ABSTRACT
A systematic literature review of Medline and Embase databases was conducted to describe rotavirus (RV) vaccine coverage for a complete series, timing of receipt of all doses in the series, and predictors of RV vaccination coverage in the US for two licensed RV vaccines (RV1, RV5). Nine publications were included in the review. RV vaccination coverage rates of under 80% suggest RV vaccines are underutilized relative to the Healthy People 2020 target and other childhood vaccines. About 50–90% of children initiating RV vaccination complete the series and coverage for a complete series is lower for black and Hispanic children (vs. whites), uninsured or Medicaid insured (vs. privately insured), and for foreign-born (vs. US-born) children. Series completion is significantly greater in children receiving DTaP, RV1 (vs. RV5), and for those receiving routine care from a pediatrician. There is a need to design and implement better RV immunization strategies for US children.
Introduction
Rotavirus (RV) is the most common cause of severe pediatric gastroenteritis that generally infects children by the age of 5.Citation1 Since 2006, RV vaccination has been recommended in the United States (US) by the Advisory Committee on Immunization Practices (ACIP) to protect children against RV gastroenteritis.Citation2 Prior to the introduction of RV vaccines in the US, there were annually about 410,000 physician visits, 205,000–272,000 emergency department (ED) visits, and 55,000–70,000 hospitalizations due to RV infections.Citation2 RV vaccination has been very impactful in preventing severe RV gastroenteritis in children, with the number of RV-positive tests performed across the US decreasing 74–90% compared with the pre-vaccine baseline.Citation1 Indirect benefits of vaccination, such as reduction in RV-related hospitalizations among unvaccinated populations, have also been observed.Citation3 Thus, RV vaccination has contributed to important public health benefits based on the reduced clinical and economic burden of RV disease in the US.Citation4
Several elements can influence receipt of vaccination, such as access to health care administration considerations (dosage form/presentation, co-administration with other vaccines, number of doses), payment (covered by insurance or not), acceptability (parent/caregiver attitude), and recommendations (local, state, federal).Citation5 The Centers for Disease Control and Prevention (CDC) monitors vaccination coverage, defined as the estimated percentage of people who have received specific vaccines, to understand how well communities are protected from vaccine-preventable diseases.Citation6 In 2016, RV vaccination coverage for the complete series of RV vaccine in the US was 74.1%,Citation7 which is below the 80% target coverage for RV vaccines in the Healthy People 2020 objectives.Citation8 It is worth noting that national vaccine coverage rates do not provide insight about the timing of receipt of doses measured as compliance rates, i.e., whether the recommended number of doses are received as per the recommended dosing schedules, or whether completion rates may be different for the two currently licensed RV vaccines in the US (RotaTeq, pentavalent RV vaccine [RV5], Merck & Co., Inc., USA, and Rotarix, monovalent RV vaccine [RV1], GSK, Belgium), one requiring two doses (RV1) and the other requiring three doses (RV5) as per the respective US prescribing information (PI) (). While the two RV vaccines have different dosing schedules in the PI, the ACIP recommends a harmonized dosing schedule for RV5 and RV1 (Citation2) and is the recommended dosing schedule in CDC’s childhood immunization schedule.Citation11 Furthermore, compared to the coverage of other childhood vaccines in the US, such as Diphtheria-Tetanus-acellular Pertussis (DTaP), coverage for RV vaccine lags behind despite having similar dosing schedules.Citation12 For instance, between 2006 and 2016, annual DTaP vaccine coverage rates among children aged 19–35 months ranged from 93.7% to 96.2%, whereas RV vaccine coverage levels increased but remained lower than DTaP vaccine coverage (43.9% in 2009 to 74.1% in 2016).Citation7,Citation13,Citation14 The age restrictions and lack of a catchup recommendation contribute to lower RV vaccine coverage compared to DTaP coverage in the US.Citation12 To help improve vaccination coverage for RV vaccines, it is important to understand vaccination series completion and compliance rates, for the two currently licensed RV vaccines in the US (RV1 and RV5). To address barriers to higher RV vaccine coverage rates and develop effective interventions to improve suboptimal coverage rates, it is also necessary to review the factors that influence coverage, completion, and compliance rates. One publication summarized and reviewed RV vaccine coverage, adherence to age recommendations and related RV-vaccine experience data for the US, but was restricted to the first three years of post-licensure data.Citation15
Table 1. Dosing schedules of RV vaccines licensed and recommended in the US
This systematic literature review was conducted with the goal to collate and describe for a population of US children (i) RV vaccine coverage rates, (ii) RV vaccine series completion rates for all recommended doses in the series, (iii) RV vaccine series compliance to the recommended dosing schedules (ACIP-harmonized schedule and US PI), and (iv) socio-demographic, health-care utilization, access-related and other factors associated with RV vaccine series completion and compliance.
Methods
This review was conducted according to guidelines in the Cochrane Handbook for Systematic Reviews of Interventions.Citation16 In-line with these guidelines, we developed a search strategy and study eligibility criteria prior to conducting the review. Following this, searches were performed and retrieved publications were assessed for eligibility in a two-phase screening process by two reviewers. Data were extracted from the final list of eligible publications. As the final step, we synthesized key findings from the data. The review methodology is detailed below.
Search source and strategy
We searched PubMed and Embase in May 2018. Search terms “(rotavirus OR rotavirus vaccines) AND (compliance or completion or adherence or predictors)” were used. Filters for the English language and human studies were applied. We further restricted the searches to studies conducted in the US, because the implementation of vaccination programs can vary by country, and published from 2006 onwards, i.e., from the year the first RV vaccine (RV5) was licensed for use in the US.
Study selection criteria
Article eligibility criteria were established a priori. The “STrengthening the Reporting of Observational Studies in Epidemiology” (STROBE) statement was used to define the review eligibility criteria.Citation17 Inclusion criteria were: (1) studies that included children identified from population-level databases, including national surveys or administrative claims; (2) studies conducted in the US; (3) vaccine intervention that included either RV vaccine licensed in the US (RV5 or RV1); (4) measured outcomes that included RV vaccination coverage rates or series completion, defined as receipt of all recommended RV vaccine doses, or series compliance to the recommended dosing schedule for RV vaccines, based on both the ACIP-harmonized schedules and the respective US PI as shown in , and/or factors associated with series completion or compliance; and (5) any observational study design that assessed completion or compliance rates relative to RV vaccine coverage. Review articles, efficacy or immunogenicity trials, effectiveness, and modeling studies were excluded.
Screening and selection
After the searches were performed, the identified publications were screened in two phases by two reviewers (PKG, JDA). The first phase included screening of titles and abstracts of all publications based on the eligibility criteria and was followed by the second phase which consisted of reviewing the full-text publications. Any discrepancies in article inclusion were resolved through a discussion between authors.
Data collection and reporting
From the selected studies, one reviewer extracted data on study population, data source, study setting, vaccine type, rates of and factors associated with RV vaccine series completion and compliance, then a second reviewer checked the quality of the extracted data. Data were extracted into Microsoft Excel 2016. In this article, we present an overview of the key findings.
Results
Overview of included studies
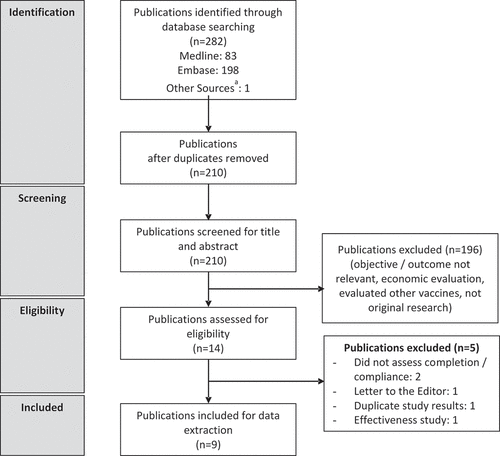
The database search identified 282 publications of which 210 were screened based on their title and abstracts after removing the duplicates. Of these 210, 14 full-text publications were assessed for eligibility after excluding studies with irrelevant outcomes such as cost analyses, DTaP studies, efficacy or safety studies, and other exclusions as detailed in , according to the Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA) checklist guidelines.Citation18 A total of nine articles were included in the review. summarizes the methodological characteristics and the main findings from the included studies.Citation7,Citation19-26 Six were retrospective cohort studies from large administrative claims databasesCitation19-22,Citation24,Citation25 and three were based on national surveys conducted annually by the CDC.Citation7,Citation23,Citation26
Table 2. Summary of included studies assessing RV vaccine series completion/compliance with schedules in the US
Vaccine coverage, completion and compliance rates from national surveys
A complete RV vaccine series (defined as either ≥2 doses of RV1 or ≥3 doses of RV5) was reported for 74.1% of children aged 19–35 months based on provider-reported vaccination data from the National Immunization Survey (NIS) for 2016 – a small decline from the previous year (73.2% in 2015).Citation7 Data from the same type of NIS survey, but for 2010–2012, showed that about 65% of children completed an RV vaccine series.Citation26 The results from these two studies show that while the coverage of the RV vaccine series has increased over time, this rate is still well below the Healthy People 2020 target of 80%. In a third study, RV completion and compliance rates for 2010–2012 were determined separately for RV1 and RV5.Citation23 About 90% of children completed the two-dose series of RV1 compared to 81% who completed the three-dose series of RV5 by 24 months of age. Also, 77% and 70% of children were compliant to the RV1 and RV5 series, respectively, by receiving all required doses on time according to the ACIP-harmonized schedule.Citation23
Vaccine completion and compliance rates for commercially-insured children
RV vaccination completion and compliance rates based on large claims databases for commercially-insured children have been determined in four studies with periods ranging from 2006 to 2012.Citation20,Citation21,Citation24,Citation25 Three of these studies compared RV vaccine completion and compliance rates for RV1 and RV5 and found that completion rates, measured as the percentage of patients receiving all required doses, were significantly higher for patients who received RV1 compared to RV5 (87% vs. 79%; 91% vs. 83%; 85% vs. 78%; p < .05).Citation20,Citation21,Citation25 Another study estimated the completion rate of RV5 at 78% among children with continuous enrollment from birth through the first year of life but did not provide a comparison to RV1.Citation24 Two of these studies also determined compliance rates as per the respective US PI and the harmonized ACIP dosing schedules, which addresses mixed series completion using both RV1 and RV5.Citation20,Citation21 In both studies, compliance rates per PI schedule were higher for RV1 compared to RV5 (69% vs. 54%, p < .05;Citation20 75% vs. 60%, p < .001Citation21). Compliance rates as per ACIP schedule in the two studies were also higher for RV1 compared to RV5 (81% vs. 66%, p < .05;Citation20 83% vs. 76%, p < .001Citation21).
Vaccine completion and compliance rates for Medicaid children
Two studies estimated RV vaccination completion and compliance rates among large cohorts of children covered by Medicaid programs.Citation19,Citation22 Medicaid is a joint federal and state program in the US, that helps with medical costs for individuals with low incomes and limited resources.Citation27 In both studies, completion and compliance were assessed based on the respective US PI and the harmonized ACIP dosing schedules. In the PI cohort, more children who received RV1 completed the series compared to those who received RV5 (55% vs. 44%, p < .0001;Citation19 65% vs. 46%, p < .0001Citation22) and were compliant with the PI schedule (55% vs. 25%, p < .0001;Citation19 65% vs. 31%, p < .0001Citation22).
Factors associated with vaccine completion and compliance
In addition to dosing schedules and vaccine characteristics, several factors influenced vaccine completion and compliance including social and geographic characteristics, family characteristics, access and provider characteristics, and immunization characteristics. These factors had a statistically significant impact on vaccine completion and compliance and are examined further.
Social and geographical characteristics
Coverage for a complete RV series was lower among black children and Hispanics than among white children (67.2% and 73.0% vs. 77.3%, p < .05), and among children living below the federal poverty level compared to children living at or above the poverty level (65.5% and 78.2%, p < .05).Citation7 A relatively small but significant difference in RV vaccine series completion by geographic region showed higher completion rates in the Midwest, South, and West regions of the US compared to the Northeast.Citation21 This association between region of residence and RV vaccine series completion was not found to be significant in another study.Citation25 However, the latter study showed that children residing outside of metropolitan areas were less likely to complete the RV vaccine series.Citation25 In another study, data from 2010 to 2012 from the NIS showed a huge disparity in RV series completion rates between 19–35-month-old foreign-born and US-born children (15.7% vs. 65.7%, p < .001).Citation26
Family characteristics
Mother’s age and number of siblings were found to be associated with RV series completion so that children born to younger mothers (<25 years; 73.9%) and children with one or more siblings less than 10 years of age (72.4–79.5%) were less likely to complete the series than mothers aged 30–34 years (81.5%) and children with no siblings (82.2%), respectively.Citation25
Access/provider characteristics
Provider type was found to be a significant factor associated with RV series completion in two studies.Citation21,Citation25 In one study, children who received routine care from a pediatrician were more likely to complete their series compared to children who were receiving routine care from a family physician (risk ratio [RR] = 1.13; 95% confidence interval [CI] = 1.11–1.14).Citation25 Similarly, the second study reported that children were less likely to complete their series when the immunization provider type was family practice compared to pediatricians (RR = 0.88; 95%CI = 0.87–0.89).Citation21 RV vaccine coverage for the completed series also varied by health insurance status with lower levels of coverage for uninsured children or those covered by Medicaid compared to those with private insurance (59.9% vs 68.7% vs 80.7%, p < .05).Citation7
Immunization characteristics
Type of RV vaccine. Children receiving the two-dose RV1 were found to be more likely to complete the vaccine series compared to children receiving the three-dose RV5 across three studies.Citation19,Citation21,Citation22 Two of these studies used data from a population of Medicaid-insured beneficiariesCitation19,Citation22 and one study assessed RV vaccine completion rates in a managed care population.Citation21
Receipt of DTaP vaccine. An overlap exists between the recommended schedules for DTaP and RV vaccination, and three studies demonstrate a significant association between DTaP receipt and RV vaccine series completion.Citation19,Citation22,Citation25 One of these studies tested the strength of the DTaP association with series completion by running two multivariable analyses – for a 2006 birth cohort and a 2009 birth cohort. While significant in both years, the strength of this association declined from 2006 (RR = 1.47; 95%CI = 1.32–1.63) to 2009 (RR = 1.24; 95%CI = 1.19–1.29).Citation25 Medicaid children vaccinated with DTaP had a higher likelihood of compliance with RV vaccination (RR = 17.8; 95%CI = 17.4–18.3).Citation22 In a population of Medicaid children, those who completed the DTaP vaccine series were 11.82 times more likely to be compliant with an RV vaccine series (RV1 and RV5) compared to those who did not complete DTaP vaccination.Citation19
Other. The association between month of series initiation and RV vaccine series completion was only evaluated in one study. This study evaluated the first 6 months of the year and children who initiated an RV vaccine series in the months of March, April, May, and June were shown to have significantly higher RV vaccine series completion rates compared to those initiating the series in January.Citation21
Discussion
This comprehensive review of available literature on RV vaccination coverage, completion and compliance rates, and factors influencing RV vaccination, suggests that RV vaccination remains underutilized in US children. Although only nine studies were eligible for inclusion, the consistent finding across these studies is that a sizable proportion of US children either do not get vaccinated against RV or do not receive vaccinations according to the recommended dosing schedules.
Despite the increase in coverage for RV vaccine over the years to 74.1% in 2016, it is still underutilized in US children relative to the Healthy People 2020 target coverage of 80%.Citation7,Citation8 RV vaccine coverage rates are lower than observed rates for most recommended childhood vaccines (≥90%) in 2016.Citation6,Citation7 Data also showed that about 50-90% of the US children initiating RV vaccination complete the RV series and compliance rates with the recommended schedules are even lower.Citation19–22,Citation24,Citation25 In addition, compliance and completion per PI and ACIP recommendations were found to be significantly greater among children receiving RV1 than those receiving RV5 among studies that are nationally representative and included both commercial and Medicaid insured children.Citation19,Citation21,Citation22 While it may seem obvious that completion and compliance with a two-dose series are easier to achieve than with a three-dose series, other factors are contributory, making vaccine coverage a complex issue.
RV coverage for a complete series is lower for black and Hispanic children (vs. whites),Citation7 uninsured or Medicaid insured (vs. privately insured),Citation7 and for foreign-born (vs. US-born) children.Citation26 Series completion and compliance are significantly greater in children receiving DTaP vaccination,Citation19,Citation22,Citation25 RV1 compared to RV5 vaccination,Citation19,Citation21,Citation22 and for those receiving routine care from a pediatrician compared to family physicians.Citation21,Citation25 The finding of higher RV vaccine completion rates for those receiving DTaP can be explained by the overlapping recommended dosing schedules for RV and DTaP vaccines, as children presenting to a provider’s office to receive DTaP vaccination at 2, 4 or 6 months of age, also have the opportunity to receive RV vaccines.Citation28 Because the receipt of DTaP vaccination was associated with higher rates of completion and compliance for the RV vaccination series,Citation19,Citation22,Citation25consideration should be given to administering the first dose of RV vaccine as soon as possible after 6 weeks of age, along with DTaP vaccination as feasible, to ensure induction of protection prior to exposure to natural RV infection.Citation29 Additional factors that influence RV vaccination coverage include region in the US,Citation21 poverty level,Citation7 family characteristics such as mother’s age, and number of siblings.Citation25These disparities indicate that improvements are needed in access to and delivery of age-appropriate immunization.
The search of online databases for this review was performed in May 2018. Since then one additional article has been published on this topic by Aliabadi et al.Citation12 Their analysis of RV coverage, timing of initiation, and completion of the vaccine series among children enrolled in seven US medical institutions that serve as active gastroenteritis surveillance sites were consistent with the findings of the studies included in this review in that coverage for RV vaccines was found to be lower than DTaP vaccine and factors that were associated with higher likelihood of RV vaccine completion were recent birth years (2013–2016) and higher maternal education. Preterm birth, African-American race, and public or no insurance were associated with lower odds of RV vaccine completion and regional differences were also observed.
This review focused on compliance, completion, and vaccine coverage rates, which are all considered important determinants of successful vaccine implementation, particularly with multi-dose vaccines.Citation30 A reduction in any of these parameters means that an individual or population has not received the full protection that is intended to be delivered through vaccination. This has adverse consequences for the individual, the at-risk population, and society at large such as reduced community immunity, decreased quality of care, and reduction in work productivity.Citation31 While the effectiveness of partial vaccination with RV5 in preventing RV-related hospitalizations and ED visits has been demonstrated,Citation32 it should also be noted that these estimates reflect short-term protection as most children in the study went on to complete the full three-dose series. In addition, there were inconsistencies reported in the effectiveness against RV-related outpatient visits such that one dose of RV5 was found to be more effective than two doses (100% vs. 40%).Citation32 Another study also demonstrated the benefits of incomplete vaccination in terms of reduced RV-disease burden compared to an unvaccinated cohort.Citation4 However, there are no clinical trial data for the efficacy of partial RV vaccination and the ACIP does not recommend incomplete vaccination. CDC’s reporting of national RV vaccination coverage is also based on full series completion (i.e., ≥2 doses of RV1 or ≥3 doses of RV5).Citation7
Even though RV vaccine programs differ in settings outside of the US, evidence from these settings tends to support high and sustained RV1 vaccination coverage. In England, a significant reduction in direct healthcare costs was reported in the first year of vaccine introduction and as high RV1 vaccination coverage (93% and 88% for one and two doses, respectively) was achieved.Citation33 Similarly, in Norway, an 86% decrease in RV gastroenteritis hospital cases was observed in children <5 years in 2016 compared to 2014–2015.Citation34 A high national coverage rate for RV1 vaccine series was documented in the first year after introduction (coverage rates of 89% and 82% for one and two doses, respectively). Among fully RV-vaccinated children, 98% received both doses within the upper age limit of 16 weeks and 90% received both doses according to the recommended schedule in Norway.Citation35 In Canada, RV1 vaccine series initiation ranged from 83.2% to 91.3%, with full series completion increasing each year of the program (Aug 2011-Jul 2014) from 73.0% to 78.5% and 84.2%.Citation36 In a study conducted in Austria, incomplete RV vaccination (RV1 or RV5) emerged as a risk factor for vaccine failure (OR 5.7; 95%CI: 4.2–7.8) underscoring the significance of complete vaccination.Citation34
Our review has several limitations. The generalizability is limited to the US and not applicable to other countries. While it describes reports on compliance, completion, and coverage to define a good level of implementation and impact of RV vaccine, there is limited evidence to support the link between these factors and clinical outcomes such as the reduction of RV gastroenteritis or RV-related hospitalizations, and that was not within the scope of this review. The link is difficult to assess for technical reasons, as cohorts with different vaccination statuses (completely vaccinated, incompletely vaccinated, unvaccinated) should be followed over time. Most of the studies included in this review have overlapping time periods and identified factors using one combined study period. It is likely that the factors may change over time, but none of the studies specifically addressed this question.
Conclusion
Despite ACIP recommendations to vaccinate children against RV, vaccine coverage in the US is not optimal. Further efforts are necessary to identify those children who are not reached through current vaccination strategies and to assess interventions to improve completion of RV vaccine series. In addition to factors such as the environment, combination with other vaccines, and timing of vaccination, data from multiple studies in different populations indicate that an RV vaccine with fewer doses may help improve vaccination coverage and, presumably, disease protection through higher rates of completion and compliance.
Abbreviations
| ACIP | = | Advisory Committee on Immunization Practices |
| CDC | = | Centers for Disease Control and Prevention |
| CI | = | confidence interval |
| DTaP | = | diphtheria-tetanus-acellular pertussis |
| ED | = | emergency department |
| NIS | = | National Immunization Survey |
| PI | = | prescribing information |
| RR | = | risk ratio |
| RV | = | rotavirus |
| RV1 | = | Rotarix, GSK, Belgium |
| RV5 | = | RotaTeq, Merck & Co., Inc., USA |
Contributorship
PKG and JDA performed the systematic literature review. All authors were involved in study conception or design. All authors participated in the analysis or interpretation of the study results. All authors took part in the development of the manuscript. All authors had full access to the data and gave final approval before submission.
Disclosure of potential conflicts of interest
All authors are employees of the GSK group of companies and hold shares in the GSK group of companies.
Trademark statement
Rotarix is a trademark owned by or licensed to the GSK group of companies. RotaTeq is a trademark of Merck & Co., Inc.
Acknowledgments
The authors thank Baudouin Standaert (GSK, Belgium) for his critical review of the manuscript and Lecia Brown (GSK, US Health Outcomes & Epidemiology) for reviewing and editing the manuscript. The authors would also like to thank the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Marie Cloes coordinated manuscript development and editorial support. Amrita Ostawal (Arete Communication UG, Berlin, Germany) provided medical writing support.
Additional information
Funding
References
- Hamborsky J, Kroger A, Wolfe S, eds. Epidemiology and prevention of vaccine-preventable diseases - rotavirus. 13th. Washington D.C: Public Health Foundation; 2015. https://www.cdc.gov/vaccines/pubs/pinkbook/rota.html
- Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2009;58:1–25.
- Rha B, Tate JE, Payne DC, Cortese MM, Lopman BA, Curns AT, Parashar U. Effectiveness and impact of rotavirus vaccines in the United States - 2006-2012. Expert Rev Vaccines. 2014;13(3):365–76. doi:10.1586/14760584.2014.877846.
- Krishnarajah G, Duh MS, Korves C, Demissie K. Public health impact of complete and incomplete rotavirus vaccination among commercially and medicaid insured children in the United States. PLoS One. 2016;11(1):e0145977. doi:10.1371/journal.pone.0145977.
- Ventola CL. Immunization in the United States: recommendations, barriers, and measures to improve compliance: part 1: childhood vaccinations. P T. 2016;41:426–36.
- Centers for Disease Control and Prevention. VaxView Vaccination Coverage. [accessed 2020 Jun 9]. https://www.cdc.gov/vaccines/vaxview/index.html
- Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y. Vaccination coverage among children aged 19-35 months - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(43):1171–77. doi:10.15585/mmwr.mm6643a3.
- U.S. Department of Health and Human Services - Office of Disease Prevention and Health Promotion. Healthy People 2020. [accessed 2018 Aug 24]. https://www.healthypeople.gov/2020/data-search/Search-the-Data#objid=4713
- United States (US) Food and Drug Administration. Highlights of Prescribing Information - Rotarix. [accessed 2018 Nov 20]. https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm133539.pdf.
- United States (US) Food and Drug Administration. Highlights of Prescribing Information - RotaTeq. [accessed 2018 Aug 24]. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM142288.pdf.
- Centers for Disease Control and Prevention. Immunization Schedules. [accessed 2020 Jun 9]. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html.
- Aliabadi N, Wikswo ME, Tate JE, Cortese MM, Szilagyi PG, Staat MA, Weinberg GA, Halasa NB, Boom JA, Selvarangan R, et al. Factors associated with rotavirus vaccine coverage. Pediatrics. 2019;143(2):e20181824. doi:10.1542/peds.2018-1824.
- Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, state, and selected local area vaccination coverage among children aged 19-35 months - United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(33):889–96. doi:10.15585/mmwr.mm6433a1.
- Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among children aged 19-35 months — United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(36):1171–77.
- Tate JE, Cortese MM, Payne DC, Curns AT, Yen C, Esposito DH, Cortes JE, Lopman BA, Patel MM, Gentsch JR, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J. 2011;30:S56–60. doi:10.1097/INF.0b013e3181fefdc0.
- Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]: the Cochrane Collaboration. Chichester (UK): John Wiley & Sons; 2011. www.handbook.cochrane.org
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–49. doi:10.1016/j.jclinepi.2007.11.008.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Calnan M, Krishnarajah G, Duh MS, Haider BA, Yermakov S, Davis M, Yan S. Rotavirus vaccination in a Medicaid infant population from four US states: compliance, vaccination completion rate, and predictors of compliance. Hum Vaccin Immunother. 2016;12(5):1235–43. doi:10.1080/21645515.2015.1136041.
- Eisenberg DF, Gu T, Krishnarajah G. Adherence to rotavirus vaccination quality measures in a commercially insured population. Hum Vaccin Immunother. 2013;9(2):389–97. doi:10.4161/hv.22877.
- Krishnarajah G, Davis EJ, Fan Y, Standaert BA, Buikema AR. Rotavirus vaccine series completion and adherence to vaccination schedules among infants in managed care in the United States. Vaccine. 2012;30(24):3717–22. doi:10.1016/j.vaccine.2011.12.077.
- Krishnarajah G, Landsman-Blumberg P, Eynullayeva E. Rotavirus vaccination compliance and completion in a Medicaid infant population. Vaccine. 2015;33(3):479–86. doi:10.1016/j.vaccine.2014.06.059.
- Kurosky SK, Davis KL, Krishnarajah G. Completion and compliance of childhood vaccinations in the United States. Vaccine. 2016;34(3):387–94. doi:10.1016/j.vaccine.2015.11.011.
- Lanes S, Quinlan SC, Mast TC, Greenland S, Holick CN. Assessing bias in administrative database studies of RotaTeq vaccine completion due to exclusion of subjects with incomplete follow-up. Emerg Themes Epidemiol. 2015;12(1):5. doi:10.1186/s12982-015-0027-6.
- Panozzo CA, Becker-Dreps S, Pate V, Jonsson Funk M, Stürmer T, Weber DJ, Brookhart MA. Patterns of rotavirus vaccine uptake and use in privately-insured US infants, 2006-2010. PLoS One. 2013;8(9):e73825. doi:10.1371/journal.pone.0073825.
- Varan AK, Rodriguez-Lainz A, Hill HA, Elam-Evans LD, Yankey D, Li Q. Vaccination coverage disparities between foreign-born and U.S.-Born children aged 19-35 months, United States, 2010-2012. J Immigr Minor Health. 2017;19(4):779–89. doi:10.1007/s10903-016-0465-4.
- Centers for Medicare & Medicaid Services. Glossary - M. [accessed 2020 Jun 9]. https://www.cms.gov/apps/glossary/default.asp?Letter=M&Language=English. .
- Centers for Disease Control and Prevention. Immunization Schedules - Child & Adolescent Immunization Schedule. Notes: diphtheria, tetanus, and pertussis (DTaP) vaccination (minimum age: 6 weeks [4 years for Kinrix or Quadracel]). [accessed 2020 Jun 9]. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html#note-dtap.
- Rotavirus vaccines WHO position paper: January 2013 - Recommendations. Vaccine. 2013;31(52):6170–71. doi:10.1016/j.vaccine.2013.05.037.
- Gallagher KE, Kadokura E, Eckert LO, Miyake S, Mounier-Jack S, Aldea M, Ross DA, Watson-Jones D. Factors influencing completion of multi-dose vaccine schedules in adolescents: a systematic review. BMC Public Health. 2016;16(1):172. doi:10.1186/s12889-016-2845-z.
- Tran AN, Husberg M, Bennet R, Brytting M, Carlsson P, Eriksson M, Storsaeter J, Österlin B, Johansen K. Impact on affected families and society of severe rotavirus infections in Swedish children assessed in a prospective cohort study. Infect Dis (Lond). 2018;50(5):361–71. doi:10.1080/23744235.2017.1416162.
- Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of an incomplete RotaTeq (RV5) vaccination regimen in preventing rotavirus gastroenteritis in the United States. Pediatr Infect Dis J. 2013;32(3):278–83. doi:10.1097/INF.0b013e318275328f.
- Thomas SL, Walker JL, Fenty J, Atkins KE, Elliot AJ, Hughes HE, Stowe J, Ladhani S, Andrews NJ. Impact of the national rotavirus vaccination programme on acute gastroenteritis in England and associated costs averted. Vaccine. 2017;35(4):680–86. doi:10.1016/j.vaccine.2016.11.057.
- de Hoog MLA, Vesikari T, Giaquinto C, Huppertz H-I, Martinon-Torres F, Bruijning-Verhagen P. Report of the 5th European expert meeting on rotavirus vaccination (EEROVAC). Hum Vaccin Immunother. 2018;14(4):1027–34. doi:10.1080/21645515.2017.1412019.
- Valcarcel Salamanca B, Hagerup-Jenssen ME, Flem E. Uptake and timeliness of rotavirus vaccination in Norway: the first year post-introduction. Vaccine. 2016;34(39):4684–89. doi:10.1016/j.vaccine.2016.08.017.
- Wilson SE, Chung H, Schwartz KL, Guttmann A, Deeks SL, Kwong JC, Crowcroft NS, Wing L, Tu K. Rotavirus vaccine coverage and factors associated with uptake using linked data: ontario, Canada. PLoS One. 2018;13(2):e0192809. doi:10.1371/journal.pone.0192809.
Appendix
Plain Language Summary
What is the context?
Currently, two vaccines against rotavirus can be administered to infants in the US: Rotarix (RV1), a two-dose-series vaccine, and RotaTeq (RV5), a three-dose-series vaccine.
Disease control is promoted by obtaining high vaccination coverage and receipt of the entire vaccine series within the recommended vaccination schedule.
What is new?
This systematic literature review summarizes published rates of rotavirus vaccine coverage, series completion, and compliance to the recommended dosing schedule among US children, and factors associated with rotavirus vaccine series completion and compliance in this population.
It shows that immunization against rotavirus is suboptimal with reported vaccination coverage below the Healthy People 2020 target of 80%.
Factors associated with rotavirus vaccine series completion include the child’s socioeconomic characteristics, insurance status and type, vaccine provider type, receipt of diphtheria-tetanus-pertussis vaccination, and rotavirus vaccine type.
What is the impact?
A better awareness of the gaps in rotavirus vaccination coverage and series completion, and understanding of factors associated with vaccine series completion or compliance to dosing schedule, could help improve protection against rotavirus disease and its complications among children in the US through the improvement of rotavirus immunization strategies.