?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective
To evaluate the rate of adverse reaction toward rabies vaccine in China from 2008 to 2019, explore its characteristics and to provide a scientific and objective basis for future policy decisions.
Methods
Literature on the rate of adverse reaction to rabies vaccine in China from 2008 to 2019 was retrieved and collected in CNKI, Wanfang, VIP databases, PubMed and Embase. A meta analysis was carried out then.
Results
Totally, 35 articles were included. The combined rate of adverse reaction to rabies vaccine was 5.6% (95% CI = 5.1% – 6.0%). Adverse reactions to rabies vaccine were 11.3% and 4.5% before and after 2011, 5.3% and 7.1% in the eastern and midwestern regions, 13.2%, 25.5% and 3.7% in the tertiary hospitals, secondary hospitals and primary medical institutions, respectively. And 13.3% were in the areas where the Changchun immortal vaccine was used. The combined rate of adverse reaction to rabies vaccine inoculated in Changsheng vaccine area was 5.1%.
Conclusion
After 2011, the rate of adverse reaction to rabies vaccine in China has decreased dramatically. The rate of adverse reaction to rabies vaccine in the midwestern regions is higher than the counterpart in the eastern regions. The primary medical institutions are lower than the counterpart in the tertiary and secondary hospitals.
Background
With the development of the economy, the number of owners keeping dogs is increasing gradually in China, which leads to the growth of the rate of rabies vaccine, and its adverse reaction becomes people’s concerns, especially the vaccine accident. On July 11, 2018, Changchun Changsheng Biotech Co. Ltd. (now referred to as “Changchun Changsheng”) reported that the company had fabricated production records in the production of human lyophilized rabies vaccine. Although clarifications made later indicating there was no issue with the quality of the vaccine, it aroused widespread public concern with respect to their vaccine quality and safety standards and discussion once again due to irregular production record.
Rabies is an acute zoonotic infectious disease caused by rabies virus infection. According to the World Health Organization (WHO), rabies causes nearly 59,000 human deaths annually in over 150 countries, with 95% of cases reported mainly from African and Asian regions.Citation1 In China, there are several reports on rabies cases. Rabies vaccines play a crucial role in preventing rabies effectively. Due to the different physical qualities of the vaccinators, patients may have varying degrees of adverse reactions during the vaccination process. The rate of adverse reactions is an essential indicator of vaccine quality. In China, adverse reaction means that harmful reactions occurred after standard vaccination procedures by qualified vaccines, which are unexpected or not related to the purpose of preventive inoculation.
Vaccines fall into two categories in China. The first type of vaccine refers to the vaccine provided free of charge by the Chinese government to their citizens and other Nationals living in China, who are vaccinated in accordance with the provisions of the government. It also includes the vaccine determined by the National Immunization Program, the vaccine added by provincial government, autonomous regions and municipalities directly under the Central Government in the implementation of the National Immunization program, and the vaccines used in emergency vaccinations or mass vaccinations organized by the Chinese government at or above the county level or their health authorities. The second type of vaccine is other vaccines which are administered voluntarily at the expense of citizens. Vaccination cost in the first type of vaccine is borne by the government. But, vaccination cost in the second type of vaccine is at the expense of the recipient or his or her guardian.
The definition of adverse reactions is premised on the presence of the vaccine in the event of a qualified vaccine quality and a specification of the vaccination process. If the recipient dies, is severely disabled or has tissue damage due to an adverse reaction in vaccination, a one-time compensation is given. If the adverse reaction caused by the first type of vaccine by vaccination, the recipient requires compensation. The compensation cost is arranged by the financial department of the people’s government in the provincial region or autonomous region or municipality, directly under the central government funds for vaccination. If the adverse reaction caused from second type of vaccine, the compensation cost is borne by the relevant vaccine manufacturer. The Chinese government encourages the establishment of mechanisms for providing compensation to the recipients having adverse reactions due to vaccinations, such as commercial insurance. Specific compensation measures for adverse reactions to vaccination can be formulated by provincial governments, autonomous regions, and municipalities directly guided the central government in China.Citation2
At present, research studies on adverse reactions to rabies vaccine in China focus primarily on the observation and care of adverse reactions from the perspective of epidemiology, for example, the symptoms of adverse reactions, the rate of adverse reactions from different types of vaccines, and the effect of the intervention, where the case samples from a single institution or hospital. Very few studies are reported on the adverse reaction rate to rabies vaccine from the perspective of management in China. Only one study conducted a meta-analysis from the 19 Chinese and English literature in total and found that the combined incidence of ADRs (adverse drug reactions) inoculated with HDCV (human diploid cell rabies vaccine) abroad was 13.44 ~ 29.19%, and the combined incidence of ADRs inoculated with domestic (China) HDCV was 0.23% ~6.98%.Citation3 However, the rate of adverse reactions to rabies vaccine in different regions and institutions in China was not analyzed. Is the rate of adverse reaction to rabies vaccine in China really as high as the public thinks? What is the difference in the rate of adverse reactions to rabies vaccine in different institutions and regions in China? In an attempt to answer these questions, we conducted a systematic literature search and a comprehensive narrative review of the literature identified. Our aim is to provide a scientific and objective basis for future policy decisions by estimating the total adverse reaction rate toward rabies vaccination in China by meta-analysis, analyzing the rate in different times, institutions, regions, especially where the rabies vaccine produced from Changchun Changsheng used.
Data and methodology
Literature search
The search keywords “rabies vaccine”, “adverse reactions”, “side effects”, “abnormal reactions” and “abnormal reactions” were retrieved from China Journal Full-text Database (CNKI), Wanfang Database (Wanfang data), VIP Database 7.0 (VIP) Search, PubMed and Embase. Taking Wanfang Database as an example, the search formula is the theme: (rabies vaccine) * (title or keyword: (adverse reaction) + title or keyword: (abnormal reaction) + title or keyword: (allergic) + title or keywords: (side effects)). Relevant literature on the rate of adverse reaction toward vaccination against rabies was collected, and the final search date was October 10, 2019.
Criteria
Inclusion
1. The type of literature is observational research;
2. The literature that gives the rate of adverse reactions to rabies vaccine or provides the data that can calculate the adverse reaction rate indirectly;
3. There is a clear mentioning of sample numbers in the text and also the samples quoted meet the standard of rabies vaccine.
Exclusion
1. The research object aims at a special group (e.g. children or the elderly);
2. Raw data that cannot be extracted or converted;
3. The literature involving man-made measures such as special nursing intervention during the vaccination process;
4. The literature that was unrelated to this study;
5. The literature appears repeatedly, a random extract was done if the data were consistent. Otherwise, the article was rejected.
Document screening and data extraction
According to the inclusion criteria, the preliminary screening steps were reading article topics, abstracts, keywords and other aspects on the literature collected in the search database. After the initial screening, the full text in the literature was read carefully, and it was followed by the secondary screening based on the exclusion criteria. The whole selection process was carried out independently by two researchers respectively. When there arose a dispute with literature, the third researcher was consulted, and then an agreement was reached in the end. The data extraction process was also conducted independently by two researchers, data such as first author, study time, number of samples, and rate of adverse reactions were entered from the final inclusion of literature.
Assessment
Although the study vaccine and potency in the selected literature are not always the same, the literature are still comparable because they were published from China. The assessments of adverse reactions in each literature were in compliance with one recognized scale, i.e. according to the national suspected vaccination requirements of the regular response monitoring program.Citation4 At present, rabies vaccination in China is post-exposure prophylaxis using the five-dose method. To control the discrepancies among literature, this paper provides a quality evaluation.
This study selected the cross-sectional study quality evaluation standardCitation5 recommended by the American Agency for Health Research and Quality (AHRQ) to evaluate the quality of the selected literature. The standard has 11 items, which are evaluated from the research object, document quality, and data processing. The total score ranges from 0 to 22, with “yes”, “no” and “do not know” as answer, corresponding to 2 points, 0 points, and 1 point, respectively. The overall quality of the relevant literature is judged by the size of its score. The higher the score is, the better is the quality of the literature. Evaluation of the literature quality was independently performed by two evaluators, and inconsistencies were determined by third-party professionals after discussion.
Data processing and statistical analysis
In this study, NoteExpress is used for document information management, Excel for data collation and Stata 15.0 for statistical analysis. If p > .01 and I2 < 50% are obtained from the results of the heterogeneity test analysis, it can be considered that there exists homogeneity among these studies, that is, the fixed-effect model was used for further analysis; contrarily, if it was considered that there exists heterogeneity among these studies, that is, the random effect model was used for meta-analysis, then subgroup analysis, and sensitivity analysis of factors that may lead to heterogeneity were carried out.
Results
Basic characteristics of literature
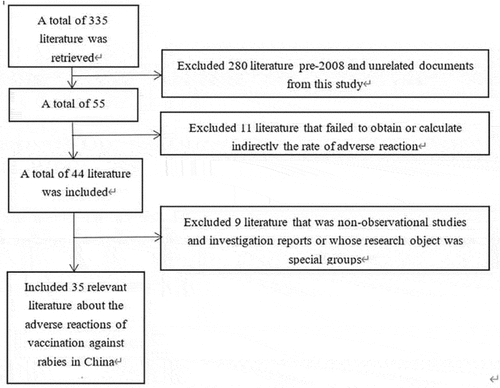
A total of 335 published papers were retrieved, 35 of which were included. The total number of samples was 117,686. The sample size varied widely in different studies, ranging from 80 to 46,212, with a median of 800. The document screening process is shown in .
According to AHRQ, the quality of the 35 literature included was evaluated. The highest quality evaluation score was 18 points, the lowest was 6 points and the average was 10.8 points. Among them, four of which scored 6 points, three of which scored 7 points, four of which scored 8 points, one of which scored 9 points, four of which scored 10 points, 3 of which scored 11 points, 6 of which scored 12 points, 1 of which scored 13 points, 5 of which scored 14 points, 1 of which scored 15 points, 2 of which scored 16 points and 1 of which scored 18 points.
The main scoreless entries came from the articles in the AHRQ cross-sectional quality evaluation criteria:
Article 2: The inclusion and exclusion criteria for the exposed group or non-exposed group was not listed;
Article 4: The source of the data was about the population, but the research object was not continuous, so the sample observation date was not continuous;
Article 7: The reasons for excluding any patients for analysis were not declared in it;
Article 9: How to address the missing data was not explained.
The general characteristics of the 35 documents included eventually are shown in and . The number of people surveyed was 171,686, of which 2887 adverse reactions occurred.
Table 1. Incorporating Basic Information Sheets in the Literature
Combined effect value and forest map
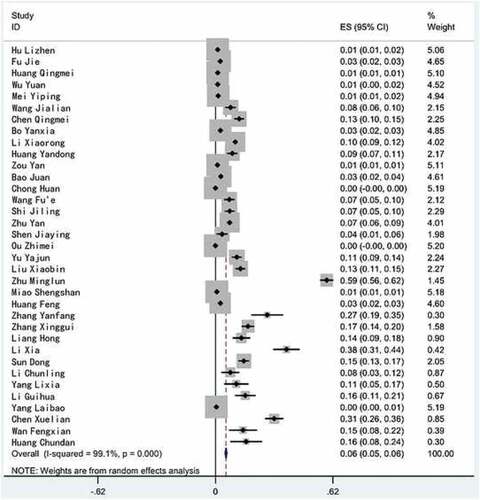
A heterogeneity test was performed on the 35 included literature, and the results showed that there was a large heterogeneity between the studies (2 = 3781.39, P < .01, I2 = 99.1%), so meta analysis using random effect model was carried out. The combined effect value showed that the rate of adverse reaction to rabies vaccine in China was 5.6% (95% CI = 5.1%~6.0%). The combined rate of adverse reaction to rabies vaccine and its confidence interval of 95% in each literature are shown in .
Subgroup analysis
According to three sets of indicators collected in the literature, including the time of vaccination against rabies (around 2011), the study area (East, Midwest China[a]) and the institutional category (the tertiary hospital, the secondary hospital and the primary medical institutions)[b], a subgroup analysis was carried out.
From the perspective of the time of vaccination against rabies, the results of subgroup analysis () showed that the combined effect value of the rate of adverse reaction to rabies vaccine in China was 10.5% (95% CI = 7.8%~13.2%) in 2011 and before, higher than the counterpart of 4.7% (95% CI = 4.3% ~5.2%) after 2011.
Table 2. Subgroup Analysis of the Rate of Adverse Reaction to Rabies Vaccine in China
From the perspective of the study area, the results of subgroup analysis showed that combined effect value of the rate of adverse reaction to rabies vaccine in the eastern region was 5.30% (95% CI = 4.4%~6.1%) in China, lower than the counterpart of 7.10% (95%CI = 6.4%~7.8%) in the Midwestern regions.
From the perspective of institutional category, the results of subgroup analysis showed that the combined effect value of the rate of adverse reaction to rabies vaccine in primary medical institutions was 3.7% (95% CI = 3.3%~4.1%) in China, lower than the counterpart of 25.50% (95%CI = 0.4%~50.6%) in secondary hospitals and 13.2% (95%CI = 10.9%~15.5%) in tertiary hospitals.Footnotea
Sensitivity analysis and publication of bias analysis
We excluded the literature with a minimum score of 6 points, calculated the combined effect value, and concluded that the combined rate of adverse reaction to rabies vaccine was 6.6% (95% CI = 6.0% to 7.1%), which was 1% higher than the previous removal.Footnoteb It can be concluded that the result of the initial combined rate was less affected. All the 35 literature were tested by the Egger method. In the Bias line of the Egger test table, it displayed that P>|t| and P < .01, suggesting that there was a certain publication bias in the results ().
Table 3. Egger Test Table in the Literature on the Rate of Adverse Reactions to Rabies Vaccine
Discussion
Why did people think the rate was very high?
According to the combined effect value in meta analysis, the total rate of adverse reaction to rabies vaccine in China was 5.6%. This result was basically consistent with the results from an earlier reported study (Li Fan,Citation3), in which the combined incidence of adverse reactions to HDCV vaccination in China was 0.23% to 6.98% from 1970 to 2018, and lower than the conclusion from other study in China (Zhang Xiaorui,Citation41), in which the rate of adverse reactions after rabies vaccine injection was 9.82% from 2000 to 2016.
A meta-analysis on the publications from United States, Thailand, Georgia, and France showed that the combined rate of adverse reactions to rabies vaccine was 13.44% to 29.19%,Citation3 which was much higher than in China. Taking the data of the U.S. National Center for Disease Control and Prevention as a reference, 60–89.5% recipients of HDCV reported to have local reactions (e.g., pain at the injection site, redness, swelling, induration). Most of the local reactions were mild and resolved spontaneously within a few days. Local pain at the injection site was the most frequently reported adverse reaction reported in 21%–77% of vaccinated patients. Mild systemic reactions (e.g., fever, headache, dizziness, gastrointestinal symptoms) were reported in 6.8–55.6% of recipients.Citation42 Compared with these data, we found that the rate of adverse reaction to rabies vaccine in China is relatively low, at least, not as high as the general public opinions or views after the vaccine development accident. But what makes the public to think that the rate of adverse reactions tends to be very high and even get panic over it? The following reasons may explain it.
Vaccine adverse event reporting system (VAERS)
In information era, medical detection system gets optimized gradually, contributing to increase in the reporting rate of adverse reactions. All clinically significant adverse events occurred following administration of rabies biologics should be reported to the Vaccine Adverse Event Reporting System (VAERS), even if causal relation to vaccination is not certain. Although VAERS is subjected to its limitations common to passive surveillance systems, including under-reporting and reporting bias, yet it is a valuable tool for characterizing the safety profile of vaccines and specifically to identify risk factors for rare serious adverse reactions to vaccines. To some degree, adverse reactions appeared more frequently, which reflects that the reporting policy was effectively implemented. Meanwhile, we should be aware that the increase in the reporting rate may lead to the enlargement of the incidence rate. Regulatory policy, socio-economic development status, public awareness and education, administrative and incentive mechanism may have impact on reporting of adverse reaction due to rabies vaccine inoculation. So, taking the real data seriously is essential to consider whether the occurrence of adverse reaction appears severely as it was reported.
In 2005, China issued the Regulations on the Circulation of Vaccines and Vaccinations, which were revised in 2016.Citation2 Among them, the compensation for abnormal reactions in vaccination has been supplemented, and compensation has been increased, which may lead to an increase in the rate of reporting adverse reactions due to the acquisition of economic compensation. In May 2019, the Notice on Doing a Good Job in Basic Public Health Services in 2019Citation43 noted that the administrative departments of primary health care at all levels should cooperate with relevant departments to strengthen the information construction of vaccination and promote the production, circulation and use of vaccines for the entire traceability management. With the interconnection and popularization of the information platform, the grass-roots data can be reported in real time and processed at the higher level. Although to a certain extent, it may increase the reporting rate of adverse reactions, but the authenticity of the data is worthy of recognition.
From the management point of view, the report of vaccine adverse reactions can be included in the assessment, thereby increasing the initiative and accuracy of medical institutions.
The supervision of adverse reactions should take the establishment of the technical system of monitoring adverse reactions of vaccine as an important part of the work, which can be included in the scope of law, perfect the relevant laws and regulations, and clarify the work responsibilities and working procedures of the vaccine adverse reaction monitoring institutions. In particular, strengthening the construction of municipal and county-level monitoring institutions for adverse reactions to vaccines is essential. It is an important measure to ensure people’s vaccine safety by establishing a unified and efficient monitoring system for adverse reactions to vaccines, which will improve the level of vaccine safety supervision.
Focus on improving the use of data analysis under the vaccine adverse reaction reporting system will help to reduce the rate of adverse reactions, thereby reducing the damage caused to people’s lives by adverse reactions. Relevant departments should take measures to encourage researchers to participate in the research of vaccine adverse reactions projects, share achievements with the administrative and other technical system personnel at the spirit of “people’s health” which is the center of the scientific research. Scientists can enhance the practical research on the occurrence of adverse reactions from social statistical factors, which may lead to targeted publicity and education activities to different specific groups, thus making them known about precautions when getting vaccinated.
Poor awareness and health education
Inadequate awareness of adverse reactions may be one of the reasons for the public panic about rabies vaccine adverse reactions. To better understand the public awareness of rabies vaccine in China, we conducted a questionnaire on the perception of the adverse reactions to rabies vaccine, which was answered by 93 people. We collected 80 valid questionnaires, of which 71.25% from women and 28.75% from men, 93.75% from who had a bachelor's degree or above, 35% from who had been vaccinated against rabies, while 57.5% were not vaccinated against rabies.
It showed that the public was relatively weak in aware of the adverse reactions to rabies vaccine. The population that filled in the questionnaire was well educated, among them 93.75% have a bachelor’s degree or above, but only 10% of them have been informed of adverse reactions, and 78.75% have never received any education about the adverse reactions to rabies vaccine. Most of them had some misunderstandings about the adverse reactions to rabies vaccine. For example, with the severity and scope of adverse reactions expanding, they all thought that these symptoms were not acceptable and that these adverse reactions were caused by substandard vaccine quality.
The awareness of adverse reactions to rabies vaccines differed between those who had received education and those who had not received education. People who have received education on adverse reactions to rabies vaccine showed a relatively high degree of acceptance of the adverse reactions, while those who have never received any knowledge about it had a significantly lower acceptance of adverse reactions, especially those systemic adverse reactions. They are most likely to panic once systemic adverse reactions occur. Details are as following .
Table 4. Acceptability Rate of Adverse Reactions
Why did the rate decrease after 2011?
China has achieved some goals in vaccine in recent years. The vaccination rate of national immunization programs in towns has reached and maintained at more than 90%. The vaccine reserve system was improved, and the production capacity reserve was laid out, the physical reserve of vaccines was added, the quality supervision and evaluation system of vaccination effectiveness were improved. Also, the quality of the vaccine for human and veterinary diseases was significantly improved, and the technical reserve of vaccines for foreign diseases and animals was enhanced. At the end of 2011, the Chinese State Council issued the Notice of the General Office of the State Council on forwarding the plan for the construction of vaccine supply system, which aims to improve the reporting system for vaccination statistics and adverse reactions. It was expected to develop the evaluation of vaccination effectiveness and safety, and the monitor system for adverse reactions to vaccines. The standardization construction of vaccine supply system may strengthen the supervision of vaccination, and help to lower the rate of adverse reactions after 2011.
Why was the rate relatively high in the midwest?
The results of subgroup analysis showed that the rate of adverse reactions to rabies vaccine in the Midwestern regions was relatively high, reaching 7.1%, higher than the counterpart of 5.3% in the eastern region, which may be related to the insufficient supervision of vaccine quality and vaccination effectiveness.
In China, the economic and social development varies from place to place, and there is also great gap in health resources and supervision between different regions. The per capita financial public health expenditure in the high-spending areas of public health is 3.18 times of that in the low-spending areas (mostly located in midwestern regions), 26.3% more hospital beds in eastern regions than in central and western regions, 16.7% more general practitioners per thousand in eastern regions than that in the midwest regions of China.Citation44 The poor health resources may lead to a defective vaccination system. For example, there are some problems in cold chain construction and medical staff recruitment in remote areas in midwestern regions of China, which may explain the high rate of adverse reaction to rabies vaccine.
Why was the rate lower in primary medical institutions?
The results of subgroup analysis showed that the rate of adverse reaction to rabies vaccine in primary medical institutions was 3.7%, which was significantly lower than the counterpart of secondary hospitals (25.5%) and tertiary hospitals (13.2%). In light of the actual situation, the usage rate of rabies vaccine in primary medical institutions is relatively high, owing to most people choose to vaccinate the rabies vaccine in the nearest rabies exposure admission clinic. District Health Bureau is the main regulatory agency of regional public health, especially for vaccination projects. The rate of adverse reactions to rabies vaccine in secondary and tertiary hospitals is significantly higher than that in primary health institutions. This may be due to the difference in approach of the administrative system of the public health institutions.
In Chinese health care system, the health institutions are divided into primary medical institutions, secondary hospitals and tertiary hospitals according to the size and functions. The primary medical institutions are affiliated to district health bureaus, while secondary hospitals affiliated to the municipal health bureau. Most of the tertiary hospitals affiliated to provincial health bureau or a university. However, although most of the hospitals are public institutions offering health services, they have hierarchical characteristics in the administrative bureaucracy. In China, the administrative bureaucracy system is divided into different levels from the top to the bottom, which is the ministry level, the vice ministry level, the department or province level, the vice department or province level, division or county level, divisional level, the vice section or township level. Generally, in a big city on the vice ministry level, the secondary and tertiary public hospitals are at or above the divisional level in the administrative hierarchy, and affiliated to the municipal or provincial health bureau. Although the district health bureaus are the direct supervisors of vaccination, it may be hard for them to regulate the secondary and tertiary public hospitals, which are on the same or above the divisional level in the hierarchy. It is much easier for them to supervise the primary institutions, which are at the lower administrative level. This administrative characteristic of Chinese public hospitals may explain why the incidence of adverse reactions to rabies vaccine in secondary and tertiary hospitals is higher than that in primary medical institutions.
From the management point of view, the factors affecting the rate of adverse reactions include public health resources and regulatory efforts. The economic and social development in midwestern regions of China is lesser compared to the eastern region, also with relatively few per capita public health resources and weaker supervision. So, this may be one of the possible reasons for the relatively high rate of adverse reactions in midwestern regions of China. In China, although the overall resources in the primary medical institutions are lower than those of hospitals, the resources of hospitals are mainly medical resources, and public health resources are not necessarily higher than that of primary medical institutions. In recent years, China has strengthened the construction of primary medical institutions, increased investment in resources; especially public health resources such as vaccination resources have been greatly improved. The public health resources of primary medical institutions are not necessarily lower than hospitals, and some are even higher than some hospitals. At the same time, the district health bureau’s supervision of hospitals at the same level or higher level is lower than that of primary health care institutions. Therefore, in the case of public health resources and supervision is not dominant, it may be explained that the rate of adverse reaction in hospitals is higher than that in the primary medical institutions.
Conclusion
The rate of adverse reaction to rabies vaccination in China was 5.6% (95% CI = 5.1%~6.0%), and it dropped from 10.5% (95% CI = 7.8%~13.2%) before 2011 to 4.7% (95% CI = 4.3% ~5.2%) after 2011. The more sensitive Vaccine Adverse Event Reporting System and poor awareness and health education may cause the public panic on the adverse reaction to rabies vaccination.
Based on our study, government should enlarge publicity to change public awareness. First, the government should increase the intensity of public health education by distributing brochures, online health lectures and training, focusing on awareness such as symptoms and treatment of adverse reactions to rabies vaccine. CDC can be a good channel in public education. Second, China can learn from the experience of developed countries. For example, hospitals or primary institutions proceed to train some social workers in community, relying on whom to offer residents one-on-one education and guidance. It’s also a great way to raise awareness among residents.
In addition, we found that the rate of adverse reaction to rabies vaccine in the midwestern regions was higher than that in the eastern region, which may be due to the unbalanced allocation of health resources and insufficient supervision in china. The incidence of adverse reactions to rabies vaccine in secondary and tertiary hospitals was higher than that in primary medical institutions, which may owe to the hierarchical characteristics of Chinese public hospitals in the administrative bureaucracy.
This systematic review found many factors related to the rate of adverse reaction to rabies vaccine. These factors should be taken into account by policymakers during the improvement of the vaccination system. In the basic framework of China’s current medical service system, government should put increasing supervision in the first place. At present, there are imperfect policies and laws, less punishment, poor implementation efficiency and other problems in adverse reaction supervision. Measures should be taken to improve the safety of vaccines and construct a perfect quality control system. Big data are increasingly being used for regulation, including information transmission and feedback, data queries and sharing. Due to special medical administration system, policies which can authorize lower-level health administration department to regulate hospitals at higher or same level need to be carried out.
Limitation
This analysis was carried out with reference to the meta analysis methodological quality scale AM-STAR, and the inclusion and exclusion of the literature were strictly carried out. At the same time, subgroup analysis and sensitivity analysis were performed based on the heterogeneity results between the literature, and the conclusions obtained were more reliable. The shortcomings of this study are as follows:
1. Vaccine sources, vaccine type and vaccination procedures may influence adverse reactions. More in-depth and comprehensive analysis can be conducted in the future.
2. The number of the primary medical institutions included in the literature is more than the counterpart in the tertiary and the secondary hospitals, which has some influence on the results of the meta analysis.
3. There is no unpublished literature or data, and there still exists some publication bias.
4. This paper includes publications published in China which had been peer reviewed in Chinese journal before publishing and whose results were consistent with other papers. The required literature in PubMed and Embase has not been retrieved. We tried to keep this sampling as accurate and informative as possible.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
Notes
a. In China, the common characteristics of the central and western regions are remote, less developed and lack of resources. The eastern region is well developed and well-resourced. Therefore, this study combines the central and western regions into midwestern regions for subgroup analysis. Subsequent statements are all midwestern regions.
b. According to the type of institution, we divide the medical and health institutions into hospitals, primary health care institutions, professional public health institutions and other medical and health institutions. Among them, hospitals include general hospitals, Chinese medicine hospitals, Chinese and Western medicine combined hospitals, ethnic hospitals, various specialized hospitals and nursing homes, excluding specialist disease prevention and control hospitals, maternal and child health care homes and nursing homes. By the health administration department sitrated, the grade is divided into first, second, third and undetermined, and so on into A, B, C and undetermined, comprehensively reflects the size of the hospital and the level of medical treatment. Primary health care institutions include community health service centers (stations), street health centers, township hospitals, village health clinics, outpatient clinics, clinics (infirmaries). This study analyzed and selected tertiary hospitals, secondary hospitals and primary medical institutions.
References
- WHO. accessed 2020 Feb 12. https://www.who.int/health-topics/rabies#tab=tab_1
- State Council. accessed 2016 Apr 25. http://www.gov.cn/zhengce/content/2016-04/25/content_5067597.htm
- Li F, Lv Q, Zhu XP. A meta-analysis on adverse drug reactions rate of human diploid cell rabies vaccine. Chin J Emergency Resusc Disaster Med. 2019 Jun;14(6):548–51.
- National Medical Products Administration. accessed 2016 Jun 3. http://www.nmpa.gov.cn/WS04/CL2079/333398_2.html.
- Yang JC, Yang ZR, Yu SQ, Zhan SY, Sun F. Introduction on ‘assessing the risk of bias of individual studies’ in systematic review of health-care intervention programs revised by the agency for healthcare research and quality. Chin J Epidemiol. 2019;40(1):106–11. doi:10.3760/cma.j..0254-6450.2019.01.021.
- Hu LZ, Wu LL, Zhang SW. Adverse reactions after receiving rabies vaccination and its nursing care. Occup Health. 2012 Mar;28(6):761–62.
- Fu J, Luo LD, Wang YF. Observation of adverse reactions to rabies vaccine after exposure. Chin Commun Doctors. 2013;12:327.
- Huang QM, Liang YQ, Pang SY. 68 observations and nursing of adverse reactions after rabies vaccination. J Mod Med Health. 2013 May 15;29(9):1399–401.
- Wu Y. Analysis of adverse reactions to rabies vaccine after exposure. Med Inf. 2013 Oct;26(10):497.
- Mei YP. Analysis of adverse reactions after rabies vaccination and corresponding nursing measures. World Health Dig. 2016;6:74.
- Wang JL. Observation and nursing of adverse reactions after rabies vaccination. China Health Care Nutr. 2015;25:301–02.
- Chen QM. Analysis of the causes of adverse reactions after rabies vaccination and nursing strategies. China Continuing Med Educ. 2016;8:250–51.
- Li XR. Observation and nursing of rabies vaccine adverse reactions. China Continuing Med Educ. 2019;9:243–44.
- Huang YD. Adverse reactions and prevention measures of rabies vaccine. All Health. 2016;10:21.
- Chong H. 55 cases of adverse reactions after rabies vaccination and nursing analysis. J Clin Med. 2017;4:8615–9616.
- Wang FE. A variety of factors rabies vaccination adverse reactions analysis and effective measures. China Health Ind. 2015;12:46–48.
- Shi JL. Brief discussion of nursing strategies for adverse reactions for patients vaccinated with rabies vaccine. Med J Chin People’s Health. 2015;27:117–18.
- Shen JY, Cang L. Discussion on various factors and effective methods of adverse reactions after rabies vaccination. Guide China Med. 2018 Jun;16(18):82–83.
- Ou ZM, Zhu KR, Chen C, Jiang D. Analysis on the effect of adverse reactions intervention of 63 patients after receiving rabies vaccination. Mod Preventive Med. 2018;45(6):1130–32.
- Yu YJ, Wang LZ, Li L, Zhang XW. Effect observation and care after 2-2-1 procedure vaccination of rabies vaccine. China Med Herald. 2008;5(32):132.
- Liu XB, Hu P, Wang L. Comparison of five-dose schedule and four dose of 2-1-1 schedule vaccine on rabies. J Occup Health Damage. 2012 Aug;27(4):233–34.
- Zhu ML, Chen JS. Observation of immune effect and adverse reactions of different procedures of rabies vaccine. China Health Care Nutr. 2013;23:4232.
- Miao SS, Li TJ, Xia YP, Sun J, Dong WB. Analysis of abnormal response to suspected vaccination of rabies vaccine in Fenghua City, 2010–2011. Shanghai J Preventive Med. 2013;25(4):178–79.
- Huang F, Xie L, Yi T. Effect and nursing intervention of “2-1-1” rabies vaccine immune program and “5-pin” immunization program. Pract Clin Med. 2014;15:103.
- Zhang YF. Rabies vaccine adverse reactions observed after inoculation. Med Inf. 2014 Sept;27(9):644.
- Zhang XG. Study on the adverse reactions of 4-pin immunization method and 5-needle immunization method. Women’s Health Res. 2015;18:23.
- Liang H. To explore the adverse reactions and immune effects of two different dosage forms of human rabies vaccine. J Clin Med Lit. 2015;2:1242.
- Li X. Analysis of adverse reactions to rabies vaccination. J Clin Med. 2015;2:3469.
- Sun D. Analysis of adverse reactions to human rabies vaccine. Guide China Med. 2015;13:67.
- Li CL, Ma JQ, Zan RN, Lin JW. Evaluation of the effect of nursing intervention on adverse reactions to vaccination of rabies vaccine. Dis Monit Control. 2015 Oct;9(10):755–56.
- Yang LX. Comparison of the efficacy of four-shot injection and five-injection injection method and nursing experience of human rabies vaccine. Nei Menggu J Traditional Chin Med. 2016;35:166–67.
- Li GH. Value analysis of the application of nursing intervention in vaccination rabies vaccine to prevent adverse reactions. World Latest Med Inf. 2017;17:201.
- Yang LB, Xiao ST, Chen HY, Fei Y. Influencing factors on adverse reactions of rabies vaccine in Pudong new area of Shanghai in 2016. Occup Health. 2017 Nov;33(21):2989–91.
- Chen XL, Lv CT. Adverse reactions of 400 cases of inoculation with freeze-dried rabies vaccine for human use and nursing effect observation. MHGN. 2018 Jun;24:1720–23.
- Wang FX. Study on the clinical effect of nursing intervention on adverse reactions to vaccination rabies vaccine. Health Guide. 2018;18:259.
- Huang CD. Effect of nursing intervention to prevent adverse reactions in rabies vaccine. J Front Med. 2018;8:388–89.
- Bao J, Lu XQ. To explore the application of nursing preventive measures in reducing adverse reactions after rabies vaccination. World Latest Med Inf. 2016;16:265.
- Zhu Y. Abnormal reaction and treatment of rabies vaccination. All Health. 2015;9:17–18.
- Bo YX. Analysis of adverse reaction care after vaccination against rabies vaccine. Fashion Baby. 2016;24:509.
- Zou Y. Discussing the adverse reactions of the people vaccinated against rabies. China Health Care Nutr. 2016;26:58.
- Zhang XR, Wu ZS, Zhang WS. Meta analysis of the incidence of adverse reactions to rabies vaccine in China. Chin J Epidemiol. 2017;38:821–27.
- Centers for disease control and prevention, rabies adverse reaction. accessed 2011 Jun 30. https://www.cdc.gov/rabies/specific_groups/doctors/adverse_reaction.html
- State Council. accessed 2019 Nov 15. http://www.gov.cn/zhengce/zhengceku/2019-11/15/content_5452431.htm
- Jiang H, Chen RJ. Study on the regional gap of china’s fiscal public health expenditure: cluster analysis and teal index analysis based on single indicator panel data. Caizheng Jiandu. 2014;21:65–67.