ABSTRACT
There is a well-known inverse association between mortality rate from infectious diseases and improvements in socioeconomic status, even though longer time-series are required to demonstrate this relationship. This general rule seems to apply to mortality from pneumonia in children in the pneumococcal conjugate vaccine (PCV) era. Two recent published secular trend studies spanning from about 30 years among Brazilians under the age of five show either no effect of PCV – not even death rate decline from pneumococcal meningitis – or a modest one (8% reduction). Time-series mortality studies from pneumonia are needed for both, developed and developing countries, those who have implemented PCV or not. Results from these studies would provide critical input and feedback to public health policy makers.
Introduction
The inverse relationship between mortality rate from infectious diseases and improvement in socioeconomic status is well known. For instance, five decades ago, two seminal papers were published on this subject.
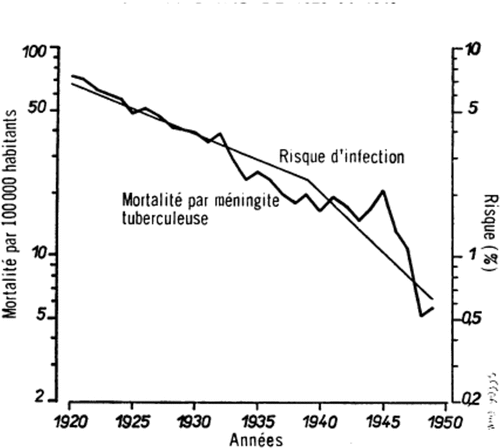
Styblo and coworkers demonstrated that from 1920 to 1949, an era when anti-tuberculous drugs were not available, in the Netherlands – a country with a small proportion of BCG-vaccinated child population and infrequent mycobacterial infections other than M. tuberculosis – the mortality rate from tuberculous meningitis among under-fours decreased around 90% (see ).Citation1
Figure 1. Mortality rate from tuberculous meningitis among children younger than 4 years old and annual risk of tuberculosis infection, The Netherlands, 1920–1949
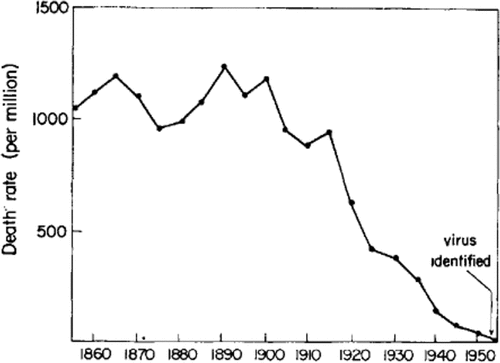
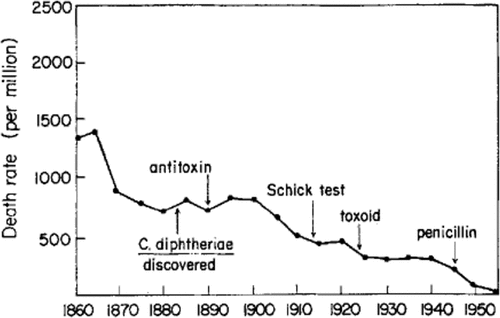
Likewise, similar data were presented by Dr Edward Kass, President, Infectious Diseases Society of America, during his address at the joint meeting of the Infectious Diseases Society of America and the Tenth Interscience Conference on Antimicrobial Agents and Chemotherapy, in October 1970. In that meeting, he presented sound numbers on mortality rate from diphtheria and measles in England and Wales, from 1860 to 1940, an era when there were no specific strategies to prevent and/or treat these diseases. Reductions were by 65% and virtually 100%, respectively, among children and adolescents younger than 15 years of age (see ).Citation2
Figure 2. Mean annual death rate from diphtheria (A) and measles (B) in children under 15 years of age, England and Wales, 1860–1950
Later on, BCG, diphtheria, and measles vaccines demonstrated their unequivocal efficacy against the three respective diseases.Citation3–5 It was expected that similar findings would apply to mortality from pneumococcal diseases after the implementation of pneumococcal conjugate vaccines (PCVs) as well, as it was shown above for tuberculosis, diphtheria and measles, but no paper from the developed world has been published on this subject so far.
According to estimates from a recently published paper, from 1990 to 2017, mortality from lower respiratory infections (LRI) among HIV-negative children younger than 5 years has declined 67.2% (63.5–70.1%) globally.Citation6 To note, such declining trend had been observed even before PCV introduction. That attainment was related to both, a reduction in exposure to household air pollution and in the prevalence of childhood wasting, as well as increased vaccine coverage, mainly against Haemophilus influenzae type b and pneumococci. The authors pointed out that there was variation by country, suggesting that no single intervention such as PCV or others would substantially reduce LRI mortality in every country.Citation6 Moreover, we should emphasize that, as that time series covered 27 years, from 1990 to 2017, in 195 countries, and PCV has been introduced since 2000 across more than 100 countries, it was estimated that PCV implementation contributed, roughly, to a modest 6.3% decrease on mortality.Citation6 It is noteworthy that a previous estimate from another GBD Group pointed that childhood wasting, by itself, was the leading risk factor for LRI mortality among children under 5 years, responsible for 61.4% of LRI deaths.Citation7
Then, before and even after PCV introduction, improvement in human development index, education, housing, sanitation, nutritional status, antibiotic therapy, may have contributed with the impressive above-mentioned mortality rate reduction from LRI, in the same fashion that those reductions reported by Styblo K et al. and Kass occurred.Citation1,Citation2
But an intriguing question emerges from these findings: why has the impact of PCV on mortality from LRI among pre-schoolers not been widely demonstrated in developed countries which implemented PCV 20 years ago, where systematic, reliable, and sophisticated epidemiological surveillance is continuously underway?
PCV was conceived and implemented having mortality rate reduction as one of its major and ultimate goals. However, the few reports published on this subject so far came from low-middle income countries. Even more surprising which allows us to re-emphasize, none of them were done in affluent societies.
This “Commentary” aims to provide a brief review of the literature on this subject, taking into account, when applicable, the multiple determinants related to the reduction of mortality rate from LRI and pneumonia among under-fives.
Literature overview
As expected, it is hard and nearly impossible to conduct a randomized trial on mortality from pneumococcal diseases. In fact, because only one randomized, placebo-controlled, double-blind trial was done in the eastern Gambia in the early 2000s, it merits a specific comment. Primary endpoint was originally all-cause child mortality, but because of practical constraints on sample size, researchers changed it to radiologically confirmed pneumonia. Then, mortality was obtained as a post-hoc analysis.Citation8
The nine-valent PCV (Wyeth Vaccines, Collegeville, PA, USA) contained serotypes 1, 4, 5, 9 V, 14, 19 F, and 23 F polysaccharides, serotype 6B polysaccharide, and serotype 18 C oligosaccharide linked to the diphtheria toxoid protein CRM197. In that trial, 529 children had been assigned to receive the intervention (PCV-9) and 568 were allocated to the control-group, vaccination ended in April, 2003, and clinical follow-up ended in April, 2004. Vaccination reduced overall all-cause mortality by 16% (95% CI, 3–28%) in the per-protocol analysis and by 14% (95% CI, 2–24%) by intention to treat analysis.Citation8 However, attention should be drawn to the width (by 24%) of the 95% confidence interval, because it reveals imprecise estimates.
Short-medium time-series studies
Published works came exclusively from developing countries from series that lasted less than 10 years.
In Peru, a 7-year time-series analysis comprising 3 years before and 4 years after PCV introduction showed that mortality due to pneumonia in children younger than 1 year old had been reduced by 35% (95% CI, 8.6–53.8%).Citation9 Similarly, a two-year population-based nested case–control study conducted in Chile found a reduction of 71.5% in deaths due to pneumonia (95% CI, 9.0–91.8%) after PCV-10 introduction.Citation10 Another study conducted in the State of Santa Catarina, Brazil, showed an 11% reduction (95% CI, minus 20% to 34%) in mortality from pneumonia in children younger than 1 year, 4 years after PCV-10 had been implemented, but diverging results were reported in different regions of that State.Citation11 Again, attention should be paid to the width of the 95% CI verified in these three studies, which also reveals imprecise estimates like previously mentioned in The Gambia trial.
A study conducted in South Africa covered the period pre-(2005–2008) and post-PCV introduction (2012–2013) in children aged up to 59 months. Estimates for reductions on mortality rate from bacteremic and non-bacteremic pneumococcal pneumonia, were 53% and 60%, respectively. However, the authors commented that reductions were likely due not only to PCV introduction but also due to improvements in HIV care and overall prevention as well.Citation12
Thus, these time-series studies lasted less than 10 years, a relatively short time span to assess the relationship between mortality and vaccine effectiveness, due to the complexity of vaccine and non-vaccine factors involved when assessing such outcome in infectious diseases as mentioned above, which leads to a potentially equivocal interpretation when quantifying solely the impact of PCV on mortality.
Longest time-series studies
The two longest ecological studies analyzed data from Brazil, a large country with huge wealth disparities, where PCV-10 was implemented in 2010.
Accessing publicly available mortality data of children aged 59 months or younger between 1980 and 2014, from the Mortality Information System of the Brazilian Ministry of Health, the first study examined long-term trends in childhood pneumonia mortality, i.e., from 1980 to 2014, and also compared mortality rates from, namely, the pre-vaccine (April 1, 2004, to March 31, 2009) and the post-vaccine period (April 1, 2010, to March 31, 2014). Reductions in annual rates of pneumonia mortality were steepest in the 1980s and 1990s, and decreased by 10 times in Brazil (90% during 1980–2010), when Human Development Index (HDI- a composite index that includes income, education, life expectancy at birth) increased from 0.55 to 0.71. Most of the decline since 1980 was achieved before the millennium and clearly preceded the introduction of Haemophilus influenzae type b vaccine in 1999, and of PCV10 in 2010.Citation13
Apart from socioeconomic developments verified through HDI, many other achievements had been reached during that period. Among them, continuous mortality rate reduction occurred concomitantly with improved education, sanitation, housing, expanded access to health care, especially antibiotic drugs, and public health policies, like school food program, promotion of breastfeeding, child and women’s health program, promulgation of the 1988 Federal Constitution leading to the creation of the Brazilian Unified Health System (that contemplate “Health as a right of citizenship”), community health-care worker’s program, family health program, and pact to reduce infant and maternal mortalityCitation13(see ).
Figure 3. National annual all-cause pneumonia mortality rate from 1980 to 2014 in children younger than 5 years and Human Development Index evolution over time in Brazil
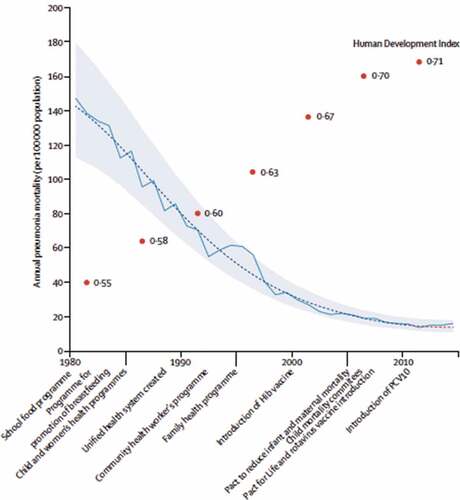
In spite of a high level of vaccine uptake (80–85% of the target population), authors found weak evidence that having introduced PCV-10 produced a further reduction in childhood pneumonia deaths of about 10% at the national level. That estimate had substantial uncertainty, and all credible intervals (CrIs) included 1 (signifying no effect): the estimated reduction was 12% (95% CrI, minus 6 to 12) for children aged 3–11 months, 12% (95% CrI, minus 6 to 13) for children aged 3–23 months, and 8% (95% CrI, minus 9 to 19) for children aged 3–59 months. However, after stratifying by socioeconomic status, authors found larger reductions in the subpopulation living in municipalities characterized by poverty or low maternal education, but no decline was observed in more affluent municipalities. The largest decrease (24%) was observed in children aged 3–23 months in municipalities with low maternal education (95% CrI, 7–35%). Although a vaccine-related decrease in childhood pneumonia mortality of 10% is less than what many expected, those estimates indicated that the vaccine had a substantially greater benefit in poorer municipalities.Citation13
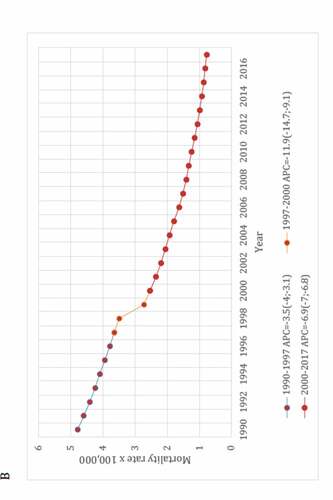
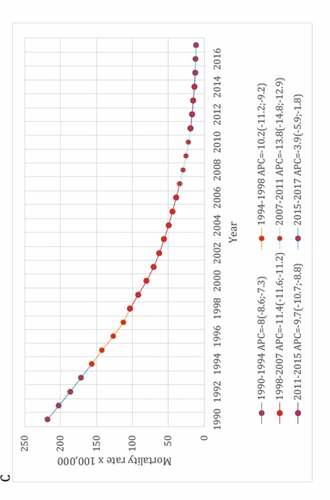
The second study covered the period 1990–2017, meaning that it included 3 years more after the end point of the first one, and used a different methodology, i.e., the well-established and widely recognized Global Burden of Disease (GBD) approach.Citation14 In this study, results were based on the GBD Study 2017 estimates which database provides data for each cause of death over time. The cause of death estimation is related to how well death and its cause were captured by the routine vital registry (VR) system, as is the official Brazilian Mortality Information System.Citation15
In the GBD cause of death estimation, issues in the ascertainment of the underlying cause, such as ill-defined codes or poorly specified causes of death, called garbage codes, were corrected and redistributed to specific causes by age, sex, location, and year using various statistical approaches. All specific causes of death data were adjusted to 100% completeness by multiplying the cause of death fraction in VR by the GBD estimates of total under-five deaths (all causes), based on data from VR, censuses, and surveys such as the Brazilian National Household Sample Survey and the Brazilian Demographic and Health Survey.Citation16,Citation17
As an attempt to control biases inherent to ecological studies, secular trend of deaths from LRI was compared with time trend from pneumococcal meningitis (PM) and diarrheal diseases, i.e., “positive and negative controls,” respectively. Both diseases were chosen because they share similar socioeconomic determinants with LRI. Moreover, etiology of PM is a laboratory-confirmed condition and then it played a role as a surrogate of the real impact of PCV-10 on invasive pneumococcal diseases. Interestingly, the lack of impact of PCV on mortality trend verified in this study parallels with the following statement by Koelman et al. in their recent published paper: “the promising decline in the incidence of pneumococcal meningitis following the introduction of vaccination seems to have been temporary. Replacement by non-vaccine serotypes illustrates that pneumococcal meningitis continues to pose a major challenge. The implementation of PCVs has resulted in a significant decrease in PCV serotype pneumococcal meningitis, but has not uniformly resulted in a decrease in pneumococcal meningitis incidence.”Citation18
Like the first study, this second one demonstrated a sustainable reduction in mortality rates for these three diseases, and especially with no relevant changes in the secular trends for LRI and PM after the PCV-10 implementation, despite a mean 91% vaccine uptake (see ). In brief, for LRI and PM a statistically significant (p < .05) annual percent mortality reduction was verified from 1990 to 2009, i.e., 20 years before countrywide vaccination, and it was comparable to that observed during the PCV era.
Figure 4. Joinpoint regression models for mortality rates from lower respiratory infections (A), pneumococcal meningitis (B) and diarrheal diseases (C) among Brazilians under-five
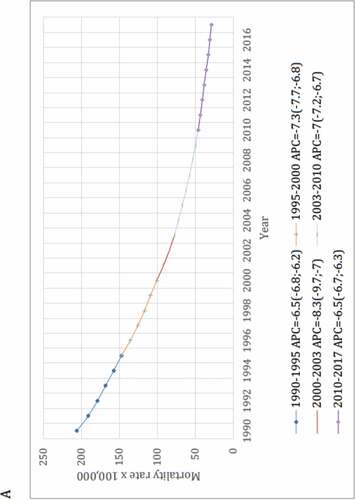
To note, the overlapping of the curve shapes generated by both studies shows that they are almost identical.Citation13,Citation14 Furthermore, it should be noted that, if – like the first study – the second one had been limited from only 6 years before (2003–2009), and after (2011–2017) PCV-10 implementation, mortality rate would reduce from around 62.5 to 32.5/100,000, i.e., by 48% and could, equivocally, be ascribed to PCV-10 effectiveness.
Similarly to the scenario reported by Schuck-Paim et al.,Citation13 during our study period, there was an unequivocal and continuous increase in the HDI (from 0.611 to 0.759, i.e., plus 24.2%), in life expectancy at birth (which increased by 10.4 years, i.e., 75.7 years in 2017), and in Gross National Income per capita (plus 28.6%).Citation14
Once again, these two studies emphasize Kass’ and Styblo’s point of views on infectious diseases and social changesCitation1,Citation2 verified in Brazil in two apparently opposite ways, namely, improvements in socioeconomic scenario lead to impressive mortality rate reductions from LRI through non-vaccine factors and poorest communities have a burden that recommends specific prevention strategies, such as PCV vaccination among others, to save children’s lives.
Countrywide or targeted vaccination?
Based on their own results and those obtained by Schuck-Paim et al., the authors of the present “Commentary” agree with the following statement: “ … although the effect of PCV on pediatric pneumonia mortality might be reduced in countries where mortality rates have already fallen because of economic and social improvements, substantial vaccine-related gains are more likely in the world’s low-income regions.”Citation13In this sense and taking into account a global perspective and from the latest available worldwide estimates, PCV priority might be given to Sub-Saharan Africa and South Asia, the regions where around 660,000 out of 810,000 (≅ 80%) of deaths from LRI occurred in 2017.Citation6
Conclusion
Time-series mortality studies from LRI, pneumonia, and pneumococcal meningitis are urgently needed across the world. They do need to last, at least, 20–25 years, allowing them to better understand trend rates and their relationship with PCV implementation. Moreover, even in countries, either developed and developing ones, that did or did not introduce PCV, results from these studies would provide crucial inputs and feed-back to public health policy makers.
Other than temporal analyses, cohort or case–control approach – using, for instance, verbal autopsy in settings where reliable vital statistics data are unavailable – may be an alternative way to assess vaccine effectiveness and causality of deaths from LRI/pneumonia, and the need to implement control measures, related or not with PCV introduction.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Styblo K, Meijer J, Sutherland I. La transmission du bacilli tuberculeux: son evolution au sein d’une collectivité humaine. Bull World Health Organ. 1969;41:137–78. [Articlein French; Summary available in English].
- Kass EH. Infectious diseases and social change. J Infect Dis. 1971;123(1):110–14. doi:10.1093/infdis/123.1.110.
- Camargos PA, Guimarães MD, Antunes CM. Risk assessment for acquiring meningitis tuberculosis among children not vaccinated with BCG: a case-control study. Int J Epidemiol. 1988;17(1):193–97. doi:10.1093/ije/17.1.193.
- Wagner KS, White JM, Andrews NJ, Borrow R, Stanford E, Newton E, Pebody RG. Immunity to tetanus and diphtheria in the UK in 2009. Vaccine. 2012;30(49):7111–17. doi:10.1016/j.vaccine.2012.09.029.
- Di Pietrantonj C, Rivetti A, Marchione P, Debalini MG, Demicheli V. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database Syst Rev. 2020 Apr 20;4:CD004407. doi:10.1002/14651858.CD004407.pub4.
- GBD 2017 Lower Respiratory Infections Collaborators. Quantifying risks and interventions that have affected the burden of lower respiratory infections among children younger than 5 years: an analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2020;20(1):60–79. doi:10.1016/S1473-3099(19)30410-4.
- GBD 2016 lower respiratory infections collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210. doi:10.1016/S1473-3099(18)30310-4.
- Cutts FT, Zaman SMA, Enwere G, Jaffar S, Levine OS, Okoko JB, Oluwalana C, Vaughan A, Obaro SK, Leach A, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365(9465):1139–46. doi:10.1016/S0140-6736(05)71876-6.
- Suarez V, Michel F, Toscano CM, Bierrenbach AL, Gonzales M, Alencar AP, Ruiz Matus C, Andrus JK, de Oliveira LH. Impact of pneumococcal conjugate vaccine in children morbidity and mortality in Peru: time series analyses. Vaccine. 2016;34(39):4738–43. doi:10.1016/j.vaccine.2016.07.027.
- Diaz J, Terrazas S, Bierrenbach AL, Toscano CM, Alencar GP, Alvarez A, Valenzuela MT, Andrus J, Del Aguila R, Hormazábal JC, et al. Effectiveness of the 10-Valent Pneumococcal Conjugate Vaccine (PCV-10) in children in Chile: a nested case-control study using nationwide pneumonia morbidity and mortality surveillance data. PLoS One. 2016;11(4):e0153141. doi:10.1371/journal.pone.0153141.
- Kupek E, Vieira IL. Impact of PCV10 pneumococcal vaccine on mortality from pneumonia in children less than one year of age in Santa Catarina State, Brazil. Cad Saúde Publica. 2016;32(3):e00131414. doi:10.1590/0102-311X00131414.
- von Mollendorf C, Tempia S, von Gottberg A, Meiring S, Quan V, Feldman C, Cloete J, Madhi SA, O’Brien KL, Klugman KP, et al. Estimated severe pneumococcal disease cases and deaths before and after pneumococcal conjugate vaccine introduction in children younger than 5 years of age in South Africa. PLoS One. 2017;12(7):e0179905. doi:10.1371/journal.pone.0179905.
- Schuck-Paim C, Taylor RJ, Alonso WJ, Weinberger DM, Simonsen L. Effect of pneumococcal conjugate vaccine introduction on childhood pneumonia mortality in Brazil: a retrospective observational study. Lancet Glob Health. 2019;7(2):e249–56. doi:10.1016/S2214-109X(18)30455-8.
- Camargos P, Nascimento-Carvalho CM, Teixeira R, França E. Lower respiratory infections mortality among Brazilians under-five before and after national pneumococcal conjugate vaccine implementation. Vaccine. 2020;38(11):2559–65. doi:10.1016/j.vaccine.2020.01.084.
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–88. doi:10.1016/S0140-6736(18)32203-7.
- GBD 2016 Mortality Collaborators. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1084–150. doi:10.1016/S0140-6736(17)31833-0.
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210. doi:10.1016/S0140-6736(17)32152-9.
- Koelman DLH, Brouwer MC, van de Beek D. Resurgence of pneumococcal meningitis in Europe and Northern America. Clin Microbiol Infect. 2020 Feb;26(2):199–204. doi:10.1016/j.cmi.2019.04.032.