ABSTRACT
Outer membrane vesicles (OMV) are exosomes naturally released from the surface of Gram-negative bacteria. Since the ’80s, OMVs have been proposed as powerful vaccine platforms due to their intrinsic self-adjuvanticity and ability to present multiple antigens in natural conformation. However, the presence of several pathogen-associated molecular patterns (PAMPs), especially lipid A, has raised concerns about potential systemic reactogenicity in humans. Recently, chemical and genetic approaches allowed to efficiently modulate the balance between reactogenicity and immunogenicity for the use of OMV in humans. Several assays (monocyte activation test, rabbit pyrogenicity test, limulus amebocyte lysate, human transfectant cells, and toxicology studies) were developed to test, with highly predictive potential, the risk of reactogenicity in humans before moving to clinical use. In this review, we provide a historical perspective on how different assays were and can be used to successfully evaluate systemic reactogenicity during clinical development and after licensure.
OMVs as vaccine platforms
Outer membrane vesicles (OMVs) are small (25–250 nm) exosomes released during growth by Gram-negative bacteria.Citation1 They resemble the composition of the outer membrane, displaying a wide range of surface bacterial antigens (i.e. LPS, membrane proteins) in native conformation and orientation, providing them natural immunogenicity, self-adjuvanticity, and possibility to be taken up by immune cells.Citation2 For these reasons, since their discoveries in the ’60s,Citation3 OMVs have been investigated preclinically as potential vaccines against several diseases,Citation2 demonstrating their strong immunogenicity,Citation4 ability to induce antibodies with bactericidal activity,Citation5 and to protect in bacterial challenge animal models;Citation6 meningococcal group B OMV-based vaccines have been extensively and successfully tested in humans for many years.Citation7–9 Indeed, as OMVs combine both adjuvant and carrier activity, they are able to increase the immunogenic properties of protein and carbohydrate antigens and have been proven superior to traditional glycoconjugate vaccines in animal models.Citation10 This is likely due to the size of OMVs that allows rapid phagocytosis by professional antigen-presenting cells and to the presence of several pathogen-associated molecular patterns (PAMPs) that stimulate both innate and adaptive immunity.Citation11,Citation12
A limitation for the use of OMVs as vaccines is their low yield; OMVs are released spontaneously by bacteria, but in relatively low quantities; additional limitations are in the fact that key protective antigens on their surface can be present in low amounts and OMVs contain endotoxins. Increased yield and reduced levels of endotoxins can be achieved by the deoxycholate extraction, followed by differential centrifugation from the homogenized bacterial bulk; OMVs prepared using this method are usually referred to as detergent-extracted OMVs (dOMVs). In the last 20 years, genetic manipulation of OMV-producing bacteria has improved the usefulness of OMVs as vaccine platforms. Bacterial strains can be engineered not only to modulate LPS endotoxicity but also to increase the blebbing,Citation4,Citation13-15 overexpress key target antigens,Citation16 and simultaneously express multiple variants or heterologous antigens (either proteins or polysaccharides).Citation17,Citation18 This enabled to produce and test, to date mainly at preclinical level, several engineered OMVs carrying either bacterial,Citation19 viral,Citation20 parasitic,Citation21 and even cancerCitation22 antigens. Finally, the development of ad hoc industrial production, purification, and characterization processes has allowed to obtain well-defined and stable vaccine products in high yield and at low costs. GSK has developed a process for the production of OMV particles called generalized modules for membrane antigens (GMMA). GMMA is an acronym that evokes the process of budding (bud in Italian translates as “gemma”), as well as to differentiate from OMV extracted with detergents.Citation23 GMMA vesicles can be produced at high yield and purity for low costs by a three-step process: fermentation of the GMMA-producing strain, followed by two consecutive tangential flow filtration steps.Citation4 This process has been successfully applied to the production of GMMA from Shigella,Citation13 Salmonella,Citation24 and NeisseriaCitation16 species, and can potentially be used for any Gram-negative bacterial species with minimal adjustments. Another industrial approach, consisting of bacterial growth under sulfate deprivation, was developed by IntraVacc,Citation25,Citation26 with many other methods continuously discovered and developed industrially, demonstrating the huge versatility of OMVs and feasibility of manufacturing at industrial scale. The ease and efficiency of production methods enabled to move promising OMVs-based vaccines to GMP production and clinical testing with appropriate in-process control and analytical tests.Citation13
Potential reactogenicity due to endotoxin and other PAMPs remains one of the main concerns for the safe use of OMVs as vaccines. The ability to predict the risk of systemic reactogenicity in humans at the preclinical stage is often cause of debate for new vaccine candidates. In this review, we focus on the different assays used as predictor of systemic reactogenicity of OMVs and on the correlation between preclinical testing and clinical tolerability. We will mainly focus on those OMV-based vaccines that have already been moved to clinical trials.
OMVs naturally contains PAMPs
The high immunogenicity of OMVs can be partially ascribed to their strong self-adjuvanticity. OMVs contain high levels of PAMPs, molecules present only in bacteria and sensed by pattern recognition receptors (PRRs), expressed instead on a wide range of mammalian cells. The interaction of PAMPs with PRRs rapidly induces host immune responses via the activation of complex signaling pathways that lead to inflammatory responses mediated by various cytokines and chemokines. There are several classes of PRRs, such as Toll-like receptors (TLRs), RIG-I-like receptors, NOD-like receptors, and cytosolic DNA receptors. TLRs represent the most important class of PRRs in the recognition of OMV components. Each TLR recognizes only specific PAMPs. In humans, 10 TLR family members have been identified, and 13 in mice (TLR1 to 9 are conserved in both species).Citation27 Those of particular importance considering the composition of OMVs are TLR4 (sensing LPS), TLR2 (that, with TLR1 and TLR6, senses lipoproteins, lipoteichoic acids, and peptidoglycan), as well as TLR5 (recognizing flagella), and TLR3, TLR7, TLR8, and TLR9 (activated by nucleic acids).Citation28 The content and type of PAMPs differ between OMVs from different pathogens and this might lead to different reactogenicity profiles.
Limited activation of the innate immune system can aid a useful immune response to the vaccine, but a strong activation could lead to adverse effects ranging from febrile response to septic shock.Citation29 Thus, a balance between immune stimulation and reactogenicity is desired in a vaccine.
Lipid A in OMVs and TLR4 engagement in different animal models
LPS can be an effective adjuvant,Citation30 but it is also highly reactogenic and one of the most abundant components present in OMVs. Thus, depending on the dose, LPS endotoxicity of OMVs must be reduced before moving into clinical testing.
Lipid A is the endotoxic part of the LPS and the portion directly interacting with TLR4. Lipid A “core structure” (called lipid IV A) is composed of a disaccharide of glucosamine acylated at 2, 3, 2ʹ, and 3ʹ position with R-3-hydroxymyristate and is conserved in most of Gram-negative bacteria. Several lipid A structures are described in different bacteria due to the action of various enzymes on lipid IV ACitation31-35 and each structure shows a different level of endotoxic activity, with the most reactogenic form of lipid A being a hexa-acylated glucosamine disaccharide phosphorylated at the 1 and 4ʹ position with 12 to 14 carbon acyl chains and an asymmetric (4/2) distribution.Citation31–33
Pathogenic bacteria use natural modifications of their lipid A structure as a strategy to evade host recognition;Citation36,Citation37 i.e. the temperature increase from 25°C in the environment to 37°C in the host induces Yersinia pestis to produce a tetra-acylated lipid A that is poorly recognized by TLR4.Citation38 Similarly, Francisella tularensis, containing mainly monophosphoryl tetra-acylated lipid A composed of three steroyls and a palmitoyl group, is neither agonist nor antagonist for human and mouse TLR4, hence providing the bacteria with the ability to evade mammalian immune defense mechanisms and to survive in the infected host.Citation39 Thus, the decrease of acylation level or changes of position and acylation type of lipid A through genetic modification can represent an attractive way for reducing the TLR4-mediated endotoxicity of OMVs.
Since most of the genes involved in the lipid A biosynthesis are essential to the integrity of the bacterial membrane and the viability of the bacterium, successful approaches to modify lipid A acylation focused on the inactivation of genes encoding late acyltransferases, resulting predominantly in penta-acylated LPS. Other modifications of lipid A that have been tested preclinically consisted in dephosphorylation, overexpression of deacylase LpxR (that causes cleavage of two fatty acid chains) or of PagL (acting as 3-O-deacylases).Citation40,Citation41
Reduction of endotoxic activity of LPS has been essential for the application of meningococcal OMV vaccines in humans and has been achieved by changing the relative quantity (by chemical treatment) or the type (by genetic manipulation of OMV-producing strain) of LPS. The approach using chemical (detergent) extraction, resulting in huge decrease (>90%) of LPS quantity (still remaining in hexa-acylated formCitation23) in dOMV, has the largest track record of clinical testing in humans, with 4CMenB- (Bexsero, GSK vaccine composed by dOMV and three recombinant proteins formulated on alum) being widely used as an effective meningococcal vaccine worldwide.Citation9,Citation42 Using the genetic manipulation approach, OMVs from meningococcal strains with modified LPS (where lpxL1 or lpxL2 genes were deleted, both resulting in penta-acylated lipid A) have been developed and demonstrated to possess attenuated endotoxic activity in humans.Citation8,Citation43 Similarly, deletion of htrB in Shigella sonnei resulted in GMMA carrying penta-acylated lipid A with a highly attenuated background;Citation44 deletion of msbB in Vibrio cholerae and ETEC leads to OMVs that exhibit 50% less endotoxicity than the wild type.Citation45 The genetic approach allows the use of native OMVs/GMMA without any need for LPS removal by detergent extraction, making them much easier to produce and more versatile as a vaccine platform.
A crucial aspect to consider is that the prediction of the effect of LPS modifications a priori is complex. In fact, similar lipid A structures could induce very different levels of TLR4 activation, and the behavior might be different when lipid A is in purified form or when in the bacterial membrane.Citation46 Moreover, recognition of lipid A by the TLR4/MD-2 complex is species-specific, with mice being less sensitive to LPS than humans, and little being known about recognition of lipid A by rabbit TLR4.Citation47 For instance, lipid IV A (tetra-acylated) is considered an antagonist of human TLR4, but an agonist of murine TLR4. This species-specificity of TLR4/MD-2 poses a problem in terms of translatability of results obtained in murine or rabbit models when testing altered LPS structures that are meant for use in humans.Citation48 Furthermore, within the same species, TLR4 is subject to polymorphism, resulting in different susceptibilities of subjects to endotoxins.Citation49 Finally, in response to LPS stimulation, neonatal human whole blood produced significantly less TNF-α and IL-1β than adult whole blood and, consistently, neonatal monocytes displayed down-regulated surface expression of CD14 and TLR4, and suppressed phosphorylation of NF-κB p65 and p38 MAPK in response to the TLR4 agonist compared with adult monocytes.Citation50 This will pose further difficulties in predicting risks of reactogenicity in different target populations.
Assays performed in vitro or in animal models to predict risks of reactogenicity in humans
A vaccine can move to clinical testing only if there is reasonable evidence that the risk and severity of adverse events will be acceptable. The prediction of this risk is crucial, especially when testing novel types of vaccines. While the toxic effects associated with contaminants or impurities in the preparation or in the formulation can be controlled, the adverse effects of a vaccine that might occur due to inherent endotoxicity of the product (like the presence of lipid A in the OMVs) need to be adequately measured. Despite the potential of the OMVs as vaccines and the abundance of preclinical data, few are the approaches to test reactogenicity in humans. Several tests can be considered for this purpose, with some being suggested by regulatory agencies as part of the package necessary to start clinical testing. These include the rabbit pyrogenicity test (RPT), the monocyte activation test (MAT), the limulus amoebocyte lysate (LAL) test, the use of specific reporter cell lines, and classical toxicology studies. Moving through this labyrinth of assays to produce data that could best predict the risks of clinical reactogenicity is often difficult, although crucial. Each of the available assays has advantages and drawbacks (described below and in ) and many tests have been differently used and often adapted ad hoc to be more suitable to testing OMV.
Figure 1. Pro and cons of the assay used as predictor for risk of systemic reactogenicity for OMV-based vaccines
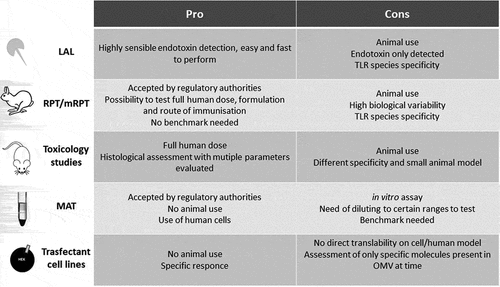
LAL test
The LAL test is based on the clotting reaction of the hemolymph of the horseshoe crab in the presence of endotoxin. It is extremely fast and sensible to quantify endotoxins. However, it does not detect non-endotoxin pyrogens.Citation51 Thus, LAL cannot replace other methods to detect pyrogens other than LPS alone. Furthermore, since LAL test is intended to quantify endotoxin in vaccines where levels of endotoxin contamination should usually be very low or absent, it is less suitable for application to bacterial vaccines such as OMVs that contain a considerable amount of LPS and other PAMPs. The assay can be applied directly just if testing OMV formulations extremely diluted (million times) to fit in the quantification range of classical LAL assays, with the risk of testing samples not being representative of the initial formulation. Furthermore, LAL assay was demonstrated to be unable to distinguish between the endotoxicity of OMVs containing wild-type and modified LPS, while the RPT and the MAT could.Citation52 This is not surprising, considering that the LAL assay is based on LPS recognition by a completely different receptor system (crab) than the mammalian TLR4/MD-2.
For the above reasons, despite being accepted by PharmacopoeiaCitation53 to determine the safety of compounds, LAL has never been used alone as predictor of OMV risk of inducing reactogenicity, and neither to evaluate lot-to-lot consistency.
Toxicology studies and mouse/rat models
The aim of a preclinical toxicology study is to determine whether the vaccine is likely to be safe for testing in clinical trials. Absorption, distribution, metabolism, and excretion (ADME) test, as well as pharmacokinetic/toxicokinetic studies, are also important to obtain a comprehensive safety evaluation of new vaccines. Although animal toxicity studies are not ideal, as there may be species-specific responses to vaccine components and the development and validation of in vitro tests is increasingly more encouraged, a repeated-dose toxicity study is still required prior to progress to clinical studies for vaccines.
In toxicology studies, the full human dose is generally tested in animals (usually mice/rats and rabbits) that should be likely to develop a vaccine response similar to that expected in humans, using the same immunization route, and n + 1 injections compared to what envisaged in clinics, going to evaluate several local and systemic parameters over the course of time.Citation11 Pretreatment values should be measured where possible. Rabbits are the animals of choice for toxicology studies since a full human dose (0.5–1.0 mL) can be administered to them, their weight is comparable to that of a child, and they show a rise in body temperature in response to (wild-type) endotoxin comparable to that of humans.Citation54
1790GAHB (GMMA from a S. sonnei ΔhtrB strain formulated on aluminum hydroxide) were well tolerated in rabbit toxicology studies (when either intranasal, intradermal, or intramuscular doses of 80, 10, or 100 µg, respectively, of vaccine were administered), with very slight-to-moderate local reactions (induration, erythema, and edema) and minimal inflammatory signs observed upon administration. The observations were consistent with pharmacological response to a vaccine, and all changes were resolved by the end of the two-weeks recovery phase.Citation13 1790GAHB in multiple phase I and II studies resulted to be well tolerated up to 100 μg/dose in adults.Citation44,Citation55
A meningococcal OMV vaccine expressing a single PorA and a single FetA (MenPF-1) formulated with aluminum hydroxide was tested in a toxicology study for local tolerance and repeated doses in rabbits to support the clinical administration of up to three intramuscular doses of vaccine, either in 25 or 50 μg protein dose levels.Citation56 MenPF-1 showed no evidence of systemic reactogenicity. The vaccine in phase I clinical trial gave no serious adverse events, with mostly mild-to-moderate transient local reactions at the site of injection; systemic symptoms such as fever were infrequent and no safety concerns were reported.Citation57
In repeated toxicology studies in rabbits conducted during preclinical evaluation of Bexsero (with 25 µg dOMV), local reactogenicity of the vaccine formulations was of a low order of magnitude, and changes were slightly exacerbated compared to the aluminum hydroxide placebo formulation. Injection site inflammation was partially to fully reversible within the 14 d recovery period and all was in line with results obtained in humans.Citation58
Despite the correlations described earlier, failures during the later clinical phases often raise the question whether the preclinical safety toxicology studies are sufficiently predictive for the human outcome.Citation59–61 Shanks et al. have shown that animal toxicity studies are predictive to a certain extent, and the predictivity varies among endpoints, with some being better predicted than others (i.e. cutaneous/local adverse events being the best predicted).Citation62 The instantaneous reactogenicity and the post-vaccination fever response that could translate in rejection of the vaccine (at least for use in children) are more difficult to predict in classical toxicology studies.
As an example, the preclinical toxicology studies on 4CMenB correlated with the local reactions found in clinics in different investigational studies;Citation7 overall, the most commonly reported adverse effects after any vaccination were injection site induration (42%), erythema (13%), and swelling (9%), and similar results were found in humans. On the other hand, the toxicology was not a good predictor of the most common adverse event, that was fever. In fact, fever (≥38.0°C) was reported for 41% to 58% of subjects (across groups); however, fever was transient, with the majority resolving within 2 d, and overall fever rates were comparable to that of the Priorix Tetra vaccine.Citation7,Citation63
In case of the 1790GAHB (GMMA-based vaccine), preclinical toxicologyCitation13 results correlated with the local reactions observed in clinical trials. In humans, mild fever was the only, although infrequent, adverse event observed after vaccine administration, in line with what was predicted by the toxicology studies.Citation44
Very recently Sheerin et al.Citation64 used a combination of RNAseq, ELISA, and temperature recording for 24 hours upon vaccination to demonstrate for the first time that a mouse model can predict with high concordance the reactogenicity profile of OMV-based vaccines in humans. These authors compared the temperature rise, cytokine profiles, and cytokine gene expression induced in mice by 4CMenB (dOMV-based vaccine), by OMVs both with wild-type or attenuated endotoxin, or by LPS alone (all formulated in alum) to what was observed in humans. A significant temperature rise upon vaccination was observed only in mice immunized with 4CMenB, wild-type OMVs, and LPS, whereas the temperature increase detected was much lower for OMV with modified lipid A; the same was confirmed looking at the profiles of IL-6 induced and at the transcriptomic profile. Overall, the pyrogenic response is much lower with OMVs with modified lipid A compared to both dOMV and OMV with wild-type lipid A, suggesting that the type and not the quantity of LPS per se makes a real difference in potentially inducing reactogenicity in humans.
Rabbit pyrogenicity test
Historically, the RPT (Ph.Eur.2.6.8Citation65) was used to screen for contamination in medicines for intravenous delivery, as the model is really sensitive to low doses of pyrogens. Also, in the field of vaccinology, RPT represents the most largely used assay to assess pyrogen content. Furthermore, the response to LPS seen in rabbits is historically believed to be more similar to that seen in humans than the ones that can be detected using experimental miceCitation54 However, a clear correlation between responses in rabbits and humans has never been established, and RPT has been validated just for wild-type lipid A.
In accordance with the Pharmacopoeias,Citation65 the classical RPT involves the intravenous infusion of the test drug into rabbits and measurement of possible rises in body temperature. The product passes the pyrogen test if the temperature increase is within a defined range over the course of a specified timeframe. The inherent variability of the in vivo system makes RPT difficult to apply with consistency unless large numbers of animals were used. Furthermore, Pharmacopoeias are unclear about the strain of rabbits and conditions to be used, whereas the sensitivity of rabbits to pyrogens differs depending on the strain, the sex, environmental conditions, and even time of the day and of the year in which the test is carried out.Citation66–68 Finally, the route of administration of vaccines to rabbits in the classical RPT (intravenous) and to humans (intramuscular, subcutaneous, intradermal, oral, or nasal) is different and therefore the results of RPT may not reflect pyrogenic response to the vaccine in a patient. Hence, the classical RPT was adapted for use in the screening of vaccines such as OMVs. One way is to dilute the vaccine to a previously established non-pyrogenic dose determined from a clinically safe batch (i.e. a batch that resulted not reactogenic or acceptably reactogenic in clinical trials) to discard any batches which are pyrogenic at the specified dilution. With this adaptation, RPT becomes a lot-to-lot consistency test, since the administered doses are not comparable between RPT and humans. By this adaptation, a vaccine was accepted if not inducing fever in rabbit when diluted (several hundred-fold) at an equivalent of 1 (for MenBvacCitation69) or 0.214 μg/per kg (for MenZBCitation70). According to Pharmacopoeias (EU, US, and JP), rabbits’ temperature needs to be monitored for 3 h; although this timeframe is optimal to detect the peak in temperature rise in response to purified endotoxin (happening between 1 and 2 h), the maximum temperature rise recorded in rabbits following vaccination with meningococcal dOMV was at 4.5 h.Citation71 These results indicate that the response to endotoxin alone is different from the response when it is presented in an OMV, further suggesting that the classical RPT is not suitable for testing vaccines like OMVs which intrinsically contain endotoxin.
A second way to perform the RPT relies on a modification of the European Pharmacopoeia RPT method that uses the administration of full human dose intramuscularly (mRPT). In this experimental setup, rabbits are injected intramuscularly with the vaccine or with an equivalent volume of sterile saline solution and body temperature is recorded continuously by an automated system from 90 min before injection until 3 h after administration and later manually for up to 24 h. The test is valid if the mean difference of the maximum temperature rise (the difference between the highest temperature measured during the 3 h upon vaccine administration and the initial temperature) of controls is ≤0.3°C, and the vaccine is considered to have an acceptable pyrogenic profile if the temperature response is moderate, transient, and returns back to normal at the end of the experiment. Different OMV-based vaccine products may have different criteria for passing the test, as, for example, 4CMenB (dOMV-based) and 1790GAHB (GMMA-based) vaccines are considered having acceptable pyrogenic profile in mRPT with a mean maximum temperature response <0.8°C in average (and <1.2°C for each single animal) in the test group. A maximum average temperature increase of 0.64°C was observed after administration of 100 µg of 1790GAHB intramuscularly (versus 0.38°C for placebo group) at 6 h post-vaccination, and temperature return to baseline after 24 h.Citation13 Similarly, in a repeated dose toxicity study in rabbits, only minimal body temperature rise (<1°C) was observed 4 h after intramuscular immunization with a human dose of NonaMen compared with placebo, and in all animals, the body temperature was restored within 24 h.Citation72 Based on these results, the pyrogenic profile of other OMV-based vaccines targeting different pathogensCitation41,Citation45,Citation73 could be assessed using mRPT using similar limits for the determination of safety.
Additionally, it has been found that absorption to aluminum salt might modulate the reactogenicity of OMV-based vaccines when tested in RPT. By continuous temperature monitoring using abdominally implanted data loggers, Kaaijk et al. compared plain and aluminum-adjuvanted MenB OMVs (NonaMen) in mRPT, detecting a transient temperature rise of approximately 0.6°C 4 h after the first immunization with both MenB vaccines, and a 0.2–0.3°C rise in body temperature at the same timepoint upon second vaccination just with plain NonaMen, with no increase in body temperature being detected with aluminum-adjuvanted NonaMen vaccine.Citation74 Rosenqvist et al. compared free endotoxin with dOMV formulated on aluminum adjuvants, demonstrating a difference in the RPT, the LAL, and in tolerability in humans, with free endotoxin giving the greatest fever response, and dOMV adsorbed to adjuvant giving the least reaction.Citation75
Moreover, it should always be considered that different vaccine delivery routes could cause different pyrogenic responses. In fact, if only very low levels (0.1 µg/rabbit) of meningococcal OMV could be given intravenously in RPT tests without inducing fever, just a small spike in temperature was caused by 25 µg OMV via intramuscular delivery, and larger doses (400 µg) of native OMV could instead be given intranasally without inducing fever.Citation76 This suggests that different administration routes could be investigated in the RPT assays by establishing different criteria for passing the test for each specific route. Therefore, tailoring of the RPT tests, as for the case of the modified model used to test 1790GAHB, may be the appropriate way to predict reactogenicity of vaccine administered by a route different from the intramuscular; for example, it might be appropriate the use of pulmonary or oral routes of pyrogenicity testing in RPT for OMV-based vaccines that are envisaged to be administered via these routes in humans to induce optimal immunogenicity.Citation73,Citation77
Monocyte activation test
Since the ’80s, scientists have worked on the development and validation of in vitro pyrogen tests that could be suitable for replacing RPT. MAT is a suitable alternative to RPT for estimating pyrogenicity of pharmaceuticals, including vaccines, accepted by regulatory authorities in 2010 (Ph.Eur.2.6.30Citation78). MAT does not involve the use of animals and is therefore in line with the 3Rs principles.Citation79 Three MAT methods (defined as A, B, and C if performed as quantitative, semi-quantitative, or reference lot comparison test, respectivelyCitation78) are described in the EU Pharmacopoeia, all involving the incubation of the compound to be assayed with human monocytes followed by the quantification by ELISA of proinflammatory cytokines released by the cells in response to stimulation. Human monocytes might come from different sources, including fresh human whole blood (WB), purified peripheric blood mononuclear cells (PBMC), or human cell lines (i.e. MM6). MAT can be applied as a fully quantitative test, making it more appropriate for vaccines which are inherently pyrogenic, and is physiologically relevant, since it uses human cells. IL-6 or TNF-alpha are the usual two readouts of the assay, being the cytokines more correlated with reactogenicity in vivo.Citation80 Method C, relying on comparison to reference lots, is the most suitable MAT method to date for testing OMV-vaccines. This is due to the fact that the OMV composition is complex and it also varies depending on the pathogen from which they are originated, and on the method and technology used for their production. Therefore, the direct comparison by MAT of OMV to endotoxins or any other single pyrogenic compound is difficult, as the latter might cause a different pro-inflammatory release kinetics compared to OMVs, and therefore result in difficult quantifications and parallelism issues.Citation81,Citation82
MAT (method C) has been validated as the most appropriate assay for measuring the pyrogenicity of the 4CMenB vaccine (Bexsero), especially for lots release.Citation71,Citation82 In the validated MAT, batches equally pyrogenic or less pyrogenic than those batches shown to be safe in a clinical trial can be also certified as safe (i.e. if results fall in a specified range of relative pyrogen units).Citation83 During validation of MAT for Bexsero, PBMCs were found to give better results with increased sensitivity as compared to WB; IL-6 was found to give the best readout, as this cytokine correlates with endotoxin-induced fever in rabbits, and it is secreted entirely into the culture medium in contrast to IL-1β and TNFα. MAT showed significantly fewer false-positive batches than mRPT whilst also providing a more meaningful result. Similarly, Hasiwa et al.Citation84 showed evidence that material classified as “safe” by RPT caused fever in humans and a high level of cytokine release; this difference in sensitivity between the two assays in predicting fever response in humans can be mainly attributed to issues of species specificity, inherent variability of animal system, and to superior ability to detect all non-endotoxin pyrogens when using MAT compared to RPT.
MAT has the potential not only to assess the absolute difference in comparison to the same compound and batch-to-batch variation but also to assess the relative difference between compounds produced with different strategies (i.e. by different genetic modification of lipid A). The latter aspect is useful for screening different vaccine candidates during discovery,Citation14,Citation15 as it does not require animal experimentation (that is more expensive and less ethical).
For N. meningitidis ΔlpxL1 GMMA and OMV overexpressing fHbp, Koeberling et al. showed reduction (around 1000-fold) in release of IL-1β, IL-6, IL-8, and TNFα from human PBMCs compared with what was obtained with wild-type OMVs; this reduction was similar to what was seen when testing dOMV (possessing relatively lower quantities of LOS, but in wild-type form).Citation16,Citation19 Similar results were obtained by Van de Waterbeemd et al.,Citation25 suggesting that also other vaccines based on detoxified OMVs targeting different pathogensCitation41,Citation45,Citation73 can be assayed with MAT to prove their reduced pyrogenicity compared to their respective wild-type OMVs.
The reduced cytokine release induced by lipid A mutants is not due to a minor lipid A content, as they produce normal amounts of LPS, but to mutated forms of lipid A that have a greatly attenuated ability to activate the TLR4/MD-2 complex. However, this effect is species-specific: on mouse cells, the reduction in activity is not as pronounced as on human cells, with ΔlpxL2 being less active than ΔlpxL1, whereas on human cells both lpxL1 and lpxL2 mutants showed similar residual activity.Citation85 The latter further confirms the different behavior of animal species, further highlighting the importance of using relevant models and human cell lines. Stoddard et al.Citation86 showed that MAT could well discriminate between different mutants of meningococcal LPS with different lipid A types, while the LAL and RPT could not. Authors showed that IL-6 and TNFα release both in human WB and PBMCs after stimulation with OMVs from lpxL1 and lpxL2 mutants is lower than the one caused by wild-type OMV (>400 fold); the same OMVs tested in RPT resulted in ΔlpxL1 OMVs being 40-fold and ΔlpxL2 OMVs 200-fold less pyrogenic than the wild-type. Fissea et al.Citation52 Zollinger et al. also tested N. meningitidis OMVs by RPT and MAT and showed that while the RPT suggested that the OMVs from lipid A mutants might be too toxic, MAT revealed that it was several hundreds fold less toxic in comparison to wild-type OMVs.
MAT assay using MM6 cells has been successfully used for testing NonaMen prior to clinical testing, either when vaccines are in adjuvanted and non-adjuvanted forms. The assay demonstrated an IL-6 release considerably lower for the non-adjuvanted next-generation NonaMen (ΔlpxL1 OMV-based) compared to both NonaMen (dOMV-based, vaccine demonstrated to be safe in humans) and reference DTwcP-IPV vaccine, with cytokine levels similar to those induced by other vaccines when tested in the alhydrogel adjuvanted form.Citation72 In the same study, the different vaccines have been tested in rabbits, and showed a similar pyrogenic response, therefore highlighting a superior potential to discriminate between different formulations and vaccine composition of MAT compared to RPT.
Like what was described for meningococcal vaccine candidates, a strong decrease of lipid A endotoxicity has been demonstrated for both htrB and msbB inactivation in other bacteria by MAT.Citation14,Citation15,Citation87-91 GMMA purified from Shigella lipid A mutants showed a reduction in their activity to stimulate cytokine production (IL-6, IL-8, IL-1β, TNF-α) in comparison to GMMA from strains with wild-type lipid A; the reduction in cytokine production was approximately 300-fold for ΔmsbB GMMA and 800-fold for ΔhtrB GMMA.Citation15 Similar levels of reduction in endotoxin activity were observed by MAT in GMMA from Salmonella typhimurium and Enteritidis strains with mutated penta-acylated lipid A (ΔpagP ΔmsbB).Citation14 By selectively blocking individual TLRs, it was possible to further dissect the TLR receptor-mediated IL-6 release by GMMA from lipid A mutants; residual TLR-mediated activity in GMMA from lipid A mutants of ShigellaCitation15 and SalmonellaCitation14 is mostly due to TLR2-activating components. The TLR4-mediated cytokine release was reduced up to a level in which the residual pro-inflammatory potential is mediated by other pathways. Furthermore, by simultaneous blocking with anti-TLR2 and anti-TLR4 antibodies, the cytokine release by PBMC in MAT was almost completely abolished (>90%), suggesting that the relative contribution of PAMPs other than lipid A and TLR2-activating components in GMMAs with modified lipid A is marginal (<10%).Citation14,Citation15
Dowling et al.Citation92 compared OMVs containing genetically attenuated endotoxin with other vaccines both using human newborn and adult blood, and various cytokines and chemokines as readout. When tested at equivalent treatment concentrations, ΔlpxL1 OMV induced a low response for most innate cytokines and chemokines tested, at a level similar to the ones induced by pediatric vaccines such as PCV13 and HBV, and lower than vaccines containing alum and TLR agonists (i.e. PedvaxHIB and EasyFive).
Since human monocytes express NOD receptors, MAT should be able to detect NOD activation by OMV, and the same should happen for RPT; however, there may be differences between activation levels of NOD receptors between rabbits and humans, and therefore MAT might be a better system to detect response in a species-specific way.
The main disadvantage of MAT is the fact that certain molecules contained in vaccine formulations (like alum) may interfere with a direct test, and there may be discrepancies depending on whether cell lines or WB are used, or on the culturing conditions used (i.e. source of serum), meaning that results are not always comparable between studies. Furthermore, to date, it is not possible to determine a priori which is the threshold level of cytokine induction in MAT to be used to deem new OMV-based vaccines as safe. Therefore, the use of an appropriate comparator is always necessary. Lastly, the interaction of the OMV with blood-derived cells, although being human cells, is not the one really happening in vivo after parenteral immunization.
Transfectant cell lines: TLR-specific assays
Human cells can be engineered ad hoc and used tools to dissect the specific activation of certain pathways, and thus determine fine characteristics of the candidate vaccine. One of the most used system for this purpose is Human Embryonic Kidney (HEK293) cells that can be stably transfected to express human TLRs and an NF-κB-inducible reporter genes. TLR stimulation results in a signaling cascade that finally activates NF-kB; the stronger is the TLR stimulation, the higher is NF-kB activation.
By using HEK293 cells stably transfected with the human TLR4 complex (TLR4/MD2/CD14), it was possible to verify that 600-fold more GMMA from Shigella ΔmsbB than the parent GMMA with wild-type lipid A were necessary to cause the same TLR4 activation. In contrast, GMMA from ΔhtrB producing strain resulted in substantially lower TLR4 stimulation than ΔmsbB GMMA and required 60,000-fold more GMMA than the GMMA with wild-type lipid A.Citation15 Similarly, a broad range of differences are observed in TLR4-specific assays when comparing different structures of lipid A, generally bigger than the ones observed by MAT.Citation16,Citation46 By using HEK293-TLR2 cells, it was demonstrated that TLR2 stimulation is similar for all GMMA from Shigella (and Salmonella), either with wild-type or modified lipid A, resembling their similar composition. By TLR5-specific assay, a TLR5-activation by Salmonella GMMA was detected, although this was not relevant in inducing residual cytokine release in MAT after TLR5-target blocking.Citation14
HEK293 can be also used to assess NOD-specific activation mediated by OMV,Citation93 that has been reported in OMV from Vibrio, Helicobacter, Pseudomonas, and Neisseria gonorrhoeae.Citation94
In other specific assays, Dowling et al.Citation92 tested immature newborn and adult DCs for production of cytokines and PGE2 (molecule whose in vitro production has been correlated with reactogenicity in vivo). DCs demonstrated significantly reduced OMV-mediated PGE2 responses for ΔlpxL1 OMV as compared to both Bexsero and dOMV formulated on alum. PGE2 and IL-1β production responses to ΔlpxL1 OMV and other licensed vaccines showed high concordance with RPT.
The main limitations of the use of transfectant cells or other specific assays are that those are unable to capture sinergies or antagonisms between receptors or within signaling, and the differences observed for certain pathways are always bigger (or smaller) than the ones detected both by MAT and RPT. Therefore, they most likely are poor direct predictor for risk of inducing systemic reactogenicity in humans if not used in the presence of well-established comparators and/or in combination with other assays. In contrast, transfectants might represent a good tool to check that each stage of the process is sufficiently sensitive.
Translation of results obtained with various assays to systemic reactogenicity
Besides Bexsero, that got by far the longest track record in terms of dOMV-based vaccines doses tested for safety and administered in humans to date,Citation9 1790GAHB is the the most advanced OMV-based vaccine widely tested in clinics for which results by all the assays described above have been obtained.Citation13 1790GAHB was well tolerated up to 100 µg after intramuscular injection (2 or 3 doses) in phase I and II clinical studies,Citation44,Citation55,Citation95 and the same tolerability profile was confirmed for intradermal or intranasal immunization.Citation44 Building on this experience, the level and type of systemic reactogenicity seen in humans could be initially correlated with the reactogenicity profile seen in different preclinical models. 1790GAHB vaccine showed an acceptable profile by mRPT, where an increase of temperature below 0.8°C peaking at 6 h was detected and resolved in few hours after the injection of full human dose.Citation13 But the results possibly better reflecting the response seen in human studies are the ones obtained with MAT conducted with human PBMC and IL-6 as readout, in which a difference of 800-fold has been observed in comparison to GMMA with wild-type lipid A. Additionally, using HEK293 transfectants, over 60,000-fold reduction in TLR4 activation was observed in comparison to GMMA with wild-type lipid A without affecting the TLR2 engagement. Finally, in rabbit toxicology studies, the vaccine resulted well tolerated, with just local inflammation detected, in line with expectations for an effective vaccine.
Wide experience with OMVs generated with genetically modified LPS in humans has been obtained also in Phase I clinical trials performed with OMV from ΔlpxL1 and ΔlpxL2 N. meningitidis mutants.Citation8,Citation43 OMVs from a ΔlpxL2 ΔsynX mutant with stabilized opcA expression given in two different doses (25 or 50 μg), with or without aluminum hydroxide, resulted well tolerated without evidence of residual endotoxin activity; reactions at injection site resulted mild or moderate, not depending on dose, and worst in those receiving alum-adjuvanted vaccine.
This opens another critical aspect to evaluate tolerability: the impact of different formulations and differential response to adjuvants between animal species. In fact, in RPT, alum-formulated vaccines are better tolerated than unformulated counterparts,Citation13,Citation74 suggesting that aluminum’s action to local antigen depot effects may indeed play a role. On the other hand, ΔlpxL1 ΔsynX OMVs with increased expression of fHbp and stabilized expression of opcA and a second copy of porA was tested at 4 doses (10–25–50–75 μg) and 3 intramuscular injections at 6-week intervals, all with aluminum hydroxide. Systemic reactogenicity in this case was slightly higher compared with what seen in the lpxL2 OMV trial, although both the amount of LPS and the in vitro endotoxic activity were lower, further complicating the story. The OMV with penta-acylated lipid A showed 100-fold reduced activity by MAT, and 200-fold reduced activity by RPT. Similarly, Gorringe et al.Citation96 showed tolerability up to 25 µg dose, with no or mild reaction, for N. lactamica OMV vaccine in a phase I study conducted with adult volunteers.
Overall, the results obtained using these assays with many candidate vaccines could be used in the future as a reference for studies with similar vaccines based on the same-production technology. For example, 1790GAHB could serve as reference for other Shigella GMMA-based vaccine or for GMMA-based vaccines from closely related Enterobactericaee like Salmonella; OMV-based vaccine against N. meningitidis could serve as reference for vaccines from close-related species, and for lot-to-lot consistency.
Discussion
The natural presence of several PAMPs, of specific (over)expressed proteins, and especially of lipid A, has often raised concerns for the safety of OMVs in humans. Despite the fact that genetic and chemical approaches can efficiently modulate the reactogenicity of OMVs, current assays to test reactogenicity during development (usually at least one between MAT and RPT, together with toxicology studies) still show strengths and weaknesses in predicting risk of inducing systemic reactogenicity in humans ().
LAL and human transfectant cells are highly sensitive and easy to perform, but respond only to specific compounds within the complex matrix of OMVs and therefore these assays can be useful just at specific stages of the development or for particular readouts and cannot be used alone as predictor of the overall tolerability. The classical RPT based on intravenous injection is not useful to test OMV-based vaccines but, building on the experience successfully applied to the development of Bexsero and 1790GAHB, adaptations may be possible to improve the predictive ability of this test especially if using the same immunization route envisaged for humans. This might be extremely relevant for testing those OMV-based vaccines for which intranasal or oral immunization routes may be envisaged. However, RPT reflects the rabbit species-specific sensitivity to LPS and to other OMV components, and requires the large use of animals to overcome the biological variability of the system. Toxicology studies still suffer from the species-specificity issues of RPT and the large animal use, although they allow to assess several parameters both at injection site and at systemic level, difficult to measure using in vitro assays. The development of ad hoc transgenic animals expressing human proteins (i.e. TLRs) holds the promise of more reliable evaluation of OMV-based vaccines, although it is difficult to have all the humanized immune system, and this still should not solve the problem of animal use and its inherent variability.
The species-specific sensitivity to OMVs can be efficiently overcome in MAT, that uses human cells. MAT, unlike LAL and RPT, can clearly discriminate between different lipid A acylation levels or types. Importantly, quantitative comparisons can be efficiently made without animal use. By choosing a minimal sensitivity for endotoxin of 50 pg/ml, MAT is at least as sensitive as the most sensitive RPT. Nevertheless, for large volume parenterals, the sensitive MAT-setups (in range of 3–6 pg/ml) offer the opportunity for pyrogen testing, where the RPT was not sensitive enough.Citation97 In head-to-head comparisons, MAT showed the same results as RPT when RPT was clearly negative or positive, whereas in cases where RPT result gave borderline results (and thus required a repeated test), MAT was able to discriminate, with false-negative results never occurring; this is critical for the safety of users, as false-positives are preferable to false-negatives in an assay used to predict tolerability. Taken together, these findings show that the MAT is more sensitive than RPT and may detect pyrogenicity earlier than RPT.Citation98
The assessment of the results coming from these tests may impose at a very initial stage an integrative evaluation of all this preclinical information to try to gain safety-related predictions with the possibility of reducing and, where possible, replacing in vivo (i.e. RPT) with other in vitro (i.e. MAT) tests,Citation82 after having performed the toxicology studies. Also, dose-escalation studies could help in understanding vaccine safety issues. However, the production of a more robust amount of safety data coming from clinical testing of OMV-based vaccine is necessary in order to support the preclinical evaluation of reactogenicity risks using the mentioned models, with the ultimate aim of correlating the preclinical results with relevant findings in humans.
All of this will culminate at later phases of clinical development and production to support the decision to use only one in vitro safety test for batch release. Indeed, this is the case for Bexsero, where the mRPT was proved as difficult method for measuring the pyrogenic content of the vaccine, and MAT (method C) was adapted and validated as an alternative.Citation82 This required setting of a specification and deciding on a procedure using multiple cell donors to cover biological variability and to finally certify the batch as safe.Citation23 However, until a much broader experience will be gained by testing tolerability in humans of several different types of OMV from different pathogens, the determination of absolute limits for OMV-tolerability in in vitro assays is dangerous, and therefore the simultaneous testing of the right comparator is necessary every time in which a novel OMV-based vaccine is evaluated. The right comparator is represented by the most similar clinically tolerated OMV in terms of the composition (i.e. protein composition and lipid A type), the detoxification strategy (i.e. detergent versus genetic), and the technology used for OMV-production, also taking into account doses, route of administration, formulation, and target population envisaged for the vaccine. In fact, tolerability by mucosal or parenteral administration might be different, and protein/lipid A composition of OMVs purified from phylogenetically distant bacterial species is different (i.e. OMV of an Enterobacteriae is different to the one of a Neisseriaceae). Furthermore, a dOMV has a different composition to an OMV directly purified from bacterial strain,Citation23 and different OMV-production processes could result in different types and levels of impurities. All of the above are aspects that could translate in markedly different behaviors and in the risk of accepting or discarding potential good candidates simply due to wrong comparator choice.
To date, the reduction of the pyrogenic activity can be achieved/increased in part using different formulation excipients. For example, the Shigella (1790GAHB)Citation13 vaccine contains GMMA formulated with alum partially to temper the reactivity of the endotoxin (“depot effect”) other than the need of an adjuvant. Similarly, aluminum-adjuvanted MenB OMVs (NonaMen) did not cause any temperature rise in rabbits at the second intramuscular vaccination.Citation74 However, mirroring a different composition of dOMV with GMMA/OMVs, the systemic reactogenicity detected is different in humans (although both possess an acceptable reactogenic profile). In fact, high fever (>38.5°C) was never induced by S. sonnei 1790GAHB (both in endemic and non-endemic populations), whereas fever is often induced (33–53% of the cases) upon vaccination with dOMVs (4CMenB) comparing similar doses (25 µg);Citation99 the same pyrogenic response is induced also when dOMVs are tested at ½ or ¼ of the dose. The fever response and other elicited reactions can be effectively controlled through the use of paracetamol concomitantly (and twice after 4–6 h) with vaccination with 4CMenB, even when administered with other pediatric vaccines, without affecting immunogenicity.Citation100 It is interesting to note how a borderline (temperature rise peaking just below 1.5°C) response in mRPT induced by 1790GAHB resulted instead in the absence of similar temperature rise in humans (increase of temperature detected was just in a minority of subjects, at a level similar to what detected in placebo group, thus most likely not due to vaccination itself). In contrast, the really infrequent and low pyrogenic response induced in humans by 1790GAHB is more in line with MAT (difference in ability of inducing IL-6 release of 800-fold compared to wild-type GMMA) assay and with the almost complete abolishment of TLR4 response (confirmed both with targeted blocking experiments and HEK TLR4/CD14/MD2 cells). On the contrary, although results in mRPT were highly supportive for absence of fever in humans (temperature rise detected upon vaccination <0.5°C), a general, although acceptable, fever response has been often detected by mass vaccinations with Bexsero.
All of this give rise to renewed confidence in OMV-based approaches for vaccination due to great immunogenicity data corroborated by tolerability in humans as demonstrated by recent clinical trials.Citation44,Citation55 Mass vaccination with dOMV-based vaccines are well tolerated and are currently changing the world by highly decreasing the burden of meningococcal meningitidis. Similarly, results obtained in terms of tolerability of GMMA are extremely satisfactory and true not only on healthy adults in developed countries but also in developing countries, where endemic factor may influence the immunogenicity/reactogenicity balance. However, due to differences in maturation of TLR in children and adults,Citation50 an open question remains regarding the risk of systemic reactogenicity in different age groups. For vaccines where children are the primary target population, this question can be fully answered only by ad hoc dose-escalation clinical trials.
The road to finally obtain reliable “safety related specification ranges” in all of these tests to predict risk of systemic reactogenicity of a new product is still long; more clinical data are needed to validate any of the approaches discussed in this review. However, potential new products that in these preclinical assays (especially MAT) would show similar or less reactogenicity than existing clinically accepted OMV-vaccines could move ahead to clinical testing with reasonable confidence.
Ethics
All animal studies were ethically reviewed and carried out in accordance with European Directive 2010/63/EEC and the GSK policy on the Care, Welfare and Treatment of Animals.
Acknowledgments
We thank Dr Allan Saul, Dr Rino Rappuoli, and Dr Francesca Micoli for suggestions and critical revision of the review.
Disclosure of potential conflicts of interest
This work was undertaken at the request of and sponsored by GlaxoSmithKline Biologicals SA. GSK Vaccines Institute for Global Health Srl is an affiliate of GlaxoSmithKline Biologicals SA.
The authors have the following conflict of interest to declare: all authors were, at the time in which the study was conducted, employee of the GSK Vaccines Institute for Global Health, part of the GSK group of companies. This does not alter the authors’ adherence to all Journal policies on data and material sharing.
Additional information
Funding
References
- Kuehn MJ. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19(22):2645–55. doi:10.1101/gad.1299905.
- van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015;10(11):1689–706. doi:10.1002/biot.201400395.
- Bishop DG, Work E. An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1965;96(2):567–76. doi:10.1042/bj0960567.
- Berlanda Scorza F, Colucci AM, Maggiore L, Sanzone S, Rossi O, Ferlenghi I, Pesce I, Caboni M, Norais N, Di Cioccio V, et al. High yield production process for Shigella outer membrane particles. PLoS One. 2012;7(6):e35616. doi:10.1371/journal.pone.0035616.
- Necchi F, Saul A, Rondini S. Development of a high-throughput method to evaluate serum bactericidal activity using bacterial ATP measurement as survival readout. PLoS One. 2017;12(2):e0172163. doi:10.1371/journal.pone.0172163.
- Baker SM, Davitt CJH, Motyka N, Kikendall NL, Russell-Lodrigue K, Roy CJ, Morici LA. A burkholderia pseudomallei outer membrane vesicle vaccine provides cross protection against inhalational glanders in mice and non-human primates. Vaccines (Basel). 2017;5(4):49
- Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381(9869):825–35. doi:10.1016/S0140-6736(12)61961-8.
- Keiser PB, Biggs-Cicatelli S, Moran EE, Schmiel DH, Pinto VB, Burden RE, Miller LB, Moon JE, Bowden RA, Cummings JF, et al. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine. 2011;29(7):1413–20. doi:10.1016/j.vaccine.2010.12.039.
- Rappuoli R, Pizza M, Masignani V, Vadivelu K. Meningococcal B vaccine (4CMenB): the journey from research to real world experience. Expert Rev Vaccines. 2018;17(12):1111–21. doi:10.1080/14760584.2018.1547637.
- Micoli F, Rondini S, Alfini R, Lanzilao L, Necchi F, Negrea A, Rossi O, Brandt C, Clare S, Mastroeni P, et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc Natl Acad Sci U S A. 2018;115(41):10428–33. doi:10.1073/pnas.1807655115.
- Sanders H, Feavers IM. Adjuvant properties of meningococcal outer membrane vesicles and the use of adjuvants in Neisseria meningitidis protein vaccines. Expert Rev Vaccines. 2011;10(3):323–34. doi:10.1586/erv.11.10.
- Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179(11):7692–701. doi:10.4049/jimmunol.179.11.7692.
- Gerke C, Colucci AM, Giannelli C, Sanzone S, Vitali CG, Sollai L, Rossi O, Martin LB, Auerbach J, Di Cioccio V, et al. Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS One. 2015;10(8):e0134478. doi:10.1371/journal.pone.0134478.
- Rossi O, Caboni M, Negrea A, Necchi F, Alfini R, Micoli F, Saul A, MacLennan CA, Rondini S, Gerke C, et al. Toll-like receptor activation by generalized modules for membrane antigens from lipid A mutants of Salmonella enterica serovars typhimurium and enteritidis. Clin Vaccine Immunol. 2016;23(4):304–14. doi:10.1128/CVI.00023-16.
- Rossi O, Pesce I, Giannelli C, Aprea S, Caboni M, Citiulo F, Valentini S, Ferlenghi I, MacLennan CA, D'Oro U, et al. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications. J Biol Chem. 2014;289(36):24922–35. doi:10.1074/jbc.M114.566570.
- Koeberling O, Ispasanie E, Hauser J, Rossi O, Pluschke G, Caugant DA, Saul A, MacLennan CA. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA). Vaccine. 2014;32(23):2688–95. doi:10.1016/j.vaccine.2014.03.068.
- Gnopo YMD, Watkins HC, Stevenson TC, DeLisa MP, Putnam D. Designer outer membrane vesicles as immunomodulatory systems – reprogramming bacteria for vaccine delivery. Adv Drug Deliv Rev. 2017;114:132–42. doi:10.1016/j.addr.2017.05.003.
- Stevenson TC, Cywes-Bentley C, Moeller TD, Weyant KB, Putnam D, Chang YF, Jones BD, Pier GB, DeLisa MP. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc Natl Acad Sci U S A. 2018;115(14):E3106–E15. doi:10.1073/pnas.1718341115.
- Koeberling O, Giuntini S, Seubert A, Granoff DM. Meningococcal outer membrane vesicle vaccines derived from mutant strains engineered to express factor H binding proteins from antigenic variant groups 1 and 2. Clin Vaccine Immunol. 2009;16(2):156–62. doi:10.1128/CVI.00403-08.
- Watkins HC, Rappazzo CG, Higgins JS, Sun X, Brock N, Chau A, Misra A, Cannizzo JPB, King MR, Maines TR, et al. Safe recombinant outer membrane vesicles that display M2e elicit heterologous influenza protection. Molecular Therapy. 2017;25(4):989–1002. doi:10.1016/j.ymthe.2017.01.010.
- Scaria PV, Rowe CG, Chen BB, Muratova OV, Fischer ER, Barnafo EK, Anderson CF, Zaidi IU, Lambert LE, Lucas BJ, et al. Outer membrane protein complex as a carrier for malaria transmission blocking antigen Pfs230. NPJ Vaccines. 2019;4(1):24. doi:10.1038/s41541-019-0121-9.
- Grandi A, Tomasi M, Zanella I, Ganfini L, Caproni E, Fantappie L, Irene C, Frattini L, Isaac SJ, König E, et al. Synergistic protective activity of tumor-specific epitopes engineered in bacterial outer membrane vesicles. Front Oncol. 2017;7:253. doi:10.3389/fonc.2017.00253.
- Ferrari G, Garaguso I, Adu-Bobie J, Doro F, Taddei AR, Biolchi A, Brunelli B, Giuliani MM, Pizza M, Norais N, et al. Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics. 2006;6(6):1856–66. doi:10.1002/pmic.200500164.
- De Benedetto G, Alfini R, Cescutti P, Caboni M, Lanzilao L, Necchi F, Saul A, MacLennan CA, Rondini S, Micoli F, et al. Characterization of O-antigen delivered by Generalized Modules for membrane antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine. 2017;35(3):419–26. doi:10.1016/j.vaccine.2016.11.089.
- van de Waterbeemd B, Streefland M, van der Ley P, Zomer B, van Dijken H, Martens D, Wijffels R, van der Pol L. Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccine. 2010;28(30):4810–16. doi:10.1016/j.vaccine.2010.04.082.
- van de Waterbeemd B, Zomer G, Kaaijk P, Ruiterkamp N, Wijffels RH, van den Dobbelsteen GP, van der Pol LA. Improved production process for native outer membrane vesicle vaccine against Neisseria meningitidis. PLoS One. 2013;8(5):e65157. doi:10.1371/journal.pone.0065157.
- Mahla RS, Reddy MC, Prasad DV, Kumar H. Sweeten PAMPs: role of sugar complexed PAMPs in innate immunity and vaccine biology. Front Immunol. 2013;4:248. doi:10.3389/fimmu.2013.00248.
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–84. doi:10.1038/ni.1863.
- Bishop RE. Polymorphic regulation of outer membrane lipid A composition. mBio. 2016;7(6). doi:10.1128/mBio.01903-16.
- De Gregorio E, Caproni E, Ulmer JB. Vaccine adjuvants: mode of action. Front Immunol. 2013;4:214. doi:10.3389/fimmu.2013.00214.
- Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U, Di Padova F, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. Faseb J. 1994;8(2):217–25. doi:10.1096/fasebj.8.2.8119492.
- Schromm AB, Brandenburg K, Loppnow H, Moran AP, Koch MH, Rietschel ET, Seydel U. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur J Biochem. 2000;267(7):2008–13. doi:10.1046/j.1432-1327.2000.01204.x.
- Seydel U, Oikawa M, Fukase K, Kusumoto S, Brandenburg K. Intrinsic conformation of lipid A is responsible for agonistic and antagonistic activity. Eur J Biochem. 2000;267(10):3032–39. doi:10.1046/j.1432-1033.2000.01326.x.
- Raetz CR, Guan Z, Ingram BO, Six DA, Song F, Wang X, Zhao J. Discovery of new biosynthetic pathways: the lipid A story. J Lipid Res. 2009;50(Suppl):S103–8. doi:10.1194/jlr.R800060-JLR200.
- Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76(1):295–329. doi:10.1146/annurev.biochem.76.010307.145803.
- Matsuura M. Structural modifications of bacterial lipopolysaccharide that facilitate gram-negative bacteria evasion of host innate immunity. Front Immunol. 2013;4:109. doi:10.3389/fimmu.2013.00109.
- Trent MS, Stead CM, Tran AX, Hankins JV. Diversity of endotoxin and its impact on pathogenesis. J Endotoxin Res. 2006;12(4):205–23. doi:10.1179/096805106X118825.
- Rebeil R, Ernst RK, Jarrett CO, Adams KN, Miller SI, Hinnebusch BJ. Characterization of late acyltransferase genes of Yersinia pestis and their role in temperature-dependent lipid A variation. J Bacteriol. 2006;188(4):1381–88. doi:10.1128/JB.188.4.1381-1388.2006.
- Hajjar AM, Harvey MD, Shaffer SA, Goodlett DR, Sjostedt A, Edebro H, Forsman M, Bystrom M, Pelletier M, Wilson CB, et al. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect Immun. 2006;74:6730–38.
- Zariri A, van der Ley P. Biosynthetically engineered lipopolysaccharide as vaccine adjuvant. Expert Rev Vaccines. 2015;14(6):861–76. doi:10.1586/14760584.2015.1026808.
- Asensio CJ, Gaillard ME, Moreno G, Bottero D, Zurita E, Rumbo M, van der Ley P, van der Ark A, Hozbor D. Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid a deacylase PagL as a novel acellular vaccine candidate. Vaccine. 2011;29(8):1649–56. doi:10.1016/j.vaccine.2010.12.068.
- Isitt C, Cosgrove CA, Ramsay ME, Ladhani SN. Success of 4CMenB in preventing meningococcal disease: evidence from real-world experience. Arch Dis Child. 2020. doi:10.1136/archdischild-2019-318047.
- Keiser PB, Gibbs BT, Coster TS, Moran EE, Stoddard MB, Labrie JE 3rd, Schmiel DH, Pinto V, Chen P, Zollinger WD, et al. A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine. 2010;28(43):6970–76. doi:10.1016/j.vaccine.2010.08.048.
- Launay O, Lewis DJM, Anemona A, Loulergue P, Leahy J, Scire AS, Maugard A, Marchetti E, Zancan S, Huo Z, et al. Safety profile and immunologic responses of a novel vaccine against Shigella sonnei administered intramuscularly, intradermally and intranasally: results from two parallel randomized Phase 1 clinical studies in healthy adult volunteers in Europe. EBioMedicine. 2017;22:164–72. doi:10.1016/j.ebiom.2017.07.013.
- Leitner DR, Lichtenegger S, Temel P, Zingl FG, Ratzberger D, Roier S, Schild-Prüfert K, Feichter S, Reidl J, Schild S, et al. A combined vaccine approach against Vibrio cholerae and ETEC based on outer membrane vesicles. Front Microbiol. 2015;6:823. doi:10.3389/fmicb.2015.00823.
- Needham BD, Carroll SM, Giles DK, Georgiou G, Whiteley M, Trent MS. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc Natl Acad Sci U S A. 2013;110(4):1464–69. doi:10.1073/pnas.1218080110.
- Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–95. doi:10.1038/nature07830.
- Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;5:316. doi:10.3389/fimmu.2014.00316.
- Noreen M, Shah MA, Mall SM, Choudhary S, Hussain T, Ahmed I, Jalil SF, Raza MI. TLR4 polymorphisms and disease susceptibility. Inflamm Res. 2012;61(3):177–88. doi:10.1007/s00011-011-0427-1.
- Li YP, Yu SL, Huang ZJ, Huang J, Pan J, Feng X, Zhang XG, Wang JH, Wang J. An impaired inflammatory cytokine response to gram-negative LPS in human neonates is associated with the defective TLR-mediated signaling pathway. J Clin Immunol. 2015;35(2):218–26. doi:10.1007/s10875-015-0128-6.
- Neun BW, Dobrovolskaia MA. Detection and quantitative evaluation of endotoxin contamination in nanoparticle formulations by LAL-based assays. Methods Mol Biol. 2011;697:121–30.
- Fisseha M, Chen P, Brandt B, Kijek T, Moran E, Zollinger W. Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect Immun. 2005;73(7):4070–80. doi:10.1128/IAI.73.7.4070-4080.2005.
- European Pharmacopeia 9.0. 9th edition Council of Europe, Strasburg, France. Chapter 2.6.14. Bacterial Endotoxin, 2017. p. 204–207.
- Greisman SE, Hornick RB. Comparative pyrogenic reactivity of rabbit and man to bacterial endotoxin. Proc Soc Exp Biol Med. 1969;131(4):1154–58. doi:10.3181/00379727-131-34059.
- Obiero CW, Ndiaye AGW, Scire AS, Kaunyangi BM, Marchetti E, Gone AM, Schütte LD, Riccucci D, Auerbach J, Saul A, et al. A Phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front Immunol. 2017;8:1884. doi:10.3389/fimmu.2017.01884.
- Norheim G, Sanders H, Mellesdal JW, Sundfor I, Chan H, Brehony C, Vipond C, Dold C, Care R, Saleem M, et al. An OMV vaccine derived from a capsular group b meningococcus with constitutive FetA Expression: preclinical evaluation of immunogenicity and toxicity. PLoS One. 2015;10(9):e0134353. doi:10.1371/journal.pone.0134353.
- Marsay L, Dold C, Green CA, Rollier CS, Norheim G, Sadarangani M, Shanyinde M, Brehony C, Thompson AJ, Sanders H, et al. A novel meningococcal outer membrane vesicle vaccine with constitutive expression of FetA: a phase I clinical trial. J Infect. 2015;71(3):326–37. doi:10.1016/j.jinf.2015.05.006.
- [accessed 2012 Nov 15]. https://wwwemaeuropaeu/en/documents/assessment-report/bexsero-epar-public-assessment-report_enpdf.
- Zbinden G. The concept of multispecies testing in industrial toxicology. Regul Toxicol Pharmacol. 1993;17(1):85–94. doi:10.1006/rtph.1993.1009.
- Baldrick P. Safety evaluation to support first-in-man investigations II: toxicology studies. Regul Toxicol Pharmacol. 2008;51(2):237–43. doi:10.1016/j.yrtph.2008.04.006.
- Monticello TM, Jones TW, Dambach DM, Potter DM, Bolt MW, Liu M, Keller DA, Hart TK, Kadambi VJ. Current nonclinical testing paradigm enables safe entry to first-in-human clinical trials: the IQ consortium nonclinical to clinical translational database. Toxicol Appl Pharmacol. 2017;334:100–09. doi:10.1016/j.taap.2017.09.006.
- Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philos Ethics Humanit Med. 2009;4(1):2. doi:10.1186/1747-5341-4-2.
- Zafack JG, Bureau A, Skowronski DM, De Serres G. Adverse events following immunisation with four-component meningococcal serogroup B vaccine (4CMenB): interaction with co-administration of routine infant vaccines and risk of recurrence in European randomised controlled trials. BMJ Open. 2019;9(5):e026953. doi:10.1136/bmjopen-2018-026953.
- Sheerin D, O’Connor D, Dold C, Clutterbuck E, Attar M, Rollier CS, Sadarangani M, Pollard AJ. Comparative transcriptomics between species attributes reactogenicity pathways induced by the capsular group B meningococcal vaccine, 4CMenB, to the membrane-bound endotoxin of its outer membrane vesicle component. Sci Rep. 2019;9(1):13797. doi:10.1038/s41598-019-50310-0.
- European Pharmacopeia 9.0. 9th edition Council of Europe, Strasburg, France. Chapter 2.6.8.. Pyrogens. 2017. p. 193–194. .
- Hull D, Vinter J, McIntyre J. The effect of endotoxin-induced fever on thermoregulation in the newborn rabbit. J Physiol. 1993;461(1):75–84. doi:10.1113/jphysiol.1993.sp019502.
- van Dijck P, van de Voorde H. Factors affecting pyrogen testing in rabbits. Dev Biol Stand. 1977;34:57–63.
- Bellentani L. Cyclic and chronobiological considerations when employing the rabbit fever test. Prog Clin Biol Res. 1982;93:329–42.
- Frasch CE, van Alphen L, Holst J, Poolman JT, Rosenqvist E. Outer membrane protein vesicle vaccines for meningococcal disease. Methods Mol Med. 2001;66:81–107. doi:10.1385/1-59259-148-5:81.
- Medsafe. Full Consent Application for the Meningococcal GroupB OMV vaccine MeNZB, by Chiron at the Rosia and Siena Sites, Italy Application in Accordance with Medicines Act 1981 and Medicines Regulations 1984, for Consent to distribute in New Zealand 2006.
- Vipond C, Findlay L, Feavers I, Care R. Limitations of the rabbit pyrogen test for assessing meningococcal OMV based vaccines. Altex. 2016;33:47–53. doi:10.14573/altex.1509291.
- Kaaijk P, van Straaten I, van de Waterbeemd B, Boot EP, Levels LM, van Dijken HH, van den Dobbelsteen GPJM. Preclinical safety and immunogenicity evaluation of a nonavalent PorA native outer membrane vesicle vaccine against serogroup B meningococcal disease. Vaccine. 2013;31(7):1065–71. doi:10.1016/j.vaccine.2012.12.031.
- Raeven RHM, Brummelman J, Pennings JLA, van der Maas L, Helm K, Tilstra W, van der Ark A, Sloots A, van der Ley P, van Eden W, et al. Molecular and cellular signatures underlying superior immunity against Bordetella pertussis upon pulmonary vaccination. Mucosal Immunol. 2018;11(3):1009. doi:10.1038/mi.2017.110.
- Kaaijk P, van der Ark AA, van Amerongen G, van den Dobbelsteen GP. Nonclinical vaccine safety evaluation: advantages of continuous temperature monitoring using abdominally implanted data loggers. J Appl Toxicol. 2013;33(6):521–26. doi:10.1002/jat.2720.
- Rosenqvist E, Hoiby EA, Bjune G, Aase A, Halstensen A, Lehmann AK, Paulssen J, Holst J, Michaelsen TE, Nøkleby H, et al. Effect of aluminium hydroxide and meningococcal serogroup C capsular polysaccharide on the immunogenicity and reactogenicity of a group B Neisseria meningitidis outer membrane vesicle vaccine. Dev Biol Stand. 1998;92:323–33.
- Shoemaker DR, Saunders NB, Brandt BL, Moran EE, Laclair AD, Zollinger WD. Intranasal delivery of group B meningococcal native outer membrane vesicle vaccine induces local mucosal and serum bactericidal antibody responses in rabbits. Infect Immun. 2005;73(8):5031–38. doi:10.1128/IAI.73.8.5031-5038.2005.
- Adriani R, Mousavi Gargari SL, Nazarian S, Sarvary S, Noroozi N. Immunogenicity of Vibrio cholerae outer membrane vesicles secreted at various environmental conditions. Vaccine. 2018;36(2):322–30. doi:10.1016/j.vaccine.2017.09.004.
- European Pharmacopeia 9.0. 9th edition Council of Europe, Strasburg, France. Chapter 2.6.30. Monocyte activation test.
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes Text with EEA relevance. Strasburg, France.Document 32010L0063. 2010. p. 33–79.
- Netea MG, Kullberg BJ, Van der Meer JW. Circulating cytokines as mediators of fever. Clinical Infectious Diseases. 2000;31(Suppl 5):S178–84. doi:10.1086/317513.
- Valentini S, Santoro G, Baffetta F, Franceschi S, Paludi M, Brandini E, Gherardini L, SerruD, Capecchi B. Monocyte-activation test to reliably measure the pyrogenic content of a vaccine: an in vitro pyrogen test to overcome in vivo limitations. Vaccine 37(2019):3754–3760.
- Vipond C, Sutherland J, Nordgren K, Kemp G, Heath A, Care R, Studholme L. Development and validation of a monocyte activation test for the control/safety testing of an OMV-based meningococcal B vaccine. Vaccine. 2019;37(29):3747–53. doi:10.1016/j.vaccine.2018.06.038.
- Studholme L, Sutherland J, Desai T, Hockley J, Care R, IK N, Vipond C. Evaluation of the monocyte activation test for the safety testing of meningococcal B vaccine Bexsero: a collaborative study. Vaccine. 2019;37(29):3761–69. doi:10.1016/j.vaccine.2018.05.073.
- Hasiwa N, Daneshian M, Bruegger P, Fennrich S, Hochadel A, Hoffmann S, Rivera-Mariani FE, Rocker C, Schindler S, Spreitzer I, et al. Evidence for the detection of non-endotoxin pyrogens by the whole blood monocyte activation test. Altex. 2013;30(2):169–208. doi:10.14573/altex.2013.2.169.
- Steeghs L, Keestra AM, van Mourik A, Uronen-Hansson H, van der Ley P, Callard R, Klein N, van Putten JPM. Differential activation of human and mouse Toll-like receptor 4 by the adjuvant candidate LpxL1 of Neisseria meningitidis. Infect Immun. 2008;76(8):3801–07. doi:10.1128/IAI.00005-08.
- Stoddard MB, Pinto V, Keiser PB, Zollinger W. Evaluation of a whole-blood cytokine release assay for use in measuring endotoxin activity of group B Neisseria meningitidis vaccines made from lipid A acylation mutants. Clin Vaccine Immunol. 2010;17(1):98–107. doi:10.1128/CVI.00342-09.
- Schilling B, Hunt J, Gibson BW, Apicella MA. Site-specific acylation changes in the lipid A of Escherichia coli lpxL mutants grown at high temperatures. Innate Immun. 2014;20(3):269–82. doi:10.1177/1753425913490534.
- Nichols WA, Raetz CRH, Clementz T, Smith AL, Hanson JA, Ketterer MR, Sunshine M, Apicella MA. htrB of Haemophilus influenzae: determination of biochemical activity and effects on virulence and lipooligosaccharide toxicity. Innate Immunity. 1997;4:163–72.
- Hone DM, Powell J, Crowley RW, Maneval D, Lewis GK. Lipopolysaccharide from an Escherichia coli htrB msbB mutant induces high levels of MIP-1 alpha and MIP-1 beta secretion without inducing TNF-alpha and IL-1 beta. J Hum Virol. 1998;1:251–56.
- Somerville JE Jr., Cassiano L, Bainbridge B, Cunningham MD, Darveau RP. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J Clin Invest. 1996;97(2):359–65. doi:10.1172/JCI118423.
- Leitner DR, Feichter S, Schild-Prufert K, Rechberger GN, Reidl J, Schild S. Lipopolysaccharide modifications of a cholera vaccine candidate based on outer membrane vesicles reduce endotoxicity and reveal the major protective antigen. Infect Immun. 2013;81(7):2379–93. doi:10.1128/IAI.01382-12.
- Dowling DJ, Sanders H, Cheng WK, Joshi S, Brightman S, Bergelson I, Pietrasanta C, van Haren SD, van Amsterdam S, Fernandez J, et al. A meningococcal outer membrane vesicle vaccine incorporating genetically attenuated endotoxin dissociates inflammation from immunogenicity. Front Immunol. 2016;7:562. doi:10.3389/fimmu.2016.00562.
- Bielig H, Rompikuntal PK, Dongre M, Zurek B, Lindmark B, Ramstedt M, Wai SN, Kufer TA. NOD-like receptor activation by outer membrane vesicles from vibrio cholerae non-O1 non-O139 strains is modulated by the quorum-sensing regulator HapR. Infect Immun. 2011;79(4):1418–27. doi:10.1128/IAI.00754-10.
- Kaparakis M, Turnbull L, Carneiro L, Firth S, Coleman HA, Parkington HC, Le Bourhis L, Karrar A, Viala J, Mak J, et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol. 2010;12(3):372–85. doi:10.1111/j.1462-5822.2009.01404.x.
- Launay O, Ndiaye AGW, Conti V, Loulergue P, Scire AS, Landre AM, Ferruzzi P, Nedjaai N, Schütte LD, Auerbach J, et al. Booster vaccination with GVGH Shigella sonnei 1790GAHB GMMA vaccine compared to single vaccination in unvaccinated healthy European adults: results from a Phase 1 clinical trial. Front Immunol. 2019;10:335. doi:10.3389/fimmu.2019.00335.
- Gorringe AR, Taylor S, Brookes C, Matheson M, Finney M, Kerr M, Hudson M, Findlow J, Borrow R, Andrews N, et al. Phase I safety and immunogenicity study of a candidate meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Clin Vaccine Immunol. 2009;16(8):1113–20. doi:10.1128/CVI.00118-09.
- Spreitzer I. Evolution and Characteristics of the monocyte activation test (MAT). In: Williams KL, editor. Endotoxin detection and control in pharma, limulus, and mammalian systems. Cham: Springer International Publishing; 2019. p. 523–35. https://doi.org/10.1007/978-3-030-17148-3_14
- da Silva CC, Presgrave OA, Hartung T, de Moraes AM, Delgado IF. Applicability of the monocyte activation test (MAT) for hyperimmune sera in the routine of the quality control laboratory: comparison with the Rabbit pyrogen test (RPT). Toxicol In Vitro. 2016;32:70–75. doi:10.1016/j.tiv.2015.12.004.
- Esposito S, Prymula R, Zuccotti GV, Xie F, Barone M, Dull PM, Toneatto D. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine, 4CMenB, in infants (II). Hum Vaccin Immunother. 2014;10(7):2005–14. doi:10.4161/hv.29218.
- Prymula R, Esposito S, Zuccotti GV, Xie F, Toneatto D, Kohl I, Dull PM. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine (I). Hum Vaccin Immunother. 2014;10(7):1993–2004. doi:10.4161/hv.28666.
