1 dose, and 10% (52) had received no doses. The vaccine effectiveness estimate for 2 RV1 doses vs 0 doses among those aged ≥6 months was 51% (95% CI 2–75). The point estimate was not lower in the age group ≥12 months compared with age 6−11 months, but for both estimates, the 95% CI included zero ().
Table 2. Vaccine effectiveness of 2 RV1 doses vs 0 doses, by age group and rotavirus-case severity status
Point estimates from Bukhara tended to increase when the rotavirus outcome was more severe illness (e.g., cases hospitalized for ≥5 d, VE = 57% [95% CI 2−81]; cases with severity score ≥11, VE = 67% [95% CI 0−89]) however, confidence intervals were wide and included zero for some categories.
Genotype results were available on a subset of the rotavirus samples from Bukhara children whose data contributed to the vaccine effectiveness estimate. Of the 79% (73/92) with results, 55% (40/73) were G2P[4], 29% (21/73) were G9P[4], 7% (5/73) were GNTP[4], 4% (3/73) were G9P[8], 1% (1/73) was G2P[NT], 1% (1/73) was GNTP[NT], and 3% (2/73) were mixed G2G12/P[4]P[8]. Of those with known P genotype, 93% were only of P[4] genotype, 4% were only P[8] and 3% were mixed P[4]P[8].
Discussion
Our data support that rotavirus vaccine is effective in reducing the likelihood of hospitalization for rotavirus gastroenteritis in young children in urban Uzbekistan and demonstrate that the proportion of children who test rotavirus-positive among those hospitalized for acute diarrhea is lower in the post-introduction period compared with that from earlier years. Data from the vaccine records indicate that the vaccine is received in a timely manner, closely aligned with the recommended schedule.
Our 2-dose RV1 vaccine effectiveness has wide confidence intervals and the point estimate is in the lower range of those published through 2016 for countries with medium and high under-5 child mortality (e.g., the 5 evaluations with a point estimate of <55% were from Brazil [one of the three evaluations], Colombia, Guatemala, Botswana and Ghana).Citation11 As commented on previously for other countries of the former Soviet Union, it is possible that the children hospitalized for diarrhea at our sites have an overall lower level of severity than those in other regions where vaccine effectiveness evaluations have been performed, and this may be one reason our effectiveness estimate is in the lower range.Citation12,Citation13 In clinical trials, the efficacy of rotavirus vaccines was higher against rotavirus disease of higher severity compared with efficacy against rotavirus disease of any severity, and the primary goal of rotavirus vaccination has been to prevent severe rotavirus morbidity and mortality.Citation14,Citation15 The concept that children hospitalized at our sites have lower severity of diarrheal illness overall is also supported by the data from the pre-vaccine period where the proportion of those aged <5 y who tested rotavirus-positive from our hospitals, 27%, was lower than the pre-vaccine mean of 38% from all regions following the WHO protocol.Citation16 Although some estimates were not statistically significant, our vaccine effectiveness point estimates tended to be higher against rotavirus illness with higher severity, which supports that vaccinated children are being protected against the more severe outcomes from rotavirus infection.
Published data on circulating genotypes in Uzbekistan before vaccine introduction are very limited; among 52 samples collected during the 2005 surveillance for which genotyping was performed, 27 (52%) were G1P[8], 10 (19%) were G2P[4], 2(4%) were G4P[8], 4 (8%) were mixed G types with P[8], and other genotypes were detected only once or with G or P not typeable.Citation6 Among the children contributing to our vaccine effectiveness results, rotavirus strains of genotype P[4] made up >90% of genotyped strains. G2P[4] and G9P[4] strains, such as those in our surveillance, usually belong to the “DS-1-like” genogroup, with all 11 genes and protein antigens typically distinct from those strains of the “Wa-like” genogroup, such as G1P[8] strains and most other P[8] strains. Some data support that the monovalent rotavirus vaccine composed of only G1P[8] strain may be less effective against strains that are heterotypic in both P and G type, such as G2P[4], compared with effectiveness against strains that contain genotype P[8].Citation17,Citation18 However, a meta-analysis of vaccine effectiveness evaluations against disease by genotype category (genotypes with same G or P type as G1P[8] vaccine vs those with different G and P type) found no statistically significant differences in effectiveness in middle and higher income settings where most comparative data were available, and other data suggest differences in those settings may be modest at most.Citation19–22 Across regions and with differences in circulating genotypes, monovalent rotavirus vaccine has demonstrated effectiveness in providing protection against severe rotavirus disease.
Our evaluation has limitations. Our vaccine effectiveness estimate is based on children from Bukhara city. However, the very low proportion of children testing rotavirus positive at the Tashkent hospital in 2015 and 2016, both of which were lower than the proportion rotavirus-positive in the introduction year of 2014, lends additional evidence that the vaccine is, as expected, also effective in the largest urban area in Uzbekistan. In Tashkent in 2016, there was no sampling strategy for enrollment (as there had been in 2014 and 2015) so the results in proportion rotavirus-positive from 2016 would be expected to be more robust compared with that from the other years. The key limitation in our analysis of trends in proportion testing rotavirus positive is the lack of rotavirus surveillance data for at least one full calendar year immediately before the introduction of the vaccine. How well the data from 2005−2009 estimate the more proximal pre-vaccine period is unknown and hence our trend analysis may be over- or under-estimating the impact of the vaccine. However, the earlier data from the same two surveillance sites are valuable, and, as mentioned above, the finding that the proportion rotavirus-positive in Tashkent for 2014 was intermediate between the pre-vaccine and 2015−2016 results supports that the data available for the pre-vaccine period were reasonable estimates.
The data from two urban areas in Uzbekistan support that the monovalent rotavirus vaccine is effective in reducing the likelihood of hospitalization for rotavirus disease and that the country’s decision to lead vaccine introduction in the region is providing an important health benefit to its children.
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to disclose.
Disclaimer
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the World Health Organization or Ministries of Health with which they are affiliated. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplemental Material
Download MS Word (35.9 KB)Acknowledgments
We acknowledge Sevinch Kurbanova, Victoria Eolian, Galina Semeiko, Elena Samoilovich, Ara Tadevosyan and Simarjit Singh for their efforts on the evaluation. We also acknowledge Elmira Flem, currently with Norwegian Institute of Public Health who, along with Renat Latipov, led the sentinel surveillance during 2005−2009.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- [accessed 2020 May 5]. https://data.worldbank.org/country/uzbekistan
- Tate JE, Burton AH, Boschi P, Parashar UD. WHO-coordinated global rotavirus surveillance network. global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;S2:S96–S105.
- Ahmedov M, Azimov R, Mutalova Z, Huseynov S, Tsoyi E, Rechel B. Uzbekistan: health system review. Health Syst Transit. 2014;16:1–137.
- [accessed 2020 May 5]. https://www.who.int/immunization/monitoring_surveillance/data/uzb.pdf
- Latipov R, Utegenova E, Kuatbayeva A, Kasymbekova K, Abdykarimov S, Juraev R, Ismailov U, Flem E. Epidemiology and burden of rotavirus disease in Central Asia. Int J Infect Dis. 2011 Jul;15(7):e464–9. doi:10.1016/j.ijid.2011.03.014.
- Flem ET, Musabaev E, Juraev R, Kerin T, Gentsch J, Glass RI, Bresee JS. Rotavirus gastroenteritis in Uzbekistan: implications for vaccine policy in central Asia. J Infect Dis. 2009 Nov 1;200(Suppl 1):S154–9. doi:10.1086/605032.
- Isakbaeva ET, Musabaev E, Antil L, Rheingans R, Juraev R, Glass RI, Bresee JS. Rotavirus disease in Uzbekistan: cost-effectiveness of a new vaccine. Vaccine. 2007 Jan 4;25(2):373–80. doi:10.1016/j.vaccine.2006.07.029.
- World Health Organization. Generic protocol for monitoring impact of rotavirus vaccination on rotavirus disease burden and viral strains. WHO, 2009 cited 2019 Dec 17. Available from: https://www.who.int/immunization/monitoring_surveillance/resources/NUVI/en/
- Bowen MD, Mijatovic-Rustempasic S, Esona MD, Teel EN, Gautam R, Sturgeon M, Azimi PH, Baker CJ, Bernstein DI, Boom JA et al. Rotavirus strain trends during the postlicensure vaccine Era: united States, 2008–2013. J Infect Dis. 2016 Sep 1;214(5):732–38. doi:10.1093/infdis/jiw233.
- Esona MD, Gautam R. Rotavirus. Clin Lab Med. 2015 Jun;35(2):363–91. doi:10.1016/j.cll.2015.02.012.
- Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006–2016. Clin Infect Dis. 2017 Sep 1;65(5):840–50. doi:10.1093/cid/cix369.
- Sahakyan G, Grigoryan S, Wasley A, Mosina L, Sargsyan S, Asoyan A, Gevorgyan Z, Kocharyan K, Avagyan T, Lopman B et al. Impact and effectiveness of monovalent rotavirus vaccine in Armenian CHILDREN. Clin Infect Dis. 2016 May 1;62(Suppl 2):S147–54. doi:10.1093/cid/ciw045.
- Gheorghita S, Birca L, Donos A, Wasley A, Birca I, Cojocaru R, Melnick A, Ciobanu S, Mosina L, Cortese MM, et al. Impact of rotavirus vaccine introduction and vaccine effectiveness in the Republic of Moldova. Clin Infect Dis. 2016 May 1;62(Suppl 2):S140–6. doi:10.1093/cid/civ1209.
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007 Nov 24;370(9601):1757–63. doi:10.1016/S0140-6736(07)61744-9.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006 Jan 5;354(1):23–33. PubMed PMID: 16394299. doi:10.1056/NEJMoa052664.
- Aliabadi N, Antoni S, Mwenda JM, Weldegebriel G, Biey JNM, Cheikh D, Fahmy K, Teleb N, Ashmony HA, Ahmed H, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under5 years of age, 2008–16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Health. 2019 Jul;7(7):e893–e903. doi:10.1016/S2214-109X(19)30207-4.
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006 Jan 5;354(1):11–22. doi:10.1056/NEJMoa052434.
- Bar-Zeev N, Kapanda L, Tate JE, Jere KC, Iturriza-Gomara M, Nakagomi O, Mwansambo C, Costello A, Parashar UD, Heyderman RS, et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis. 2015;15:422–28. doi:10.1016/S1473-3099(14)71060-6.
- Leshem E, Lopman B, Glass R, Gentsch J, Bányai K, Parashar U, Patel M. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis. 2014 Sep;14(9):847–56. doi:10.1016/S1473-3099(14)70832-1.
- Pitzer VE, Bilcke J, Heylen E, Crawford FW, Callens M, De Smet F, Van Ranst M, Zeller M, Did Large-Scale MJ. Vaccination drive changes in the circulating rotavirus population in Belgium? Sci Rep. 2015 Dec 21;5:18585. doi:10.1038/srep18585.
- Matthijnssens J, Zeller M, Heylen E, De Coster S, Vercauteren J, Braeckman T, Van Herck K, Meyer N, PirÇon J-Y, Soriano-Gabarro M, et al. Higher proportion of G2P[4] rotaviruses in vaccinated hospitalized cases compared with unvaccinated hospitalized cases, despite high vaccine effectiveness against heterotypic G2P[4] rotaviruses. Clin Microbiol Infect. 2014 Oct;20(10):0702–10. doi:10.1111/1469-0691.12612.
- Roczo-Farkas S, Kirkwood CD, Cowley D, Barnes GL, Bishop RF, Bogdanovic-Sakran N, Boniface K, Donato CM, Bines JE. The impact of rotavirus vaccines on genotype diversity: A comprehensive analysis of 2 decades of Australian surveillance data. J Infect Dis. 2018 Jul 13;218(4):546–54. doi:10.1093/infdis/jiy197.
