ABSTRACT
Coxsackievirus A16 (CV-A16), one of major etiological agents of hand, foot and mouth disease (HFMD), causes outbreaks of the disease in young children all over the world. In order to promote the prevention and control of HFMD, the research and development of CV-A16 vaccine have been carried out in China. However, due to lacking of a recognized CV-A16 antigen detection method, the evaluation and quality control (QC) of vaccine effectiveness are greatly limited. In this study, we established a quantitative enzyme-linked immunosorbent assay (Q-ELISA) to determine the antigen concentration in CV-A16 vaccines that can be applied in manufacturing in China. A neutralizing antibody 16E1 was used as a capture antibody that can bind to various CV-A16 antigens of different subgenotypes, and an antiserum from CV-A16-immunized rabbit conjugated by HRP was suitable for detecting and quantifying CV-A16 antigens. The Q-ELISA was validated for specificity, linearity, accuracy, precision and robustness by using the CV-A16 antigen national standard (NS). Furthermore, we utilized the Q-ELISA to quantify antigen contents of vaccine bulks from six manufacturers and other intermediate products from one manufacturer. The results indicated that the Q-ELISA can satisfy the requirements of QC for all manufacturers involved.
Introduction
Hand, foot and mouth disease (HFMD), a common pediatric disease, can lead to central nervous system (CNS) complications or even death.Citation1 The main etiological agents of HFMD are the coxsackievirus A16 (CV-A16) and human enterovirus A71 (EV-A71), with EV-A71 being associated with more severe outcomes.Citation2 HFMD caused by CV-A16 infections is generally considered mild and self-limiting. However, several severe and fatal cases involving CV-A16 were recently reported and attracted greater attention on CV-A16 research.Citation3 Studies have shown that co-infection with CV-A16 and EV-A71 can cause serious complications in the CNS.Citation4 Genomic reassortments often occur within a serotype and between different serotypes of CV-A16 and EV-A71, and these may be related to the large HFMD outbreak in Mainland China in 2008.Citation5,Citation6 EV-A71 inactivated vaccines were approved in 2015 in China which can effectively reduce HFMD caused by EV-A71, but not other pathogens, such as CV-A16. For these reasons above all, more and more studies have focused on the development of CV-A16 vaccines and related diagnostic reagents in China.Citation7
Antigen content detection is a key means of quality control (QC) and is used for evaluating effective constituents in vaccine development and production, and needs to be accurately measured in intermediate products such as harvest, purified virus and final bulk. Vaccine manufacturers need to develop a rapid and precise assay to monitor the production process. Nowadays, many CV-A16 antigen quantification methods have been developed by each manufacturer with local technology and virus strains. There is a need to streamline the detection method and provide a uniform tool for comparing the CV-A16 antigen content in vaccines from different manufacturers.
In this study, we established and validated a quantitative enzyme-linked immunosorbent assay (Q-ELISA) to determine the concentration of the CV-A16 antigen using CV-A16-specific antisera and a CV-A16 neutralizing monoclonal antibody. The Q-ELISA has been successfully used to assess antigen content in intermediate products vaccine bulks from six manufacturers in China.
1. Materials and methods
1.1. Cells and viruses
Africa green monkey kidney (Vero) cell and rhabdomyosarcoma (RD) cell were purchased from ATCC. The CV-A16/V-24 strain (subgenotype B1a) that was clinically isolated from Hangzhou, Zhejiang Province was provided by Sinovac Biotech Co. The CV-A16/00190 strain (subgenotype B1b, GenBank No.MF177223), the clinical isolate, was obtained from Taiwan by Xiamen University in 2011. The CV-A16/731 strain (subgenotype B2b, GenBank No.KF924762) and CV-A16/BJCA08 strain (subgenotype C1, GenBank No.JX481738) were preserved in the laboratory. These virus stocks were collected from the supernatants of infected Vero cells 3-days post-infection (DPI).
1.2. The CV-A16 antigen standard and other materials
The CV-A16 antigen national standard (NS) of China (2016 NBS No. 0031) contained inactivated and purified CV-A16 (2000 U/ml), developed by the Chinese National Institutes for Food and Drug Control (NIFDC) in 2016.Citation8 Inactivated EV-A71, Hepatitis A virus (HAV) and Polio virus (PV) antigens were provided by NIFDC. CV-A16 vaccine intermediate products and bulks (inactivated whole virus) were provided by Sinovac Biotech Co. Ltd, Beijing, PR China; WuHan Institute of Biological Products, Co. Ltd, Wuhan, PR China; National Vaccine and Serum Institute, Beijing, PR China; Chinese Academy of Medical Sciences, Kunming, PR China; Aimei Convac BioPharm (Jiangsu) Co. Ltd, Taizhou, PR China; and Beijing Zhifei Lvzhu Biopharmaceutical Co. Ltd., Beijing, PR China. These manufacturers were set as Lab 1–6 randomly, and the subgenotypes of CV-A16 included B1a, B1b, B2a and B2b. A Horseradish peroxidase (HRP) conjugation kit was purchased from Pierce (Rockford, IL, USA). DMEM and MEM medium and fetal bovine sera (FBS) were purchased from Gibco (ThermoFisher, Waltham, MA). Protein A was purchased from the GE Co (GE Healthcare, Chicago, IL). Goat-anti-mouse second IgG antibody conjugated with horseradish peroxidase (HRP) was purchased from Dingguo Changsheng Biotech (Beijing, China). Other chemical reagents used in this study were of analytical grade and purchased from Sigma Chemical (MO, USA).
1.3. Polyclonal antibody (pAb) and ELISA measurement of antibody titer
CV-A16/V-24 were inactivated with formalin and the protein content was measured using the BCA method. These inactivated virus were used as immunogen to immunize New Zealand rabbits, and pAb were collected. The pAb was purified using the staphylococcal protein A (SPA) method and authentication was undertaken using SDS-PAGE. The pAb was conjugated with HRP using the sodium periodate oxidation method, and added the same volume of glycerin, and stored at −20°C. The pAb conjugated with HRP (pAb-HRP) was identified with indirect ELISA and the titer was calculated.
1.4. Monoclonal antibodies (mAb)
Mouse ascites containing monoclonal antibodies were developed using CV-A16/00190 strain and the hybridoma technique, and supernatant was precipitated using caprylic acid and saturated ammonium sulfate, and purified using protein A (GE Healthcare, Chicago, IL) affinity chromatography. The mAbs were conjugated with horseradish peroxidase using the sodium periodate oxidation method and the same volume of glycerin as harvest mAbs was added, and the mAbs conjugated with HRP (mAbs-HRP) were stored at −20°C.
1.5. Neutralizing titer determination of mAbs
MAbs was diluted to 10 mg/ml and further diluted serially 1:100, 1:200, 1:400, 1:800, 1:1600, 1:3200, 1:6400 and 1:12800, and mixed 50 μl mAbs of each concentration with CV-A16/00190 or CV-A16/731 strain (100CCID50/50 μl) in a 96-well plate separately. After incubation at 37°C for 2 hours, these mixtures were added to 96-well plates containing RD cells (10000 cells/well). Mixtures were incubated at 35°C for 7 days, after which the CPE was observed, and neutralizing titer was calculated according to the Reed-Muench method.
1.6. Determining the binding ability of mAbs
Three strains of CV-A16 including subgenotype B1a, B1b and B2b were inactivated, and the protein content was measured using the BCA method. The ELISA plate (Costar, catalog number: 3590) was coated with these inactivated CV-A16 at 1 μg per well at 4°C overnight and blocked with PBS containing 0.05% (v/v) Tween-20 added to 5% bovine serum albumin. After washing, these mAbs were added at 1 μg per well separately. A secondary antibody, HRP-conjugated goat anti-mouse IgG (Dingguo Changsheng Biotech, Beijing, China) was diluted 1:8000 and added to the wells after washing. The optical density (OD) value was detected at 450/630 nm after color development.
1.7. Protective effects of mAbs in mice
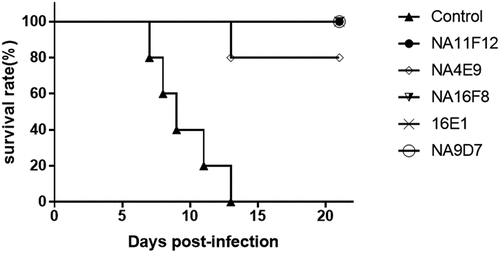
To test the protective effect of mAbs, newborn BALB/c mice were challenged intraperitoneally with CV-A16/BJCA08 (54CCID50 per mouse).Citation9,Citation10 A dosage of 10 μg/g antibody of five mAbs were inoculated separately as treatment groups and MEM medium was inoculated as a control group on day 1 after challenging. The mice were monitored daily for survival.
1.8. Parameters used for standardization of the ELISA
For standardization of the assay, four parameters were considered. (1) Optimal concentrations of capture and detection antibodies were determined. 1 μg, 2 μg, 4 μg and 8 μg per well of mAb and 1:1000, 1:2000, 1:4000 and 1:8000 gradients of pAb were evaluated. (2) Select an appropriate concentration range of antigen standard with the best linearity. Concentrations tested included 400, 200, 100, 50, 25, 12.5, 6.25 and 3.125 U/ml. (3) Two kinds of blocking solutions were compared, including PBS containing 0.05% (v/v) Tween-20 added to 10% (v/v) FBS and 5% (w/v) sucrose, and PBS containing 0.05% (v/v) Tween-20 added to 5% (w/v) bovine serum albumin (BSA). (4) Incubation time and temperature of coating were optimized, including 37°C for 1 h or 4°C overnight. For detection antibody incubation, incubation was conducted at 37°C for 120, 60 and 30 min. Furthermore, three types of ELISA plates were compared, including Nunc (catalog number: 468667), Costar (catalog number: 3590) and Greiner (catalog number: 655061).
1.9. Protocol for standardization of the ELISA
(1) Prepare coating buffer (0.05 M Na2CO3, 0.01 M NaN3, pH 9.6), blocking solution (0.01 M PBS containing 0.05% (v/v) Tween-20 and 10% (v/v) FBS and 5% (w/v) saccharose), diluent Ⅰ (0.01 M PBS containing 10% (v/v) FBS), washing liquor (0.01 M PBS containing 0.05% (v/v) Tween-20), diluent Ⅱ (0.01 M PBS containing 1% (w/v) casein and 10% fetal FBS and 0.05% (v/v) Tween-20). (2) The 16E1 (one of the CV-A16 mAbs) was diluted to 10 μg/mL with coating buffer, and added into the ELISA plate (Costar, catalog number: 3590) with 100 μl and stored at 4°C overnight. (3) The coated solution was discarded, and the plate was rinsed thoroughly with washing liquor and patted dry. (4) The plate was blocked at 37°C for 1 h. (5) The plate was rinsed with washing liquor and dried naturally at room temperature for 4 hours and sealed in a vacuum individually. The anti-CV-A16 monoclonal antibody-coated ELISA plate obtained can be stored at 4°C for 12 months. (6) Plates need to be equilibrated at room temperature for 20–30 minutes prior to opening when needed for conducting this experiment. (7) CV-A16 antigen NS (2000 U/ml) was diluted into 200, 100, 50, 25 and 12.5 U/ml with diluent Ⅰ, and samples were diluted into appropriate gradients by using a serial two-fold method with the same diluent. (8) These liquids were inserted in wells for 100 μl in duplicate and incubated for 1 h (time 1) at 37°C. (9) The plate was rinsed with washing liquor and patted dry. (10) The pAb-HRP was diluted to 1:4000 with diluent Ⅱ and added into wells for 100 μl, and incubated at 37°C for 1 h (time 2). (11) The plate was rinsed with washing liquor and patted dry. (12) TMB substrate reagents were added and incubated at 37°C for 10 minutes (time 3). OD values were measured at 450/630 nm.
1.10. Evaluation of the validation parameters
All validation data were tested by using the CV-A16 antigen NS.
1.10.1. Specificity
Specificity was determined by detecting DMEM, MEM, FBS, 1000 ng/ml and 100 ng/ml of inactivated EV-A71, HAV, PV antigen, in comparison with the CV-A16 antigen NS (200 U/ml and 50 U/ml) in triplicates. S/CO values were calculated as the following formula: S/CO = OD of samples/OD of cutoff values, and the cutoff value refers to value of negative control multiplied by 2.1
1.10.2. Linearity
The concentrations of the CV-A16 antigen NS used in this standard curve were 200, 100, 50, 25 and 12.5 U/ml. The Q-ELISA was undertaken in six independent assays. Linearity was calculated from the linear regression of the results of the standard curve.
1.10.3. Accuracy
For evaluations of accuracy, three dilutions of the CV-A16 antigen NS (150, 50 and 20 U/ml) were tested in the same run in six independent assays. The criteria of acceptance of recovery rate could not exceed 20%. Accuracy values (recovery rate) were calculated as the following formula:
Accuracy = experimental average value of concentration/theoretical value of concentration.
1.10.4. Precision
The precision study was divided into repeatability (intra-assay) and intermediate (inter-assay) precision. To evaluate both parameters, the CV-A16 antigen NS was diluted into high (150 U/ml), medium (50 U/ml) and low (20 U/ml) contents. To assess repeatability, the ELISA was carried out in six replicates. To assess intermediate precision, the Q-ELISA was executed in three independent assays by each of the two technicians. The criteria of acceptance were as follows: The coefficient of variation (CV) of repeatability could not exceed 10%, whereas the CV of the intermediate precision could not exceed 20%.
1.10.5. Robustness
To evaluate the robustness of the ELISA, the CV-A16 antigen NS was diluted into high (150 U/ml), medium (50 U/ml) and low (20 U/ml) contents. The Q-ELISA was carried out by adjusting reaction times of times 1, 2 and 3 in 2.8. Different reaction temperatures were also tested. The criteria of acceptance of recovery rate could not exceed 20%. Robustness ability values (recovery rates) were calculated as the following formula:
Robustness ability = experimental average value of concentration/theoretical value of concentration.
1.11. Applicability of the ELISA
To evaluate the applicability of the ELISA, a full set of reagents and the CV-A16 antigen NS were assigned to six manufacturers in China. These manufacturers carried out this Q-ELISA to detect the antigen content of local inactivated vaccine bulks including B1a, B1b, B2a and B2b subgenotypes according to the protocol in 1.9. Furthermore, harvests and purified virus in lab 3 were evaluated using this kit. Linearity was calculated from the linear regression of the results of the standard curve. The R2 value needed to be higher than 0.98. “Parallel line assay” was opened in Statistical Analysis software (ISHIDA, 1.0.0.0), Log(y) was selected and “parallel” parameters appeared.
2. Results
2.1. MAbs and pAb-HRP use in the ELISA
Five neutralizing monoclonal antibodies of CV-A16 were involved in this study, including NA11F12, 16E1, NA4E9, NA9D7 and NA16F8.Citation10–12 To select a good mAb for establishing the Q-ELISA, the neutralizing and binding ability, and protective effect were compared. The results showed that five mAbs could robustly neutralize CV-A16/00190 and CV-A16/731 except NA16F8 (). However, only 16E1 could react relatively well to all inactivated CV-A16 including subgenotypes B1a, B1b and B2b (). At the same time, 16E1 could simultaneously protect 100% of the newborn mice against the lethal challenge of the CV-A16 ().
Table 1. Neutralizing ability of five mAbs to different CVA16 strains
Table 2. Optical density (OD) values of mAbs reacted to inactivated CV-A16
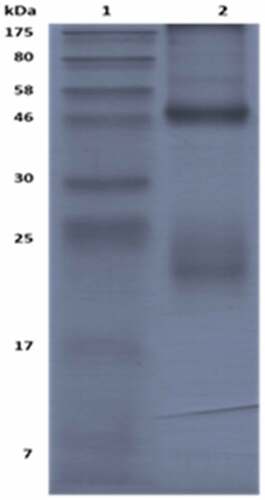
For pAbs used in the Q-ELISA, we used the neutralizing antibody testing method and ELISA method to detect the titer of pAbs. Results indicated that the neutralizing titer of pAb was 1:6144, and the titer of the indirect ELISA method was approximately 106, which represented that the pAb had robustly neutralizing and binding ability enough for Q-ELISA establishment. The pAb were purified using the Staphylococcal Protein A (SPA) method and evaluated by SDS-PAGE. Two main protein bands were heavy chain (48kd) and light chain (24kd) of the pAb, and purity was over 96.5% when analyzed by a gel imager ().
2.2. Q-ELISA parameter optimization
The best pairing was 1 μg per well of 16E1 as capture antibody and HRP-conjugated pAb diluted to 1:2000 as detection antibody. The detecting range was set as 12.5 U/ml-200 U/ml. The blocking solution of PBS containing 0.05% Tween-20 (PBS-T) added to 10% FBS and 5% sucrose showed the best results with the lowest background values and the highest sensitivity (data not shown). Incubation of the coating time and temperature were set as 4°C overnight and these resulted in the best stability and sensitivity (data not shown). Finally, the ELISA plate from Coastar showed significantly higher sensitivities compared with three other ELISA plates (data not shown).
2.3. Validation of the Q-ELISA
2.3.1. Specificity of the ELISA
Specificity was determined by detecting inactivated EV-A71, HAV and PV antigens and DMEM, MEM and FBS, in comparison with the CV-A16 antigen NS. Experiments were conducted strictly according to the protocol in triplicates, and S/CO values were calculated. As shown in , the Q-ELISA can detect CV-A16 antigens specifically and cannot cross-react with other picornavirus, HAV and culture mediums.
Table 3. Specificity of the Q-ELISA
2.3.2. Linearity of the ELISA
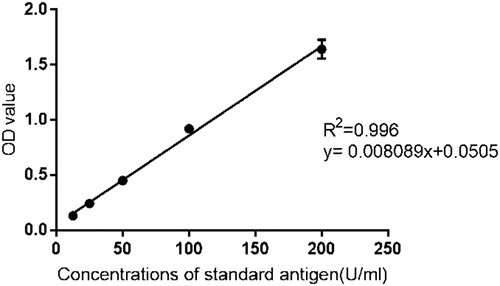
Linearity was tested for six independent assays, and the values of the correlation coefficients of the linear regression (R2) were calculated. Results indicated that R2 for six assays were all above 0.990 (95% CI = 0.987–0.996), and linearity for the analytical range was 12.5–200 U/ml, as shown in .
2.3.3. Accuracy of the ELISA
Accuracy was tested by quantifying high (150 U/ml), medium (50 U/ml) and low (20 U/ml) antigen concentration CV-A16 antigen NS and manifested as the recovery rate. Recovery rate values of six independent assays of each of the concentrations evaluated by the ELISA were between 80% and 110%, as shown in .
Table 4. Accuracy of the ELISA in high, medium and low concentrations of CV-A16 NS
2.3.4. Precision of the ELISA
Precision was analyzed by repeatability and intermediate precision. For both parameters, high (150 U/ml), medium (50 U/ml) and low (20 U/ml) concentrations of CV-A16 antigen NS were used. To assess repeatability, the experiment was conducted in six independent assays and the intermediate precision was evaluated by two technicians in three independent experiments separately. Results of the precision study are shown in . The CV for repeatability and intermediate precision were determined and found to be between 1.5–10% and 5–15%, respectively, certifying the precision of the Q-ELISA.
Table 5. Results of the repeatability and intermediate precision studies
2.3.5. Robustness of the ELISA
Concentrations of 150 U/ml, 50 U/ml and 20 U/ml were tested. To lengthen or shorten 5 min of reaction time could not significantly affect the experimental results, and the same results occurred for rises and falls of 2°C of incubation temperature. The recovery rates were between 80% and 120%, as shown in .
Table 6. Robustness study of the ELISA
2.4 Applicability study of the ELISA
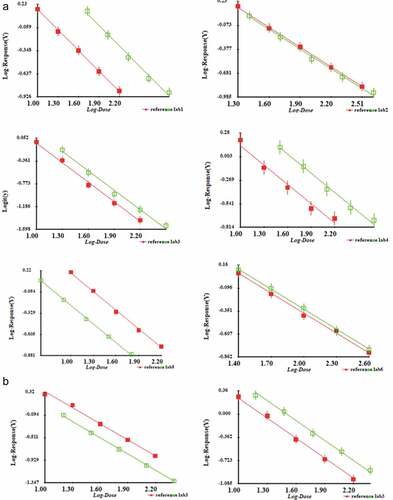
To test whether the Q-ELISA could be used for the CV-A16 inactivated vaccine bulks of the different manufacturer or not, complete sets of detection reagents were dispensed to six labs. Each manufacturer completed repetitive experiments for local bulks. Results showed good parallelism and linearity relationships between CV-A16 antigen NS and each of the bulks (). However, final bulks were products after the inactivation of the purified virus. Therefore, to understand the ability of the Q-ELISA to quantify intermediate products before inactivation, the harvested and purified virus from lab 3 were evaluated by this kit, and the parallelism was acceptable, suggesting the ELISA system had good applicability ().
Figure 4. Applicability of the Q-ELISA quantifying the CV-A16 antigen in bulks (inactivated purified virus) of six labs (A) and other intermediate products including harvest and purified virus from lab 3(B). Parallelism and linearity were analyzed by multiple parallel line comparison. The statistical validity of the parallelism and linearity of the assays were assessed by analysis of variance tests (A): Lab 1: parallel F = 0.07 (P > .05), linearity F = 1.48 (P > .05); Lab 2: parallel F = 0.55 (P > .05), linearity F = 0.76 (P > .05); Lab 3: parallel F = 0.00 (P > .05), linearity F = 1.53 (P > .05); Lab 4: parallel F = 0.53 (P > .05), linearity F = 2.01 (P > .05); Lab 5: parallel F = 1.33 (P > .05), linearity F = 2.89 (P > .05); Lab 6: parallel F = 0.46 (P > .05), linearity F = 0.49 (P > .05); (B): Harvest products: parallel F = 2.58 (P > .05), linearity F = 3.07 (P > .05); Concentrated products: parallel F = 0.28 (P > .05), linearity F = 2.16 (P > .05); Purified products: parallel F = 4.53 (P > .05), linearity F = 0.41 (P > .05)
3. Discussion
Inactivated EV-A71 vaccines developed in Mainland China were approved in 2015. Clinical trial results showed that the EV-A71 vaccines could provide sound protection against EV-A71-associated HFMD or herpangina in infants and young children, and the vaccine efficacy against HFMD with CNS complications was 100%.Citation13–15 After the EV-A71 vaccine went onto the market in China in 2015, deaths caused by HFMD decreased every year. The number of deaths in 2018 was 93% lower than before the vaccine was launched (average deaths between 2010 and 2015) in China.Citation16 However, the CV-A16 vaccine is currently unavailable for public use. Success in the development of the EV-A71 vaccines will provide valuable insights for CV-A16 vaccines. Recently, several types of CV-A16 vaccines, especially inactivated CV-A16 vaccine, have been evaluated in animals but not yet tested in a clinical trial. β-propiolactone-inactivated whole-virus vaccines were able to induce CV-A16-specific antibody and IFN-secreting T-cell responses in mice.Citation17–19 Furthermore, recombinant CVA16-VLPs can also elicit high-titer neutralizing antibodies to protect mice from the lethal CV-A16 challenge and robust Th1/Th2 cytokines, including secretion of IFN-γ, IL-2, IL-4 and IL-6.Citation20,Citation21 Therefore, the potential of the inactivated CV-A16 whole virus and CV-A16-VLPs were demonstrated to be promising CV-A16 vaccine candidates that should be included together with EV-A71 to create a bivalent vaccine for HFMD.
Antigen content is a key parameter for active components in vaccine research and development. The accuracy, repeatability and applicability for vaccines prepared by different strains of antigen content test are particularly important. With no universally accepted quantitative assay for CV-A16 antigen available, CV-A16 vaccine manufacturers have quantified the antigen content with different ELISA kits developed by themselves with different acceptance criteria. With going on development of CV-A16 vaccines, establishing a uniform verified assay is needed for CV-A16 antigen quantitation. It is significant for the evaluation of the effectiveness and standardization of QC of CV-A16 vaccines, which could promote the CV-A16 vaccine research and development powerfully.
MAbs are widely used for ELISA due to the sound specificity and definition of the binding site. As a result, screening a suitable mAb is essential for ELISA establishment. However, CV-A16 and EV-A71 have highly similar genomes and proteins, and the nucleotide identities for these were 62.50–66.80%, and the deduced amino acid identities were 70.00–72.70% in the VP1 encoding gene.Citation2 Existence of similar epitopes between these two viruses significantly restrained the research progress of the CV-A16 antigen detecting method. Moreover, the 3R (replacement, reduction and refinement) principle should be implemented to reduce the usage of experimental animals when assessing vaccine potency. For instance, an antigen quantification method regarding the HAV vaccine was published, and indicated the relationship between the immunogenicity assay and ELISA using the neutralizing monoclonal antibody.Citation22 As a result, only by means of screening out specific neutralizing monoclonal antibodies can we establish a high-specific and immunogenicity-related CV-A16 antigen detecting system.
A neutralizing antibody 16E1 was screened out from five CV-A16 neutralizing antibodies, and it can bind conformational epitopes (data not shown) and highly neutralize CV-A16/00190 strain (neutralizing titer: 9600). MAb 16E1 (10 μg/g) could simultaneously protect 100% of newborn mice against the lethal challenge of the CV-A16/BJCA08 at day 1 after challenging. Therefore, the results could be correlated to the in vivo protective effect of the CV-A16 antigen to some extent. Besides, 16E1 could specifically recognize epitope of CV-A16, and did not cross-react to other enteroviruses. As a result, good specificity and compatibility could be guaranteed at the same time when using mAb (16E1) as capture antibody and pAb-HRP as detection antibody cooperatively. In addition, accuracy (ranging from 80% to 110%), linearity (R2 > 0.99), stability (data not shown), precision (CV<15%) and robustness (ranging from 80% to 120%) were tested, and the results could satisfy the requirement of detection.
Moreover, 16E1 is the only one of five antibodies that could extensively and robustly react to multi-subgenotypes of CV-A16 (), including B1a, B1b and B2b, and it not only neutralize subgenotype B1b CV-A16/00190 strain, but also neutralize subgenotype B2b CV-A16/731 (neutralizing titer: >12800). The screening experiment could ensure the maximum binding ability with vaccine bulks of multi-manufacturers. To validate the applicability to different CV-A16 whole-virus inactivated vaccines, complete sets of detection reagents were dispensed to six manufacturers, and they completed the assay according to protocol whose virus strains, inactivation and purified methods were all different. The results indicated that the Q-ELISA could be competent for quantitation on different original vaccine bulks of subgenotype B1a, B1b, B2a, B2b, and showed good parallelisms and proximate slopes. Harvest and purified CV-A16 which are intermediates before inactivation during vaccine production were also detected of its content of CV-A16 antigen. Therefore, this Q-ELISA can be used as a QC and a uniform kit to monitor and control the antigen contents of CV-A16 inactivated vaccines, which has potential values as a pharmacopoeial method in China.
However, there are some limitations about the Q-ELISA. MAb 16E1 has a good ability to neutralize and bind with multiple CV-A16 strains of subgenotype B which is the most pandemic strains, giving the applicability of the Q-ELISA to quantitate different CV-A16 antigens to maximum extent. Subgenotype A of CV-A16, G10 prototype strain for instance, is an original epidemic isolate and uncommon now, so we have not studied the neutralizing and binding ability of 16E1 with subgenotype A strains. It could be supplemented if needed. Moreover, the Q-ELISA could be applicable for the quantitation of CV-A16 inactivated vaccine of six manufacturers, and whether it could be used for the detection of other types of vaccines should be studied further.
Above all, a quantitative enzyme-linked immunosorbent assay of the CV-A16 antigen for vaccine was established and validated in this study. At the same time, the Q-ELISA can quantify inactivated CV-A16 antigen of multiple subgenotypes and CV-A16 vaccine bulks of different technology. This uniform Q-ELISA can meet the requirement of QC of vaccines from most manufacturers in China and lay the foundation for CV-A16 vaccine research and development.
Acknowledgments
Manufacturers devoted to developing CV-A16 inactivated vaccine were involved in this applicability validation of this study, including WuHan Institute of Biological Products Co. Ltd, Wuhan, PR China; National Vaccine and Serum Institute, Beijing, PR China; Chinese Academy of Medical Sciences, Kunming, PR China; Aimei Convac BioPharm (Jiangsu) Co. Ltd, Taizhou, PR China; and Beijing Zhifei Lvzhu Biopharmaceutical Co. Ltd., Beijing, PR China.
Additional information
Funding
References
- Koh WM, Badaruddin H, La H, Chen MI, Cook AR. Severity and burden of hand, foot and mouth disease in Asia: a modelling study. BMJ Glob Health. 2018;3(1):e000442. doi:10.1136/bmjgh-2017-000442. PMID: 29564154 eCollection 2018.
- Li Y, Zhu R, Qian Y, Deng J, Sun Y, Liu L, Wang F, Zhao L. Comparing Enterovirus 71 with Coxsackievirus A16 by analyzing nucleotide sequences and antigenicity of recombinant proteins of VP1s and VP4s. BMC Microbiol. 2011;11(1):246. doi:10.1186/1471-2180-11-246. PMID: 22050722.
- Goto K, Sanefuji M, Kusuhara K, Nishimura Y, Shimizu H, Kira R, Torisu H, Hara T. Rhombencephalitis and coxsackievirus A16. Emerg Infect Dis. 2009;15(10):1689–91. doi:10.3201/eid1510.090594. PMID:19861078
- Pan H, Zhu YF, Qi X, Zhang YJ, Li L, Deng F, Wu B, Wang SJ, Zhu FC, Wang H. Analysis on the epidemiological and genetic characteristics of enterovirus type 71 and Coxsackie A16 virus infection in Jiangsu, China [Article in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30(4):339–43. PMID: 19731523
- Yip CC, Lau SK, Zhou B, Zhang MX, Tsoi HW, Chan KH, Chen XC, Woo PC, Yuen KY. Emergence of enterovirus 71 “double-recombinant” strains belonging to a novel genotype D originating from southern China: first evidence for combination of intratypic and intertypic recombination events in EV71. Arch Virol. 2010;155(9):1413–24. doi:10.1007/s00705-010-0722-0. PMID: 20549263; Epub 2010 Jun 13.
- Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, Mao N, Xu S, Zhu S, Cui A, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J. 2010;7(1):94. doi:10.1186/1743-422X-7-94. PMID: 20459851.
- Mao Q, Wang Y, Bian L, Xu M, Liang Z. EV-A71 vaccine licensure: a first step for multivalent enterovirus vaccine to control HFMD and other severe diseases. Emerg Microbes Infect. 2016;5(7):e75. doi:10.1038/emi.2016.73. PMID: 27436364
- Mao Q, Gao F, Wang Z, Li X, Gao Q, An W, Yang E, Bian L, Du R, Cui B, et al. Collaborative study on calibration and applicability of national antigen standard for coxsackievirus A16 vaccine [Article in Chinese]. Zhongguo Bing Du Bing Za Zhi. 2018;8(6):59–64. doi:10.16505/j.2095-0136.2018.0107.
- Mao Q, Wang Y, Gao R, Shao J, Yao X, Lang S, Wang C, Mao P, Liang Z, Wang J. A neonatal mouse model of coxsackievirus A16 for vaccine evaluation. J Virol. 2012;86(22):11967–76. doi:10.1128/JVI.00902-12. PMID: 22951825. Epub 2012 Sep 5.
- Du R, Mao Q, Hu Y, Lang S, Sun S, Li K, Gao F, Bian L, Yang C, Cui B, et al. A potential therapeutic neutralization monoclonal antibody specifically against multi-coxsackievirus A16 strains challenge. Hum Vaccin Immunother. 2019;15(10):2343–50. doi:10.1080/21645515.2019.1565266. PMID: 30735461
- Ye X, Yang L, Jia J, Han J, Li S, Liu Y, Xu L, Zhao H, Chen Y, Y L, et al. Development of sandwich ELISAs that can distinguish different types of coxsackievirus A16 viral particles. Appl Microbiol Biotechnol. 2016;100(6):2809–15. doi:10.1007/s00253-016-7296-z. PMID: 26767830
- He M, Xu L, Zheng Q, Zhu R, Yin Z, Zha Z, Lin Y, Yang L, Huang Y, Ye X, et al. Identification of antibodies with non-overlapping neutralization sites that target coxsackievirus A16. Cell Host Microbe. 2020;27(2):249–61. doi:10.1016/j.chom.2020.01.003. PMID: 32027857
- Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370(9):818–28. doi:10.1056/NEJMoa1304923. PMID: 24571754
- Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370(9):829–37. doi:10.1056/NEJMoa1303224. PMID: 24571755
- Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381(9882):2024–32. doi:10.1016/S0140-6736(13)61049-1. PMID: 23726161; Epub 2013 May 29.
- National outline of epidemic situation of statutory infectious diseases [Internet]. [cited 2015 Aug 11]. http://www.nhc.gov.cn/jkj/s3578/201901/7ba54ed285484474a881584606aa8c94.shtml.
- Cai Y, Liu Q, Huang X, Li D, Ku Z, Zhang Y, Huang Z. Active immunization with a Coxsackievirus A16 experimental inactivated vaccine induces neutralizing antibodies and protects mice against lethal infection. Vaccine. 2013;31(18):2215–21. doi:10.1016/j.vaccine.2013.03.007. PMID: 23499596. Epub 2013 Mar 13.
- Li J, Liu G, Liu X, Yang J, Chang J, Zhang W, Yu XF. Optimization and characterization of candidate strain for coxsackievirus A16 inactivated vaccine. Viruses. 2015;7(7):3891–909. doi:10.3390/v7072803. PMID: 26193302
- Lim H, In HJ, Lee JA, Sik Yoo J, Lee SW, Chung GT, Choi YK, Chung JK, Cho SJ, Lee JW. The immunogenicity and protection effect of an inactivated coxsackievirus A6, A10, and A16 vaccine against hand, foot, and mouth disease. Vaccine. 2018;36(24):3445–52. doi:10.1016/j.vaccine.2018.05.005. PMID: 29739716. Epub 2018 May 5.
- Liu Q, Yan K, Feng Y, Huang X, Ku Z, Cai Y, Liu F, Shi J, Huang Z. A virus-like particle vaccine for coxsackievirus A16 potently elicits neutralizing antibodies that protect mice against lethal challenge. Vaccine. 2012;30(47):6642–48. doi:10.1016/j.vaccine.2012.08.071. PMID: 22959985. Epub 2012 Sep 7.
- Ku Z, Liu Q, Ye X, Cai Y, Wang X, Shi J, Li D, Jin X, An W, Huang Z. A virus-like particle based bivalent vaccine confers dual protection against enterovirus 71 and coxsackievirus A16 infections in mice. Vaccine. 2014;32(34):4296–303. doi:10.1016/j.vaccine.2014.06.025. PMID: 24950363. Epub 2014 Jun 17.
- Poirier B, Variot P, Delourme P, Maurin J, Morgeaux S. Would an in vitro ELISA test be a suitable alternative potency method to the in vivo immunogenicity assay commonly used in the context of international Hepatitis A vaccines batch release? Vaccine. 2010;28(7):1796–802. doi:10.1016/j.vaccine.2009.12.006. PMID: 20018270 Epub 2009 Dec 16.