ABSTRACT
Rotavirus (RV) vaccines have been available in Turkey since 2006. These vaccines are not funded by the National Health System, so consequently their coverage rate in children has reached only 13–18%. We conducted a retrospective record review including all children ≤60 months of age presenting to study hospitals with suspected or clinical acute gastroenteritis (AGE) between 2012 and 2018. During the study, 109,605 children ≤60 months of age were admitted to pediatric out-patient clinics and pediatric emergency room, of which 15,501 (14%) were diagnosed with AGE. Incidence of RV-positive AGE decreased from 4.47 per 1,000 children in 2012 to 2.48 per 1,000 in 2018. A total of 4,805 (31%) such children were hospitalized with RV-positive AGE, a decrease from 1.9 per 1,000 children in 2012 to 0,45 per 1,000 in 2018. The length of hospital stays (LOS) of RV-positive AGE was 2.47 ± 1.15 days compared to LOS of RV-negative AGE 1.59 ± 1.17 days (p < .001). The overall cost of RV-positive AGE ($335 ± 200) was higher than that for RV-negative AGE ($280 ± 148) cases (p = .015). Vaccine effectiveness against any case of RV-positive AGE was 75,1% (95% CI: 65–86%). Despite the low level of vaccine coverage, the introduction of RV vaccination had a positive impact on the incidence of RV-positive AGE and related hospitalizations in young children.
Introduction
Acute gastroenteritis (AGE) is the second-leading cause of morbidity and mortality after pneumonia in children under the age of five years.Citation1 AGE is associated with an estimated 1.3 million deaths annually with most occurring in resource-limited countries – the youngest children are most vulnerable; the incidence of severe gastroenteritis is highest in the first two years of life.Citation2 AGE is caused by a wide range of etiological agents including viruses, bacteria, and parasites. In low- and middle-income countries, bacterial enteritis is very common.Citation3 Rotavirus (RV) is the most common cause of AGE in children both in upper-income countries and low/middle-income countries.Citation3 RV is generally spread by direct contact; thus, improvements in water quality are unlikely to have a significant impact on transmission. RV is estimated to account for ~39% of diarrhea-related hospitalizations and an estimated 199,000 deaths each year mostly in children under the age of two years.Citation4 Most of the mortality is reported from low-income countries in Africa and Asia.Citation5 By the age of five years, nearly every child will have experienced a symptomatic infection with the peak incidence occurring among children aged 4–23 months.Citation3
RV infection is also a common cause of diarrhea-related hospital admissions in countries with low childhood mortality.Citation3 Thus, RV vaccines are a primary strategy for reducing the mortality and morbidity associated with this infection. By the end of 2006, two live oral RV vaccines had been licensed in more than 100 countries and prequalified by the World Health Organization (WHO). RotaTeq (Merck and Co) is a three-dose pentavalent bovine-human reassortant RV vaccine, and Rotarix (GSK Biologics) is a two-dose monovalent human G1P [8] RV vaccine. More than 80 countries have introduced RV vaccines into their national routine infant vaccination programs. Clinical trials of RV vaccine efficacy (RotaTeq and Rotarix) and post-licensure effectiveness evaluations in high- and upper-middle-income countries demonstrated that the vaccines were >90% effective in preventing severe RV infection. However, clinical trials in countries with a higher child mortality found that RV vaccines were less efficacious in these settings with published efficacy estimates ranging from 50–64%.Citation6 Post-licensure vaccine effectiveness (VE) studies demonstrated that the Rotarix VE was 84% in countries with low levels of child mortality, 75% in countries with medium levels of child mortality, and 57% in countries with high levels of child mortality. Furthermore, the median RotaTeq VE was 90% in countries with low levels of child mortality and 45% in countries with high levels of child mortality.Citation7
In Turkey, both monovalent (RV1, Rotarix, GSK) and pentavalent (RV5, Rotateq, Merck) vaccines were licensed in 2006. These vaccines are not funded by the Turkish national health system and are not included in the Turkish National Immunization Program (NIP) but are available in the private market. Although not included in the routine national immunization program, the estimated national coverage of full-dose RV vaccination in Turkey after the introduction of RV vaccines increased from 8% in 2012 to approximately 16% in 2018. The WHO recommends the use of RV vaccines in all national immunization programs globally – particularly in countries with high diarrheal mortality among children.Citation8 However, by the end of 2018, RV vaccines had been introduced on the national immunization schedule in 101 countries worldwide.Citation9 In the rest of the world, the vaccine is either not licensed or is only offered after private pay. Immunization efficacy data have evaluated the incidence of infection before and after introduction of RV into the national schedule. However, the efficacy of RV vaccination in self-funded conditions and/or situations with very low rates of vaccine coverage are unknown.Citation10 It is important to know whether the self-funded RV vaccination strategy or the low rate of vaccination across the country is meaningful. To date, there is no data related to RV VE in Turkey. Thus, our goal here was to study the impact of self-financed RV vaccination on the rate of AGE and related hospitalizations and to determine the causes of AGE in children ≤ 60 months of age in Turkey.
Materials and methods
We conducted a retrospective record review including all children ≤ 60 months of age presenting to study hospitals with suspected or clinical AGE between 2012 and 2018. This study was performed at Ataşehir Memorial Hospital and Şişli Memorial Hospital. These are located in two different districts of Istanbul (population > 16 million, approximately 20% of Turkey’s population). Both hospitals are large primary and tertiary care hospitals that focus on work with private health insurance systems. The cases were children aged 1 to 60 months of age with a diagnosis of AGE in our study hospitals’ pediatric outpatient and emergency room (ER) units from January 1, 2012, to December 31, 2018. These cases were identified according to the International Statistical Classification of Diseases and Related Health Problems-10th Revision (ICD-10 codes): A08.0, rotaviral enteritis; A08.1, acute gastroenteropathy due to Norwalk agent (norovirus); A08.2, adenoviral enteritis; A08.3, other viral enteritis; A08.4, viral intestinal infection, unspecified; A08.5, other specified intestinal infections; A09.0, other and unspecified gastroenteritis and colitis of infectious origin; or A09.9, gastroenteritis and colitis of unspecified origin.
Admission and discharge dates were used to calculate the length of hospital stay for RV-positive AGE and RV-negative AGE. The costs of RV-positive and RV-negative AGE cases were drawn from hospital information systems that stored patient invoices sent to insurance companies. We determined RV VE using a case-control study design with RV-positive cases and RV-negative controls identified throughout the study period. We enrolled all RV-positive cases (n = 220) with randomly selected RV-negative controls at a 2:1 ratio (n = 440). All cases and controls whose vaccination records were documented had either a complete vaccination schedule for either RV1 or RV5. The vaccine efficacy was calculated as (1-OR) x 100 (%). Although RV vaccines have been available for private purchase since 2006, private market sales data were obtained from the respective manufacturers for the study period, and coverage was estimated to be low (<15%) during this time. The control group considered the incidence of acute appendicitis that is unlikely to be related to RV vaccination during the study period for children ≤ 60 months. The study was approved by the Şişli Memorial Hospital Ethical Committee.
Stool analysis
The detection of RV and adenovirus was performed by chromatographic immunoassay (Rapid-Viditest, Vidia spool. S.r.o., Czech Republic). The detection of norovirus was performed using an immunochromatic rapid test (Rida Quick Norovirus, R-Biopharm AG, Darmstadt, Germany), and the detection of Entamoeba histolytica antigens in stool samples was performed using an immunochromatographic one-step test (Operon, S. A, Zaragoza, Spain).
Statistical analysis
Data were entered into Microsoft Office Excel 2007 (Microsoft, Redmond, WA, USA) and analyzed using Stata 10.0 Statistics/Data Analysis (StataCorp, Lakeway, Drive, TX, USA). The annual incidences were calculated as the total number of cases divided by 1000 population. We used the Pearson correlation coefficient (r) to measure the linear correlation between the study period (years) and the incidences of AGE and AGE-related hospitalizations. A t-test was used to determine if there was a difference between RV-positive and RV-negative AGE in the mean length of hospital stay and all-medical cost. A p-value <0.05 was considered significant.
Results
From January 1, 2012, to December 31, 2018, 109,605 children ≤ 60 months of age were admitted to pediatric outpatient clinics and pediatric emergency rooms (mean: 15,657 children/year; range: 11,844–18,140 children/year) ().
Table 1. Incidence of Acute Gastroenteritis with respect to causative agents and years
Frequency of acute gastroenteritis
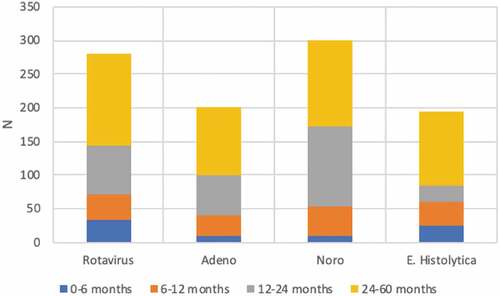
A total of 15,501 (14%) children were diagnosed with AGE (mean: 2,214 children/year; range: 1,814–2,546 children/year) (). Of the children diagnosed with AGE, 3,447 (22%, mean: 492 children/year, range: 480–521 children/year) provided stool samples for microbiological examination (). The rate of diagnosis of AGE did change from 16% to 13% between 2012 and 2018 (). Among the children who provided stool samples, 281 were positive for RV (8.1%, mean: 40 children/year, range: 45–53 children/year); 212 were positive for adenovirus (6.0%, mean: 30 children/year, range: 21–44 children/year); 301 were positive for norovirus (8.7%, mean: 43 children/year, range: 9–91 children/year); and 194 were positive for E. histolytica (5.6%, mean: 28 children/year, range: 16–35 children/year).
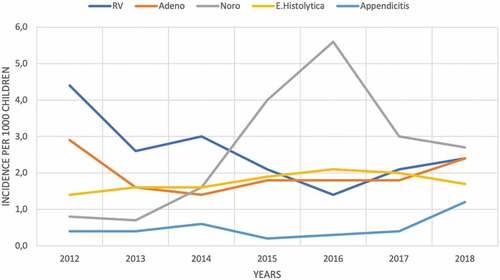
summarizes the etiological agent distribution of AGE with respect to age groups. When we included all children with a diagnosis of AGE (children who provided a stool sample and who did not have an included diagnosis), the incidence of RV-positive AGE decreased from 4.47 in 1,000 per child in 2012 to 2.48 in 1,000 per child in 2018 . Furthermore, the incidence of adenovirus-positive AGE, the incidence of E. histolytica-positive AGE, and the incidence of norovirus-positive AGE did not change with respect to time. In the control group, the incidence of acute appendicitis in children ≤ 60 months of age did not change from 2012 to 2018 (0.42/1,000, 0.41/1,000, 0.64/1,000, 0.19/1,000, 0.37/1,000, 0.44/1,000, 1.21/1,000, in 2012, 2013, 2014, 2015, 2016, 2017 and 2018, respectively.) ().
Hospitalization rates due to acute gastroenteritis
A total of 4,805 (31%) children were hospitalized with AGE during the study period (mean: 673 children/year; range: 536–887 children/year) (). The percentage of hospitalized children ≤ 60 months of age with AGE with respect to time is shown in . The rate of child hospitalization due to AGE did not change significantly with respect to time. Of the 281 children with rota-positive AGE, 94 were hospitalized (33%, mean: 13 children/year, range: 8–22 children/year). Of the 212 children diagnosed with adenovirus-positive AGE, 52 were hospitalized (25%, mean: 7 children/year, range: 5–10 children/year). Among the 301 children with norovirus-positive AGE, 108 were hospitalized (36%, mean: 14 children/year, range: 3–30 children/year). Of the 194 children with E. histolytica-positive AGE, 32 were hospitalized (16%, mean: 5 children/year, range: 1–9 children/year). When we included all the children with a diagnosis of AGE (children who provided a stool sample and who did not have an included diagnosis), the incidence of hospitalization for RV-positive AGE decreased from 1.9 per 1,000 children to 0.45 per 1,000 children. Also, the incidence of hospitalization for adenovirus-positive AGE, the incidence of hospitalization for E. histolytica-positive AGE, and the incidence of hospitalization for norovirus-positive AGE did not change significantly (). There was no mortality reported because of AGE in any of the subjects during this study period.
Table 2. Incidence of hospitalization of children with respect to causative agents and years
Rate of intussusception
summarizes the incidence of intussusception with respect to study years, and the incidence of intussusception ≤ 60 months of age did not change from 2012 to 2018 .
Length of hospital stay and cost of acute gastroenteritis
The length of hospital stay (LOS) between RV-positive AGE and RV-negative AGE was compared. The mean LOS of RV-positive AGE was 2.47 ± 1.15 days (range: 1–7 days), which was significantly longer than the LOS of RV-negative AGE by 1.59 ± 1.17 days (range: 1–9 days) (p < .001; 95% CI: −1.5 to 0.60). We then compared the overall cost of AGE between RV-positive AGE (n = 229; mean 335 ± 200 USD) and RV-negative AGE (n = 306; mean 280 ± 148 USD) cases – the RV-positive AGE cases cost significantly more than RV-negative cases (p = .015, 95% CI: 10.12–93.87). In Turkey, the average exchange rate during the surveillance period was 1 USD = 1.78, 1.87, 2.18, 2.72, 3.01, 3.64, and 4.81 Turkish liras for 2012, 2013, 2014, 2015, 2016, 2017, and 2018, respectively.
Vaccine effectiveness
Of the 220 RV-positive AGE cases, RV vaccination status could not be determined in 115 (52%) children; 70 (32%) of them had documented RV vaccination, and 35 (16%) of them did not receive RV vaccine. In the 440 control cases, 187 (43%) of those RV vaccination statuses could not be determined, 225 (51%) of them did receive the RV vaccine, and 28 (6%) of them did not receive the RV vaccine. The unknown RV vaccine status between the RV-positive AGE group and the RV-negative AGE control group did not differ. VE against any case of RV-positive AGE was calculated at 75.1% (95% CI: 65–86) ().
Table 3. Vaccine effectiveness against rotavirus diseases
Discussion
To the best of our knowledge, this is the first study in Turkey to assess the impact of RVs on the incidence of AGE and hospitalization due to AGE in children ≤ 60 months of age following the introduction of RV into the private Turkish market. In Turkey, both RV1 and RV5 were introduced in 2006 as nongovernment-funded vaccines. Unfortunately, to date, none of the RVs have been introduced into the NIP of Turkey. Our country birth cohort was nearly one to 1.3 million each year. When we consider our study period, the total number of RV vaccine doses sold in the private market for each year were 237,000, 271,000, 349,000, 357,000, 395,000, 422,000, and 416,000 for 2012, 2013, 2014, 2015, 2016, 2017, and 2018, respectively. These data suggest a RV vaccination rate between 12% and 17% during the study period in Turkey. Even with this lower RV vaccination rate, we showed a reduction in RV-positive AGE and RV-related hospitalizations in children ≤ 60 months of age for the 7-year observation period.
The analysis revealed that the incidence of RV-positive AGE and RV-related hospitalizations decreased in children ≤ 60 months of age with each passing year after the introduction of RVs into the private Turkish market. The incidence of RV-positive AGE decreased by 4.4 per 1,000 population in 2012 to 2.48 per 1,000 population in 2018 – a nearly 50% reduction. RV-related hospitalization decreased from 1.9 per 1,000 children in 2012 to 0.45 per 1,000 children in 2018 – a more than 50% reduction.
The prevalence of RV in children younger than 5 years of age admitted with acute gastroenteritis to hospitals or emergency units decreased by nearly 40% in countries after the introduction of RV vaccines into their national immunization programs. No such reduction was observed in regions where vaccines were not introduced. Previous studies have shown declines in RV-positive AGE-related hospitalizations of 43–70% in Africa, 67–69% in Europe, 59–81% in Latin America, and 40% in eastern Mediterranean countries.Citation11 There are limited details on the effect of self-funded RV vaccination.
Gil-Prieto el al. investigated the impact of non-routine RV vaccination on hospitalizations for diarrhea and RV infections in Spain where RV vaccines have been available since August 2006 (Rotarix) and January 2007 (RotaTeq) and where reported vaccination coverage was 17% in 2007, 35% in 2008 and 38% in 2009. The authors compared the rates between 2005–2006 (pre-vaccine period) and 2008–2009 (post-vaccine period) and showed that the hospitalization rates for all-cause diarrhea, RV infection, and diarrhea of undetermined etiology in children under five years of age in 2009 were 35%, 37% and 36% lower than 2005–2006. This decrease was greater in children <12 months of age: 42% reduction in all-cause diarrhea and 43% reduction in RV and diarrhea of undetermined etiology.Citation12
Another study from Spain by Orrico-Sanchez and colleagues investigated the long-term impact of self-financed RV vaccines on RV-associated hospitalizations and costs in Valencia. Here, RVs are available in the private market and are not funded by the National Health System (NHS). The coverage rate reached 40–50%.Citation13 The authors reported that the risk of RV-positive AGE hospitalizations decreased by 67% and 68% (95% CI: 18–92%) in children between 0 and 1 year of age and those between 1 and 4 years of age, respectively, versus unvaccinated children. The vaccination coverage was between 40% and 42%. They further showed that overall hospital-related costs were reduced by approximately 6 million EUR per 105 children over 7 years.
Hashizume et al. evaluated the impact of nongovernment-funded RV vaccination in Japan where RV1 and RV5 have been marketed since November 2011 and July 2012, respectively. The authors reported a 57% reduction in the number of laboratory-confirmed RV cases in 2009 and 2014 when the presumed uptake rate of RV vaccines was at least 39% of the national average.Citation14 Trimis et al. investigated the impact of limited RV vaccine uptake through the private sector via hospital-based surveillance on RV infections and hospitalizations in Greece. RV vaccines were introduced into the private market in 2007 but not into the Greek NIP during the study period (2006–2010) when the RV vaccination coverage was 30%. A comparison of RV-positive AGE rates between 2008–2010 (post-vaccine period) and 2006–2008 (pre-vaccine period) showed that the likelihood of hospitalization due to RV infection was significantly reduced (OR 0.64; 95% CI: 0.49–0.84; p < .001). They further detected a significant decrease in RV-positive AGE cases in infants aged 0–11 months but with no evidence for a change in RV-positive AGE cases in older age groups.Citation15
Studies in countries with limited vaccine uptake such as Finland and France have also shown that RV vaccination reduces hospitalizations.Citation16,Citation17 The RV coverage in our cohort (approximately 17%) was the lowest among those previously described. Nevertheless, there was a 50% reduction in the incidence of RV-related hospitalizations and a 50% reduction in the incidence of RV-positive AGE in children ≤ 60 months of age.
Previous studies and our study have shown that RV vaccine reduces RV infection in developing and developed countries even if it has not been included in the national immunization program. Thus, this vaccine should definitely be presented in the immunization schedule (family-funded) in such countries and should be recommended to families. In addition, despite the low immunization rate, the decrease in RV incidence once again indicates the effect of the vaccine on herd immunity. Vaccines often require 70% to 86% coverage for herd immunity – especially for infections with fecal/oral transmission such as cholera and hepatitis A. However, even a lower vaccination rate can have a positive effect on herd immunity.Citation18-22
The second important finding of our study is the determination of the effectiveness of RV vaccines in Turkey, which is a middle-income country. The effectiveness of any dose of RV vaccine to prevent RV infection was 75%, which is the same as that in previous reports of VE from countries with similar profiles to ours. The effectiveness of RV vaccines may vary from country to country. The VE varied according to the income of the countries and ranged from approximately 85% to 100% in high- or middle-income countries and from approximately 48% to 61% in low-income countries.Citation23
The third important finding of our study is that the mean LOS of RV-positive AGE was 2.47 ± 1.15 days, which is significantly longer than the LOS of RV-negative AGE (1.59 ± 1.17 days). More than 1/3 of RV-positive AGE cases were hospitalized, and the mean hospitalization duration was nearly 2.5 days, which is similar to findings from two Middle Eastern countries near Turkey.Citation24,Citation25 Consequently, RV-positive AGE leads to a longer hospital stay than other viral causes of AGE, which leads to increased costs.Citation25-29 Here, we calculated the direct cost of RV-positive AGE by including invoices issued to insurance companies without adding indirect costs as the number of non-work days of parents. Even with the inclusion of direct costs, we showed that overall RV-positive AGE (mean 335 ± 200 USD) cases cost significantly more than RV-negative cases (mean 280 ± 148 USD). The cost of RV-positive AGE may vary from country to country because of health service pricing. For example, in the USA, the overall cost of RV infection was 3363 USD;Citation30 in England, the estimated average cost per case of acute illness was approximately £30 for RV infection;Citation31 and in the Netherlands, the average cost of RV infection was found to be 244 EUR.Citation32 In Russia, the average cost of RV AGE was found to be 143 USD, which is close to our results.Citation33
This study has several limitations. First, the study population does not include the entire population of the country’s children but only the largest city Istanbul (nearly 20% of the country’s population). In Istanbul, there are more than 100 hospitals including state and private hospitals. Our cohort was from Ataşehir Memorial Hospital located in the Asian part of Istanbul and Şişli Memorial Hospital in the European sector. Both are large hospitals that work extensively with private health insurance systems. Nevertheless, this population is likely representative of Istanbul, which allows us to obtain an approximation of the profile of Turkey. This offers data on the impact of self-financed RV on AGE.
Second, we could not calculate the true incidence of AGE- and AGE-related hospitalizations in our study for several reasons: (1) We did not have a population of children younger than 5 years of age in our hospital regions; (2) Our study hospitals are medical units serving the population in their regions; and (3) We used rapid antigen tests to detect etiological agents of AGE (not PCR-based assays) due to costs and because the results of those PCR-based assays are generally obtained within a few days. Third, we obtained stool specimens from only 22% of children because most of the AGE cases were mild. Children could not give stool specimens immediately after being examined by the doctor, and most of the parents did not want to wait for stool to be obtained. Furthermore, none of the guidelinesCitation34 related to the diagnosis and treatment of AGE in children recommended routine stool examination. Some doctors did not routinely demand stool tests unless the child appeared sick and/or had a history of blood in the stool. Fourth, we had no pre-RV-period cohort with which to compare our study population; therefore, we studied our results according to time period. We could only investigate the impact of RV in countries with a low rate of vaccine coverage (<15%); RV was only given when the parents paid for it in private hospitals. As summary in addition to these limitations, we found that the incidence of RV-positive AGE cases and the incidence of RV-related AGE hospitalizations decreased with each passing year.
Although RV infection is transmitted via a fecal/oral route, studies on cholera suggest that its contagiousness is not strictly dependent on the infrastructure or income level of the country. RV infection can result in identical hospitalization rates in high- and low-income countries, and we think that bias that will occur in our study population between those whose family could afford the vaccine and those who could not.Citation35,Citation36
References
- Global Burden of Disease 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71.
- Global Burden of Diarrhoeal Diseases Collaborators. Estimates of global, regional, national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:909–48. doi:10.1016/S1473-3099(17)30276-1.
- Mokomane M, Kasvosve I, de Melo E, Pernica JM, Goldfarb DM. The global problem of childhood diarrhoeal diseases: emerging strategies in prevention and management. Ther Adv Infect Dis. 2018 Jan;5(1):29–43. doi:10.1177/2049936117744429.
- Munos MK, Walker CLF, Black RE. The effect of rotavirus vaccine on diarrhoea mortality. Int J Epidemiol. 2010;39(Suppl. 1):i56–i62. doi:10.1093/ije/dyq022.
- WHO. Global use of rotavirus vaccines recommended (News releases). Media Centre. [accessed 2020 Feb 18]. http://www.who.int/mediacentre/news/releases/2009/rotavirus_vaccines_20090605/en//
- Burnett E, Parashar U, Tate J. Rotavirus vaccines: effectiveness, safety, and future directions. Paediatr Drugs. 2018 Jun;20(3):223–33. doi:10.1007/s40272-018-0283-3.
- Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006–2016. Clin Infect Dis. 2017 Sep 1;65(5):840–50. doi:10.1093/cid/cix369.
- WHO. Rotavirus vaccines. WHO position paper—January 2013. Wkly Epidemiol Rec. 2013;88:49–64.
- [accessed 2020 Apr 4]. https://www.who.int/gho/immunization/rotavirus/en/.
- Pérez-Vilar S, Díez-Domingo J, López-Lacort M, Martínez-Úbeda S, Martinez-Beneito MA. Effectiveness of rotavirus vaccines, licensed but not funded, against rotavirus hospitalizations in the Valencia Region, Spain. BMC Infect Dis. 2015;15:92. doi:10.1186/s12879-015-0811-5.
- Aliabadi N, Antoni S, Mwenda JM, Weldegebriel G, Biey JNM, Cheikh D, Fahmy K, Teleb N, Ashmony HA, Ahmed H, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008-16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Health. 2019 Jul;7(7):e893–e903. doi:10.1016/S2214-109X(19)30207-4.
- Gil-Prieto R, Gonzalez-Escalada A, Alvaro-Meca A, Garcia-Garcia L, San-Martin M, González-López A, Gil-de-Miguel A. Impact of non-routine rotavirus vaccination on hospitalizations for diarrhoea and rotavirusinfections in Spain. Vaccine. 2013 Oct 9;31(43):5000–04. doi:10.1016/j.vaccine.2013.05.109. Epub 2013 Jul 30.
- Orrico-Sanchez A, López-Lacort M, Pérez-Vilar S, Díez-Domingo J. Long-term impact of self-financed rotavirus vaccines on rotavirus-associated hospitalizations and costs in the Valencia Region, Spain. BMC Infect Dis. 2017 Apr 11;17(1):267. doi:10.1186/s12879-017-2380-2.
- Hashizume M, Nakagomi T, Nakagomi O. An early detection of decline in rotavirus cases during the 2013/2014 season in Japan as revealed by time-series analysis of national surveillance data. Trop Med Health. 2015 Sep;43(3):177–81. doi:10.2149/tmh.2015-23.
- Trimis G, Koutsoumbari I, Kottaridi C, Palaiologou N, Assimakopoulou E, Spathis A, Lebessi E, Konstantopoulos A, Kafetzis D, Karakitsos P, et al. Hospital-based surveillance of rotavirus gastroenteritis in the era of limited vaccine uptake through the private sector. Vaccine. 2011 Oct 6;29(43):7292–95. doi:10.1016/j.vaccine.2011.07.092.
- Räsänen S, Lappalainen S, Halkosalo A, Salminen M, Vesikari T. Rotavirus gas- troenteritis in finnish children in 2006–2008, at the introduction of rotavirus vaccination. Scand J Infect Dis. 2011;43(1):58–63. doi:10.3109/00365548.2010.508462.
- Gagneur A, Nowak E, Lemaitre T, Segura JF, Delapierre N, Abalea, Abalea L, Poulhazan E, Jossens A, Auzanneau L, et al. Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: the IVANHOE study. Vaccine. 2011;12:3753–59. doi:10.1016/j.vaccine.2011.03.035.
- Wierzba TF. Oral cholera vaccines and their impact on the global burden of disease. Hum Vaccine Immunother. 2019;15(6):1294–301. doi:10.1080/21645515.2018.1504155.
- Belmaker I, Dukhan L, Yosef Y, Leventhal A, Dagan R. Elimination of hepatitis a infection outbreaks in day care and school settings in southern Israel after introduction of the national universal toddler hepatitis a immunization program. Pediatr Infect Dis J. 2007;26(1):36–40. doi:10.1097/01.inf.0000247105.45185.13.
- Rha B, Tate JE, Payne DC, Cortese MM, Lopman BA, Curns AT, Parashar UD. Effectiveness and impact of rotavirus vaccines in the United States - 2006–2012. Expert Rev Vaccines. 2014;13(3):365–76. doi:10.1586/14760584.2014.877846.
- Paulke-Korinek M, Kundi M, Rendi-Wagner P, de Martin A, Eder G, Schmidle-Loss B, Vecsei A, Kollaritsch H. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine. 2011;29(15):2791–96. doi:10.1016/j.vaccine.2011.01.104.
- Giaquinto C, Jackson AE, Vesikari T. Report of the second European expert meeting on rotavirus vaccination. Vaccine. 2012;30(13):2237–44. doi:10.1016/j.vaccine.2011.12.002.
- Bennett A, Bar-Zeev N, Cunliffe NA. Measuring indirect effects of rotavirus vaccine in low income countries. Vaccine. 2016;34:4351–53. doi:10.1016/j.vaccine.2016.07.001.
- Al Awaidy SA, Bawikar S, Al Busaidy S, Baqiani S, Al Abedani I, Varghesem R, Abdoan H, Al Abdoon H, Bhatnagar S, Al Hasini K. Considerations for introduction of a rotavirus vaccine in Oman: rotavirus disease and economic burden. J Infect Dis. 2009;200(Suppl 1):S248–S253. doi:10.1086/605339.
- Muhsen K, Shulman L, Rubinstein U, Kasem E, Kremer A, Goren S, Zilberstein I, Chodick G, Ephros M, Cohen D. Incidence, characteristics, and economic burden of rotavirus gastroenteritis associated with hospitalization of Israeli children <5 years of age, 2007–2008. J Infect Dis. 2009;200(Suppl1):S254–S263. doi:10.1086/605425.
- Giaquinto C, Van Damme P, Huet F, Gothefors L, Maxwell M, Todd P, da Dalt L. Clinical consequences of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. J Infect Dis. 2007;195(Suppl 1):S26–S35. doi:10.1086/516717.
- Mast TC, Walter EB, Bulotsky M, Khawaja SS, DiStefano DJ, Sandquist MK, Straus WL, Staat MA. Burden of childhood rotavirus disease on health systems in the United States. Pediatr Infect Dis J. 2010 Feb;29(2):e19–25. doi:10.1097/INF.0b013e3181ca7e2e.
- Fischer TK, Nielsen NM, Wohlfahrt J, Paerregaard A. Incidence and cost of rotavirus hospitalizations in Denmark. Emerg Infect Dis. 2007;13:855–59. doi:10.3201/eid1306.061432.
- Fourquet F, Desenclos JC, Maurage C, Baron S. Acute gastro-enteritis in children in France: estimates of disease burden through national hospital discharge data. Arch Pediatr. 2003;10:861–68. doi:10.1016/S0929-693X(03)00459-7.
- Krishnarajah G, Demissie K, Lefebvre P, Gaur S, Sheng Duh M. Clinical and cost burden of rotavirus infection before and after introduction of rotavirus vaccines among commercially and Medicaid insured children in the United States. Hum Vaccine Immunother. 2014;10(8):2255–66. doi:10.4161/hv.29511.
- Tam CC, O’Brien SJ. Economic cost of campylobacter, norovirus and rotavirus disease in the United Kingdom. PLoS One. 2016 Feb 1;11(2):e0138526. doi:10.1371/journal.pone.0138526. eCollection 2016.
- Mangen MJ, Bouwknegt M, Friesema IH, Haagsma JA, Kortbeek LM, Tariq L, Wilson M, van Pelt W, Havelaar AH. Cost-of-illness and disease burden of food-related pathogens in the Netherlands, 2011. Int J Food Microbiol. 2015 Mar 2;196:84–93. doi:10.1016/j.ijfoodmicro.2014.11.022.
- Lobzin YV, Kharit SM, Goveia MG, O’Brian MA, Podkolzin AT, Blokhin BM, Bekhtereva MK, Rudakova AV, Tikunova NV. Burden of childhood rotavirus disease in the outpatient setting of the Russian Federation. Pediatr Infect Dis J. 2017 May;36(5):472–76. doi:10.1097/INF.0000000000001472.
- Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, et al. 2017 infectious diseases society of america clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017 Nov 29;65(12):1963–73. doi:10.1093/cid/cix959.
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization–Coordinated Global Rotavirus Surveillance Network. Global, regional, and national estimates of rotavirus mortality in children <5 years of age- 2000–2013. Clin Infect Dis. 2016 May 1;62(Suppl 2):S96–S105. doi:10.1093/cid/civ1013.
- Church JA, Rogawski McQuade ET, Mutasa K, Taniuchi M, Rukobo S, Govha M, Lee B, Carmolli MP, Chasekwa B, Ntozini R, et al. Enteropathogens and rotavirus vaccine immunogenicity in a cluster randomized trial of improved water, sanitation and hygiene in rural Zimbabwe. Pediatr Infect Dis J. 2019 Dec;38(12):1242–48. doi:10.1097/INF.0000000000002485.