ABSTRACT
Aim
Pertussis continues to be a common worldwide infection in pediatric and adult populations.
We aimed to study epidemiological and clinical characteristics of infants and children admitted for pertussis to a tertiary-care hospital and to investigate the risk factors for pediatric intensive care unit (PICU) admission.
Materials and Methods
With a retrospective study, we analyzed all medical reports of patients admitted to Bambino Gesù Children’s Hospital in Rome from January 2011 to December 2018 with a diagnosis of pertussis.
Results
We examined 195 patients. The majority of hospitalized children (66.15%) were <3 months of age. No mother had received pertussis containing vaccine during pregnancy. Ten cases required admission in PICU. The age at admission was lower in PICU patients with respect to ward patients (42.8 vs 240 days; p < .0007), length of hospital stay was longer in PICU group (24.7 vs 7.52 days; p < .003). Patients who needed PICU admission had greater white blood cell count at hospital admission compared with those hospitalized in the pediatric ward. One infant died and one had encephalitis.
Conclusions
Pertussis is a remerging disease. In infants, it is associated with significant morbidity and mortality. In recent years, many countries have implemented different vaccination strategies and public health measures to prevent the increase in pertussis cases. Maternal vaccination has been shown to be highly protective for infants <3 months of age before they can develop their own immunity via vaccination.
Introduction
Pertussis is an endemic, underdiagnosed, bacterial respiratory infection caused by Bordetella pertussis (B.pertussis). Despite a widespread vaccination program, pertussis continues to be a common worldwide infection in pediatric and adult populations peaking every 2 to 5 years. In 2018, according to the World Health Organization estimates, pertussis was still causing around 89,000 deaths. With 95% of the reported cases occurring in developing countries, epidemics have also recently been reported in Europe, USA, Canada, and Australia.Citation1 The European Center for Disease Prevention and Control, in 2017, reported the highest notification rate among infants <12 months of age (53.9 cases per 100,000 population), a group too young to have completed their primary vaccination schedule, followed by the age group 10–14-years-old (25.0 cases per 100,000 population).Citation2
It has been demonstrated that infants acquire pertussis from adults and adolescents, who may be susceptible to pertussis infection due to waning immunity. The switch from whole-cell pertussis vaccines to acellular products has led to more rapidly waning immunity.Citation3 In addition, unvaccinated children are also a reservoir of infection.
Classical manifestations of pertussis include catarrhal, paroxysmal and convalescent stage. However, in infants, cases with atypical clinical presentations are frequent and may be often unrecognized, especially during the winter season, when other respiratory viruses circulate.Citation4 Serious complications (neurological, respiratory, and nutritional), even if relatively rare, can be fatal. Encephalitis is particularly alarming since it is associated with death or permanent sequelae. Pulmonary complications are the most frequent (approximately 10% of the cases) and associated with most of the deaths. These severe clinical pictures are more frequent in children <1 year of life, too young to have already received all the primary doses of vaccine or in those who delay the immunization.Citation5
In Italy, the pertussis acellular vaccine is offered free of charge in the first year of life (with doses at 3, 5, and 11 months) and it is included in the hexavalent vaccine in combination with diphtheria, tetanus, polio, hepatitis b and Haemophilus influenzae type b. A booster dose, with a reduced antigen content vaccine, is scheduled at 6 and 12 years of age in association with diphtheria and tetanus (dTap) and every 10 years.
In recent years, decreasing vaccination coverage rates during childhood have been described in many countries.Citation6–9 In Italy, in 2007–2008 pertussis vaccine coverage was 96.7%, while in 2016 it reached 93.55%.Citation10 Because of that, in July 2017, Italy approved a new law to make 10 vaccinations mandatory, including the vaccination against pertussis.Citation11 Additionally, a dTpa booster has been recommended in the third trimester of pregnancy.Citation11
We aimed to study the epidemiological and clinical characteristics of infants and children admitted for B. pertussis to a tertiary-care university hospital and to investigate the risk factors for pediatric intensive care unit (PICU) admission.
Materials and methods
With a retrospective study, we analyzed all medical reports of patients admitted to the Pediatric Academic Department of Bambino Gesù Children’s Hospital in Rome from 1st of January 2011 to the end of December 2018 with diagnosis of pertussis.
All children 0 to 18 years of age hospitalized with laboratory-confirmed pertussis were eligible for the study. Laboratory confirmation included isolation of B. pertussis in nasopharyngeal aspirates by detection of B. pertussis DNA by real-time polymerase chain reaction (RT-PCR). The samples were obtained within 24 hours of admission by trained nurses and processed within 48–72 hours. Patients with diagnosis of pertussis based only on the clinical-epidemiological criteria, without laboratory confirmation, were excluded.
For the extraction of DNA from the samples, the “QIAgen EZ 1 Virus Mini Kit v 2.0” kit (QIAgen Italia) and the automatic extractor “QIAgen EZ 1 Advanced XL” (QIAgen Italia) were used. The too thick aspirates were pretreated with PBS and homogenized by vortexing. The extraction procedure involved treating 200 µl of the sample with the Internal Control provided by the kit; at the end of the extraction reaction, an eluate of 90 µl was obtained. For the Amplification Reaction a Bordetella R-Gene Kit (BioMerieux – Argene, Marcy-l’Étoile, France), was used which amplifies for the IS481 region. At the end of the amplification, the reaction curves were analyzed and the samples with a Cycle Treshold up to 40 cycles were considered positive, the samples that showed an amplification beyond the 40th cycle were considered negative.
For patients with acute respiratory symptoms or conditions such as cough, rhinorrhea, bronchiolitis, dyspnea, we also collected nasopharyngeal specimens tested for viruses generally associated with respiratory infections (RV). For RV detection, samples were processed immediately or after storage at −80°C by AllplexTM Respiratory Panel Assays (Seegene, Korea). Nucleic acids were extracted using the STARMag Universal Cartridge kit (Seegene, Korea) on the automated Nimbus IV platform, as recommended by the manufacturer. RT-PCR was performed on CFX96 Thermalcycler (Bio Rad Laboratories, Italy) with respiratory panel assay kit made up of 3 mixes which allow the identification of 16 different viruses (Influenza A and B virus, Respiratory syncytial virus A and B, Adenovirus, Enterovirus, Parainfluenza virus 1, 2, 3 and 4, Metapneumovirus, Bocavirus, Rhinovirus, Coronaviruses NL63, Coronavirus 229E and Coronavirus OC43). An internal control was included in each sample to check both extraction efficiency and PCR inhibition. The results were analyzed automatically using Seegene software (Seegene Viewer V2.0).
The medical records of subjects were retrospectively reviewed. For each case, we collected demographic data, gestational age, maternal vaccination during pregnancy, comorbidities, immunization status, clinical presentation, date of onset of symptoms, admission diagnosis, length of illness, length of hospital stay (LOS), oxygen saturation at the pulse oximeter, laboratory test results, concurrent infections, complications, need for oxygen supplementation with noninvasive and invasive methods and therapy.
Data processing was performed with the Microsoft Excel 2018 software.
Statistical analysis
Descriptive statistics were calculated, including means and standard deviations (SDs).
For categorical variables, either Χ2 or Fisher exact test was used to test the statistical difference, as appropriate. Means were compared using Student’s t-test. P-values of 0.05 were considered statistically significant.
Formal consent is not required for this kind of retrospective study: any personal data were protected accordingly to the Helsinki Declaration and to Italian law (Legislative Decree of June 30, 2003, n. 196. Code on the protection of personal data).
Results
From 1st of January 2011 to the end of December 2018, 195 patients (116 males, mean age 230.83 days) with a diagnosis of B. pertussis confirmed by a positive RT-PCR, were admitted to our hospital. The admission diagnosis of patients is shown in . Pertussis was clinically suspected in only 68 patients (34.87%). In addition, in children, less than 3 months of age, the percentage of clinical suspicion was significantly lower (p = .0006), since pertussis was misdiagnosed with bronchiolitis.
Table 1. Diagnosis at admission. Data are shown as a number of cases (%)
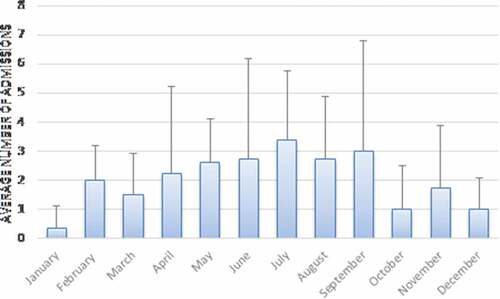
report the number of hospitalizations per year and per month, respectively. In our population study, pertussis incidence was cyclical, with a peak of hospitalization in 2012–2013 and in 2016–2017. Analyzing the seasonal trend of our patients, the maximum frequency was between May and September with a winter peak in February.
Figure 1. Pertussis cases by year and season. Number of children hospitalized by year and season from 2011 to 2018
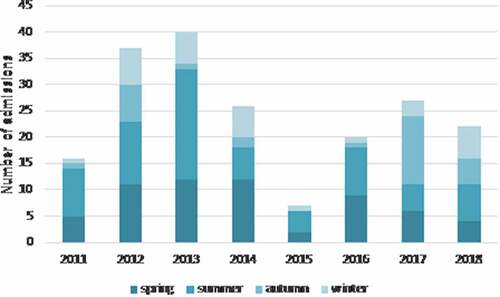
Figure 2. Pertussis cases by month. Average number of children hospitalized each month from 2011 to 2018. Error bars show standard deviation
Demographic, clinical, and laboratory data of study patients are represented in . Of 195 children analyzed, 129 (66.15%) were <3 months of age. Eighteen cases (9.23%) were born preterm (<37 weeks). Moreover, 6 patients had comorbidities: 3 had genetic syndrome, 1 esophageal atresia, 1 congenital cytomegalovirus infection, and 1 cerebral palsy. About vaccination status, 85 patients (43.58%) were too young to be vaccinated; 73 (37.43%) did not respect the timeliness of vaccination; only 32 (16.41%) had received an age-appropriate number of pertussis doses and 2 patients were unvaccinated as per their parent’s decision. For 3 patients, vaccination status was unknown (). None of the patients’ mothers had received dTap vaccine within 2 years before or during the current pregnancy.
Table 2. Demographic, clinical, and laboratory data of study patients. Data are shown as the number of cases (%) or mean (± SD). Percentages may not equal 100, because of rounding
Table 3. Vaccination status of patients according to age. Data are shown as the number of cases (%)
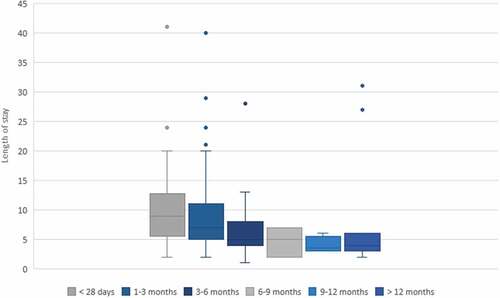
The mean length of time from onset to hospitalization was 9.73 ± 10.04 days. The average duration of stay in the hospital was 8.4 ± 6.7 days. When we compared LOS in different ages, we found that infants <3 months of age had the highest length of hospitalization ().
Figure 3. Length of hospital stay in different ages. The box shows the median, the lower and the upper quartiles, while the whiskers indicate the lowest and the highest data point excluding any outliers. The circles indicate outliers
Viral coinfection was detected in 90 children (46.15%): the most frequently isolated agent was Rhinovirus (26.15%) followed by Adenovirus (7.17%). We did not find any significant differences in clinical disease severity between patients with B. pertussis as monoinfection and patients with pertussis plus RV infection ().
Table 4. Demographic, clinical, and laboratory data of patients infected by B. pertussis only and with viral coinfections (RV, respiratory virus). Data are shown as the number of cases (%) or mean (± SD)
All patients were treated with macrolide antibiotics. Pulmonary complications were the most common; 18 (9.23%) patients reported pneumonia and 39 (20%) had respiratory distress and needed oxygen supplementation. Ten cases required admission in Pediatric Intensive Care Unit (PICU). The mean duration of hospitalization of PICU’s group was 24.7 days; 4 of them were premature while none had comorbidities. No patients admitted in PICU received vaccination because they were too young to be vaccinated. All patients admitted to PICU needed respiratory support: 5 received invasive and noninvasive ventilation, 5 only noninvasive ventilation. Two patients underwent leukapheresis treatment. One of them, a 17-girl-day-age patient presented white blood cell (WBC) count of 53.170/mm3 at admission, pneumonia, respiratory insufficiency, tachypnea, and tachycardia. She required treatment with invasive mechanical ventilation, vasoactive, and inotropic drugs and extracorporeal membrane oxygenation (ECMO). After less than 48 hours, pulmonary hypertension and cerebral edema occurred and she died. Encephalitis was verified in one girl aged 6 months. RT-PCR for B. pertussis was investigated on the cerebrospinal fluid and a low positivity was detected, confirmed by a second test. Moreover, RT-PCR for B. pertussis on the nasopharyngeal swab was positive.
Comparison between ward vs PICU group
In our series, the mean age at admission was significantly lower in PICU patients with respect to ward patients (42.80 days in PICU vs 240.98 days in ward; p < .0007) while prematurity was slightly higher in PICU patients (40% vs 7.56%; p < .0038). LOS was also longer in the PICU group (mean 24.70 days vs 7.52 days; p < .003). Patients who needed PICU admission had greater WBC count (mean 38831.50/mm3) and absolute lymphocyte count (mean 19013.5/mm3) at hospital admission compared with those hospitalized in the pediatric ward (mean 17221.08/mm3 and 10202.61/mm3; p < .02). No differences were identified between the two groups of children with regards to gender, vaccination status, and viral coinfection ().
Table 5. Demographic, clinical, and laboratory data of ward vs PICU group. Data are shown as number of cases (%) or mean (± SD)
Discussion
In recent years, pertussis has been resurging worldwide and is again an important public health issue. The disease is increasing due to many factors, including greater awareness of the disease, genetic changes in circulating B. pertussis strains,Citation8 improved diagnostic ability through the use of new molecular techniques, reduction of vaccination coverage, and evidence that neither natural infection nor vaccination gives life-long immunity.Citation12,Citation13 In particular, waning vaccine immunity has made adolescents and adults a reservoir for B. pertussis and the source of infection to the unvaccinated newborns.
In this study, we showed that in the last 7 years, 195 children have been admitted for pertussis in our hospital, most of them less than 3 months of age. Infants <3 months of age are vulnerable to pertussis infection as they are too young to be vaccinated. Furthermore, in this age group, we also reported the most serious incidence of complications, such as length of stay and admission to PICU.
Pertussis most often presents clinically as whooping cough but signs and symptoms at presentation can vary from youngest infants to adolescents. Clinical suspicion has a low sensitivity in children with less than 1 year of age: the three stages (catarrhal, paroxysmal, and convalescent) are not often recognizable; cough may be or may not be “paroxysmal,” apneic episodes can be the first sign and clinical manifestations can overlap with those of bronchiolitis.Citation14 In our study, pertussis was clinically suspected only in 34.8% of the patients at admission and the suspicion was even lower in children under 3 months. This data is in accordance with our previous work.Citation4 Several studiesCitation15,Citation16 have shown the presence of B.pertussis and RV in suspected pertussis as well as in patients who present with low respiratory symptoms without the suspicion of pertussis. Clinically differentiating B. pertussis patients from those with RV is still a challenge. Some clinical (paroxysmal cough, wheezing) and laboratory (leukocytosis) characteristics suggested the etiologies,Citation16,Citation17 but they were not pathognomonic; it supports the need for RV and B.pertussis research in this clinical circumstance.Citation18 Furthermore, the role of coinfections between B.pertussis and RV remains debated. We did not find any significant differences between patients with B.pertussis as monoinfection and patients with pertussis plus RV infection. This data is in accordance with our previous workCitation4 and with a recent study of Frassanito et al. which demonstrated no associations between clinical severity and pertussis with or without coinfections.Citation19 Consequently, we decided to include patients with viral coinfection.
It is well known that pertussis outbreaks worldwide every 3–5 years.Citation20–22 Official data indicated a peak of incidence in 2012 across Europe.Citation23–25 During this year, the notification rate of pertussis case was more than twice as high as in the previous year. A significant increase was reported also in Italy,Citation23,Citation26 as confirmed by our data. Indeed, in our series the highest incidence was seen in 2012–13 and 2016–2017. Our seasonal distribution of cases also matched the distribution already described by Gonfiantini et al. about the pertussis seasonality in Italy peaked between March and August.Citation27
Common complications of classic pertussis include pneumonia, otitis media, seizures, and encephalopathy. We report a case of B. pertussis related encephalitis in a girl aged 6 months. To our knowledge, our case is the first report of detection of B. pertussis by RT-PCR on cerebrospinal fluid in infants with acute encephalopathy.
In our work, according to literature findings,Citation28–30 younger patients had an increased risk of complications and infants <3 months of age had the highest length of hospitalization. Young age, prematurity, and high WBC count with lymphocytosis were significantly more represented in PICU patients. A recent review identified risk factors for fatal cases:Citation31 the most important is leukocytosis with lymphocytosis. No particular value of leukocytosis accurately predicts death or survival, but any rapid rise in the total WBC count over the course of the disease should represent a warning signal. Other factors relating to death include: birth weight, gestational age, young age, early onset of pneumonia, and increased pulse and respiratory rates. In our series we had only one fatal case so we cannot determine specific risk factors but, according to what previously reported, our patient presented most of the warning parameters (leukocytosis, young age, pneumonia, tachycardia, and tachypnea). The cause of death in pertussis is irreversible pulmonary hypertension associated with aggregates of WBC in pulmonary arterioles due to extreme leukocytosis.Citation32
Early identification of risk factors and prompt treatment with antibiotic are important in preventing death.Citation31 Currently, no effective therapy exists for the treatment of severe pertussis. Antibiotic therapy must be started as soon as possible. Antimicrobial treatment, commenced early in the catarrhal phase, may decrease the severity of symptoms and duration of illness and will accelerate the clearance of the organism from the nasopharynx. Unfortunately, diagnosis is rarely done in this stage, and treatment is started later when it does not impact the clinical course of the disease.Citation13,Citation33,Citation34 For this reason, the Centers for Disease Control and Prevention encourage clinicians to start antibiotic treatment based on their clinical judgment and even before laboratory results are known.Citation35 Macrolide antibiotics (e.g., erythromycin, clarithromycin, or azithromycin) have been effective and constitute the mainstay of treatment for patients with pertussis as well as for postexposure prophylaxis.Citation36 All of our patients were treated with macrolide antibiotics (azithromycin and clarithromycin). Nobody received erythromycin because of its link with infantile hypertrophic pyloric stenosis.Citation37–39
In infants, severe cases associated with leukocytosis and pulmonary hypertension, the exchange of blood transfusion is the best treatment available. Exchange transfusion may be effective by lowering the total WBC count and by removing pertussis toxin. Recently CherryCitation40 suggested new criteria for exchange blood transfusion and monitoring for the procedure for infants ≤120 days of age. In our series two patients underwent to leukapheresis treatment.
The resurgence of pertussis as a public health concern, changes in the epidemiology of the disease, and the increasing attitude of parents in delaying or missing vaccination for their children, highlight the urgent need for integrated approaches to prevent this potentially deadly childhood disease. They include the development of new therapies and vaccines, changing timing and number of doses in immunization schedules, vaccinating all close contacts of young infants immediately after delivery (cocooning), and favoring immunization of pregnant women.
Although there are many different schedules in use throughout the world, the World Health Organization suggests to start pertussis immunization at 6 weeks of age and to complete the three-dose schedule within 6 months.Citation41 Improving the timeliness of the primary infant schedule allows to complete the cycle quickly, after which protection against pertussis is guaranteed.Citation42 The solutions adopted by the various countries for the implementation of the primary vaccination cycle often depend on tradition and local decisions made for immunization against other diseases. In any case, at least three vaccine doses are to be administered during the first 12 months. In Italy, the three doses are scheduled at 3, 5, and 11 months. In our study population, 37.43% of the cases had not received an age-appropriate number of pertussis doses. These data highlight the importance to observe the timeliness of vaccination for preventing pertussis disease. The appropriate timing of the vaccination schedule is especially relevant in preterm who are at an increased risk for vaccine-preventable infections and associated complications.Citation43,Citation44 The US Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of these high-risk infants on the basis of evidence that vaccines induce protective immunity and are safe and well-tolerated in this population.Citation45,Citation46 Preterm infants who are medically stable should be vaccinated at the same chronological age and according to the same schedule as term infants. Infants born preterm could have a higher risk of pertussis than full-term infants because of incomplete transfer of maternal antibodies and an immature immune system.Citation47 Furthermore, increased risk of pertussis hospitalization and pertussis-related mortality have been observed in preterm infants.Citation48 In our study population, 18% were preterm, and 40% of them were admitted in PICU.
Another important feature, for preventing severe pertussis disease, is the immunization of adolescence and adults. Reported increases in pertussis incidence in these age groups have led many countries to introduce a routine adolescent pertussis booster as well as every 10 years in adults using the combined dTpa vaccine. One barrier to this strategy is to achieve a high coverage rate.Citation49
Cocooning refers to the vaccination of mothers and other contacts of newborns and infants. Cost-effective cocooning is difficult to implement since a successful program implies very high numbers of contacts to be vaccinated in order to reach a significant impact on severe infant pertussis.Citation50
Maternal immunization during the third trimester of pregnancy is to be considered the best strategy for infant protection from pertussis, especially in the first 3 months of life.Citation51–53 Amirthalingam et al. demonstrated that pertussis vaccination in pregnancy at least 7 days before delivery could prevent up to 91% of pertussis disease in infants age <3 months. The safety of the maternal pertussis immunization program has also been supported by the findings of a large observational study in the United Kingdom, which did not identify an increased risk for a range of maternal, fetal, and neonatal outcomes.Citation54 In 2011, ACIP recommended the combined Tdap vaccination in the third trimester of pregnancy for women who had never received a Tdap vaccine prior to pregnancy.Citation55 This recommendation was modified in 2012 to include all women, regardless of prior receipt of Tdap and with every pregnancy, following evidence of waning vaccine immunity after vaccination in the first trimester of pregnancy or in a previous pregnancy.Citation56 Since that, antenatal vaccination has been recommended in a variety of countries worldwide.Citation57–59 In Italy maternal vaccination against pertussis was introduced in our Immunization Plan in 2017 but to date, the coverage is still suboptimal. Even if we do not have any official data, two Italian studiesCitation60,Citation61 demonstrated a very low rate of pertussis vaccination (<1.4%) in pregnancy and a poor attitude toward pertussis immunization among pregnant women. In our study, no mother had received dTap vaccine during the current pregnancy. Furthmore, we cannot provide any data to support the role of maternal immunization in reducing pertussis in infants. During the last two years, more information campaign has been implemented in Italy in order to increase the level of knowledge and the uptake in pregnant women and we hope that the situation will improve in the coming years. It was hypothesized that if the rate of immunization of pregnant women will be close to 100%, pertussis deaths in the first months of life will be relegated to the past.Citation31
A number of studies have assessed the cost effectiveness of antenatal vaccination in different countries: it depends highly on disease incidence. A recent US study found antenatal vaccination to be cost-effective, in contrast to vaccinating a second parent or vaccinating either parent post partum.Citation62 Economic analysis in New Zealand also found that the addition of pertussis vaccination to the New Zealand national antenatal immunization program was a cost-effective or even a cost-saving decision.Citation63 Also, the World Health Organization considers vaccination of pregnant women as more cost-effective than “cocooning strategy”Citation64
This study has some limitations. Firstly, the retrospective data collection from medical records could contain inaccuracies regarding clinical information, but objective parameters were analyzed in order to reduce the possibility of bias. Secondly, our study is limited by its small sample size, and our results should be interpreted appropriately. As this is a single-center study, generalizability of our data may be limited. It was conducted in a restricted hospital setting that would not be representative of the whole country and also potentially biased by the selection criteria requiring hospitalization.
Conclusions
Pertussis is a remerging disease; in young infants, it is associated with significant morbidity and mortality. Early diagnosis and treatment are important in order to start immediately specific antibiotic treatment, and leukocyte monitoring during hospital admission. This study highlights the burden of B. pertussis in a tertiary level hospital, confirming a high incidence of complications, especially in the age group that is too young to receive the vaccination.
In recent years, many countries have implemented different vaccination strategies and public health measures to prevent the increase in pertussis cases.
Maternal Tdap vaccination has shown to be highly protective, safety, and effective to reduce the morbidity and mortality in neonates and young infants before they receive their primary immunization against pertussis.
List of abbreviations
Authors’ contributions
CDC and ACV designed the study and participated in the writing process and data review, LA performed the statistical analysis, CCiarlitto collected the clinical information of patients, GL and CConcato processed the laboratory data, LL and AV analyzed and interpreted the patient data regarding the infectious disease. All authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
Alberto Villani participated at Advisory Board for Abbvie, GlaxoSmithKline, Sanofi Pasteur MSD, and Pfizer. The other authors have no conflicts of interest to declare.
Additional information
Funding
References
- WHO. Pertussis. [accessed 2019 July 25]. https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/pertussis/en/.
- ECDC. Pertussis. http://ecdc.europa.ue/sites/portal/files/documents/AER_for_2017-pertussis.pdf
- Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367(11):1012‐1019. doi:10.1056/NEJMoa1200850.
- Vittucci AC, Spuri Vennarucci V, Grandin A, Russo C, Lancella L, Tozzi AE, Bartuli A, Villani A. Pertussis in infants: an underestimated disease. BMC Infect Dis. 2016 Aug 15;16(1):414. doi:10.1186/s12879-016-1710-0.
- Saadatian-Elahi M, Plotkin S, Mills KHG, Halperin SA, McIntyre PB, Picot V, Louis J, Johnson DR. Pertussis: biology, epidemiology and prevention. Vaccine. 2016;34(48):5819‐5826. doi:10.1016/j.vaccine.2016.10.029.
- Signorelli C, Odone A, Cella P, Iannazzo S, D’Ancona F, Guerra R. Infant immunization coverage in Italy (2000-2016). Ann Ist Super Sanità. 2017;53(3):231–37. doi:10.4415/ANN_17_03_09.
- Bozzola E, Spina G, Russo R, Bozzola M, Corsello G, Villani A. A Mandatory vaccinations in European countries, undocumented information, false news and the impact on vaccination uptake: the position of the Italian pediatric society. Ital J Pediatr. 2018 Jun 14;44(1):67. doi:10.1186/s13052-018-0504-y.
- Esposito S, Stefanelli P, Fry NK, Fedele G, He Q, Paterson P, Tan T, Knuf M, Rodrigo C, Weil Olivier C, et al. World Association of Infectious Diseases and Immunological Disorders (WAidid) and the Vaccine Study Group of the European Society of Clinical Microbiology and Infectious Diseases (EVASG). Pertussis Prevention: reasons for Resurgence, and Differences in the Current Acellular Pertussis Vaccines. Front Immunol. 2019 Jul 3;10:1344.
- WHO. 2018. Official country reported coverage estimates time series. Immunization, vaccines and biologicals. Data, statistics and graphics. http://www.who.int/immunization/monitoring_surveillance/data/en/.
- Epicentro. 2018. Le vaccinazioni in Italia. www.epicentro.iss.it/vaccini/dati_Ita
- http://: www.gazzettaufficale.it/eli/id/2017/06/07/17G00095/sg
- Preston A. The role of B. pertussis vaccine antigen gene variants in pertussis resurgence and possible consequences for vaccine development. Hum Vaccin Immunother. 2016 May 3;12(5):1274–76. doi:10.1080/21645515.2015.1137402.
- Di Mattia G, Nicolai A, Frassanito A, Petrarca L, Nenna R, Midulla F. Pertussis: new preventive strategies for an old disease. Paediatr Respir Rev. 2019 Feb;29:68–73. doi:10.1016/j.prrv.2018.03.011.
- Cherry JD, Tan T, Wirsing von Konig C-H, Forsyth KD, Thisyakorn U, Greenberg D, Johnson D, Marchant C, Plotkin S. Clinical definitions of pertussis: summary of a global pertussis initiative roundtable meeting, February 2011. Clin Infect Dis. 2012;54(12):1756–64. doi:10.1093/cid/cis302.
- Van den Brink G, Wishaupt JO, Douma JC, Hartwig NG, Versteegh FG. Bordetella pertussis: an underreported pathogen in pediatric respiratory infections, a prospective cohort study. BMC Infect Dis. 2014 Sep 30;14(1):526. doi:10.1186/1471-2334-14-526.
- Ferronato AE, Leite D, Vieira SE. The role of respiratory virus infection in suspected pertussis: A prospective study. J Microbiol Immunol Infect. 2019 Jul 23. [Epub ahead of print]. doi:10.1016/j.jmii.2019.06.009.
- Stefanelli P, Buttinelli G, Vacca P, Tozzi AE, Midulla F, Carsetti R, Fedele G, Villani A, Concato C. Severe pertussis infection in infants less than 6 months of age: clinical manifestations and molecular characterization. Hum Vaccines Immunotherapeutics. 2017;13(5):1073–77. doi:10.1080/21645515.2016.1276139.
- Nuolivirta K, Koponen P, He Q, Halkosalo A, Korppi M, Vesikari T, Helminen M. Bordetella pertussis infection is common in nonvaccinated infants admitted for bronchiolitis. Pediatr Infect Dis J. 2010;29:1013–15.
- Frassanito A, Nenna R, Nicolai A, Pierangeli A, Tozzi AE, Stefanelli P, Carsetti R, Concato C, Schiavoni I, Midulla F, et al. Infants hospitalized for Bordetella pertussis infection commonly have respiratory viral coinfections. BMC Infectious Diseases. 2017;17(1):492. doi:10.1186/s12879-017-2567-6.
- Hochwald O, Bamberger E, Srugo I. The return of pertussis: who is responsible? What can be done? Isr Med Assoc J. 2006;8:301–07.
- Gregory DS. Pertussis: a disease affecting all ages. Am Fam Physician. 2006;74:420–26.
- Crespo I, Broner S, Soldevila N, Martínez A, Godoy P, Sala-Farré M-R, Company M, Rius C, Domínguez A, Group of Catalonia TPW, et al. Characteristics of pertussis outbreaks in Catalonia, Spain, 1997 to 2010. Hum Vaccin Immunother. 2015;11(1):231–35. doi:10.4161/hv.36156.
- European Centre for Disease Prevention and Control. Pertussis. Annual epidemiological report 2016. Stockholm (Sweden): ECDC; 2019. p. 1–2.
- Tan T, Dalby T, Forsyth K, Halperin SA, Heininger U, Hozbor D, Plotkin S, Ulloa-Gutierrez R, Wirsing von König CH. Pertussis across the globe: recent epidemiologic trends from 2000 to 2013. Pediatr Infect Dis J 2015;34(9):222–32. doi:10.1097/INF.0000000000000795.
- World Health Organization (WHO). Global and regional immunization Profile: European region.www.who.int/immunization/monitoring_surveillance/data/EUR/en/
- Fiasca F, Gabutti G. Trends in hospital admissions for pertussis infection: a nationwide retrospective observational study in Italy, 2002–2016. Int J Environ Res Public Health. 2019 Nov 15;16(22):4531. doi:10.3390/ijerph16224531.
- Gonfiantini MV, Carloni E, Gesualdo F, Pandolfi E, Agricola E, Rizzuto E, Iannazzo S, Ciofi Degli Atti ML, Villani A, Tozzi AE, et al. Epidemiology of pertussis in Italy: disease trends over the last century. Euro Surveill. 2014;19(40):20921. doi:10.2807/1560-7917.ES2014.19.40.20921.
- Heininger U, Klich K, Stehr K, Cherry JD. Clinical findings in Bordetella pertussis infections: results of a prospective multicenter surveillance study. Pediatrics. 1997 Dec;100(6):E10. doi:10.1542/peds.100.6.e10.
- Hodge G, Hodge S, MARKUS C, LAWRENCE A, HAN P. A marked decrease in L-selectin expression by leucocytes in infants with Bordetella pertussis infection: leucocytosis explained? Respirology. 2003 Jun;8(2):157–62. doi:10.1046/j.1440-1843.2003.00459.x.
- Murray EL, Nieves D, Bradley JS, Gargas J, Mason WH, Lehman D, Harriman K, Cherry JD. Characteristics of Severe Bordetella pertussis Infection Among Infants ≤90 Days of Age Admitted to Pediatric Intensive Care Units - Southern California, September 2009-June 2011. J Pediatric Infect Dis Soc. 2013 Mar;2(1):1–6. doi:10.1093/jpids/pis105.
- Cherry JD. The prevention of severe pertussis and pertussis death in young infants. Expert Review of Vaccines. 2019;18(3):205–08. doi:10.1080/14760584.2019.1581065.
- Paddock CD, Sanden GN, Cherry J, Gal A, Langston C, Tatti K, Wu K-H, Goldsmith C, Greer P, Montague J, et al. Pathology and pathogenesis of fatal bordetella pertussis infection in infants. Clin Infect Dis. 2008 Aug 1;47(3):328–38. doi:10.1086/589753.
- Dierig A, Beckmann C, Heininger U. Antibiotic treatment of pertussis: are 7 days really sufficient? Pediatr Infect Dis J. 2015;34(4):444–45. doi:10.1097/INF.0000000000000567.
- Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev. 2007;(3):CD004404..
- Prevention. Pertussis: treatment. Atlanta (GA): CDC; 2013. http://www.cdc.gov/pertussis/clinical/treatment.html.
- Tiwari T, Murphy TV, Moran J, National Immunization Program CDC. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54(RR-14):1–16.
- Lozada LE, Royall MJ, Nylund CM, Eberly MD. Development of pyloric stenosis after a 4-day course of oral erythromycin. Pediatr Emerg Care. 2013;29(4):498–99. doi:10.1097/PEC.0b013e31828a3663.
- Honein MA, Paulozzi LJ, Himelright IM, Lee B, Cragan JD, Patterson L, Correa A, Hall S, Erickson JD. Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromycin: a case review and cohort study. Lancet. 1999;354(9196):2101–05. doi:10.1016/S0140-6736(99)10073-4.
- Lebel MH, Mehra S. Efficacy and safety of clarithromycin versus erythromycin B for the treatment of pertussis: a prospective, randomized, single blind trial. Pediatr Infect Dis J. 2001;20:1149–54. doi:10.1097/00006454-200112000-00011.
- Cherry JD, Wendorf K, Bregman B, Lehman D, Nieves D, Bradley JS, Mason WH, Sande-Lopez L, Lopez M, Federman M, et al. An observational study of severe pertussis in 100 infants ≤120 days of age. Pediatr Infect Dis J. 2018;37(3):202–05. doi:10.1097/INF.0000000000001710.
- WHO Publication. Pertussis vaccines: WHO position paper-recommendations. Vaccine. 2011;29:23556.
- Amirthalingam G. Strategies to control pertussis in infants. Arch Dis Child. 2013;98(7):552–55. doi:10.1136/archdischild-2012-302968.
- Dennehy PH, Cortese MM, B??gu?? RE, Jaeger JL, Roberts NE, Zhang R, Rhodes P, Gentsch J, Ward R, Bernstein DI, et al. A case-control study to determine risk factors for hospitalization for rotavirus gastroenteritis in U.S. children. Pediatr Infect Dis J. 2006;25(12):1123–31. doi:10.1097/01.inf.0000243777.01375.5b.
- Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012;207(suppl3):S3–S8. doi:10.1016/j.ajog.2012.06.068.
- Kroger AT, Duchin J, Vazquez M. Special situations. general best practice guidelines for immunization: best practices guidance of the Advisory Committee on Immunization Practices (ACIP). [accessed 2019 Jan 10]. www.cdc.gov/vaccines/hcp/acip-recs/general-recs/special-situations.html.
- Carbone T, McEntire B, Kissin D, Kelly D, Steinschneider A, Violaris K, Karamchandani N. Absence of an increase in cardiorespiratory events after diphtheria-tetanus-acellular pertussis immunization in preterm infants: a randomized, multicenter study. Pediatrics. 2008;121(5):e1085–e1090. doi:10.1542/peds.2007-2059.
- Bonhoeffer J, Siegrist C-A, Heath PT. Immunisation of premature infants. Arch Dis Child. 2006;91(11):929–35. doi:10.1136/adc.2005.086306.
- Haberling DL, Holman RC, Paddock CD, Murphy TV. Infant and maternal risk factors for pertussis-related infant mortality in the United States, 1999 to 2004. Pediatr Infect Dis J. 2009;28(3):194–98. doi:10.1097/INF.0b013e31818c9032.
- Gabutti G, Azzari C, Bonanni P, Prato R, Tozzi AE, Zanetti A, Zuccotti G. Pertussis. Hum Vaccin Immunother. 2015;11(1):108–17. doi:10.4161/hv.34364.
- Swamy GK, Wheeler SM. Neonatal pertussis, cocooning and maternal immunization. Expert Rev Vaccines. 2014;13(9):1107–14. doi:10.1586/14760584.2014.944509.
- Winter K, Zipprich J, Harriman K. Pertussis in California: A tale of 2 epidemics. Pediatr Infect Dis J. 2018 Apr;37(4):324–28. doi:10.1097/INF.0000000000001761.
- Dabrera G, Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Fry NK, Ramsay M. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012-2013. Clin Infect Dis. 2015;60(3):333–37. doi:10.1093/cid/ciu821.
- Cherry JD. Editorial commentary: tetanus-diphtheria-pertussis immunization in pregnant women and the prevention of pertussis in young infants. Clin Infect Dis. 2015;60(3):338–40. doi:10.1093/cid/ciu823.
- Amirthalingam G, Campbell H, Ribeiro S, Fry NK, Ramsay M, Miller E, Andrews N. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin Infect Dis. 2016 Dec 1;63(suppl 4):S236–S243. doi:10.1093/cid/ciw559.
- CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months. Advisory Committee on Immunization Practices (ACIP), 2011. MMWR. 2011;60:1426.
- CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women – advisory Committee on Immunization Practices (ACIP), 2013. MMWR. 2012;62:131–35.
- Vizzotti C, Neyro S, Katz N, Juárez MV, Perez Carrega ME, Aquino A, Kaski Fullone F. Maternal immunization in Argentina: a storyline from the prospective of a middle income country. Vaccine. 2015;33(47):6413–19. doi:10.1016/j.vaccine.2015.07.109.
- Maertens K, Caboré RN, Huygen K, Hens N, Van Damme P, Leuridan E. Pertussis vaccination during pregnancy in Belgium: results of a prospective controlled cohort study. Vaccine. 2016;34(1):142–50. doi:10.1016/j.vaccine.2015.10.100.
- Immunize Australia Program. 2015. Pregnant women. www1.health.gov.au/internet/main/publishing.nsf/Content/cda-cdi3903c.htm.
- Agricola E, Gesualdo F, Alimenti L, Pandolfi E, Carloni E, D’Ambrosio A, Russo L, Campagna I, Ferretti B, Tozzi AE, et al. Knowledge attitude and practice toward pertussis vaccination during pregnancy among pregnant and postpartum Italian women. Hum Vaccin Immunother. 2016 Aug 2;12(8):1982–88. doi:10.1080/21645515.2016.1188242.
- D’Alessandro A, Napolitano F, D’Ambrosio A, Angelillo IF. Vaccination knowledge and acceptability among pregnant women in Italy. Hum Vaccin Immunother. 2018 Jul 3;14(7):1573–79. doi:10.1080/21645515.2018.1483809.
- Atkins KE, Fitzpatrick MC, Galvani AP, Townsend JP. Cost-effectiveness of pertussis vaccination during pregnancy in the United States. Am J Epidemiol. 2016;183(12):1159–70. doi:10.1093/aje/kwv347.
- Poirrier JE, Mungall B, Lee IH, Terlinden A, Curran D. Cost-effectiveness of maternal immunisation for pertussis in New Zealand. Value Health. 2014;17(7):A806. doi:10.1016/j.jval.2014.08.520.
- World Health Organization. Summary of the Pertussis vaccines: WHO position paper-September 2015. www.who.int/immunization/documents/pertussis_pp_2015_summary.pdf
