?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In 2019, Bangladesh has grappled with a record-breaking surge in dengue fever, experiencing the highest number of dengue cases since the year 2000. Together, the intensification of dengue fever combined with a lack of dengue vaccines and appropriate medicines is expected to further the public and government’s interests in appropriate and potential dengue vaccines to control the epidemic. We considered people’s characteristics, dengue experience, and knowledge to assess their willingness-to-accept (WTA) and willingness-to-pay (WTP) for a hypothetical dengue vaccine and ex-post treatment in Bangladesh (June–July 2019). This study implemented a contingent valuation (CV) method with 3,251 respondents in 10 different locations of Bangladesh. All respondents participated in a hypothetical dengue vaccine scenario consisting of 65% (vaccine A), 80% (vaccine B), and 95% (vaccine C) effectiveness levels with three doses of each vaccine and ex-post dengue treatment. Around 71.2% of respondents were willing to pay for at least one of the hypothetical vaccines: A, B, or C. The average WTPs of the three vaccines amounted to US$ 47.0, US$ 66.0, and US$ 89.0, which were defined as the total cost of the doses necessary to obtain immunity. In Bangladesh, there is a significant demand for low-priced dengue vaccines, which was proven by people’s higher acceptance of vaccination practices. Though dengue vaccines are not yet available in Bangladesh, this study provides significant support that both the government and private sectors should work together to develop a reliable and affordable dengue vaccine.
Introduction
Dengue disease is an Aedes type mosquito-borne viral disease that has rapidly spread around the world, particularly in Asia,Citation1,Citation2 and it currently accounts for significant morbidity and mortality in Bangladesh. The dengue virus is conducted to humans by infected female mosquitoes, predominantly those from the Aedes aegypti (Ae. aegypti) species, and there are four distinct viral strands: DEN-1, DEN-2, DEN-3, and DEN-4Citation3 (DEN-The dengue virus). In recent decades, the incidence of dengue disease has increased dramatically; the World Health Organization (WHO) estimates that 52% of the people at risk live in Asian and Latin American countries.Citation4,Citation5 Globally, 390 million cases have been reported to the WHO in recent years, of which 96 million manifests clinically,Citation1 with 3.9 billion people in 128 countriesCitation6 being at risk for infection with dengue. The dengue disease burden increased by 30 times in the past 50 years that reported in the Asia-Pacific region.Citation7–9
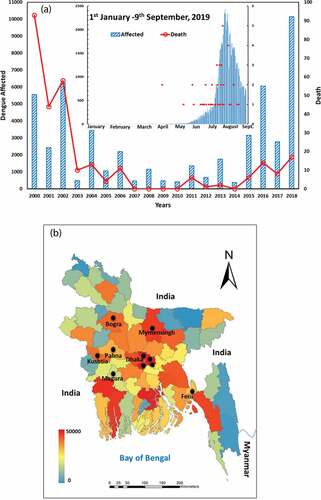
Bangladesh, a country in South Asia that is bordered by India and Myanmar, experienced its first epidemic of dengue fever in 2000, and since then, records have been updated each year.Citation10 The dengue disease is characterized by annual outbreaks (). From 2000 to 2017, there were 40,476 cases recorded, with 49.7% of cases occurring from May to August and 49.2% from September to December.Citation11 However, since 2017, these trends have been changing. The country suffered a large-scale outbreak again in 2018, with more than 10,000 infections and 17 deaths. Then, in the deadliest scenario to date, 77,230 infections and 197 deaths (of which 101 were confirmed) were recorded between January 1st and September 9th of 2019 (Health Emergency Operation Center and Control Room, DGHS, Bangladesh) ()). Sharmin et al.Citation12 have shown that 92% of the dengue cases in Bangladesh took place between August and September, and that 94% of the cases were in the capital city, Dhaka. Overall in Bangladesh, most dengue cases are associated with the intermittent rainy season, rapid and unplanned urbanization, high population density, and uncontrolled mosquito populations that are characterized by a high density of larva and pupa.
Figure 1. (A) Combined bar and line graph to show the number of reported dengue cases from 2000–2019 (blue color bar chart presents dengue cases and red color line graph shows death cases). The dengue cases (blue) with death (red) for 2019 (January1st–September 9th) is represented by an inset diagram. (B) Map of Bangladesh indicating dengue-affected people in 64 districts from January 1st–September 9th, 2019 (health emergency operation center and control room, DGHS, Bangladesh) and survey locations (black-filled circle)
Vaccines are the safest and most effective way to control risks from well-known infectious diseases, and they also have the longest-term therapeutic value. Researchers are currently investigating several promising dengue vaccines, with several of these in development and testing stages.Citation13,Citation14 The world’s first dengue vaccine – Dengvaxia by Sanofi Pasteur – was licensed in 2015 and has been commercially introduced in 11 countries: Mexico, Philippines, Indonesia, Brazil, El Salvador, Costa Rica, Paraguay, Guatemala, Peru, Thailand, and Singapore.Citation15–20 The dengue vaccine provides countries with a new way of preventing the spread of dengue, and WHO-SAGECitation21 recommends that countries’ health authorities consider vaccination as a part of their dengue control programs.Citation22 However, Dengvaxia has been associated with enhanced disease in subjects who have not been exposed to dengue virus; thus, for countries considering vaccination as part of their dengue control program, the World Health Organization recommends a pre-vaccination screening strategy as the preferred option, in which only dengue-seropositive persons are vaccinated. Recently, Takeda’s vaccine verified protection against virologically confirmed dengue, and the vaccine trials ended.Citation23,Citation24 However, a vital policy question that arises in this context is whether the vaccine should be distributed privately or publicly. If individuals are to pay for the vaccine, a central question is what an appropriate price to pay would be for individuals in low- or middle-income developing countries such as Bangladesh. Alternatively, if the vaccine is to be administered through a public program, it would be important to assess the trade-off between the social burden of dengue infections and the social benefit of preventing them. Our research attempts to quantify the willingness-to-pay (WTP) for dengue vaccines and ex-post treatment by suggesting three different vaccinations with varying levels of effectiveness.
From a socio-economic-demographic perspective, we evaluate people`s demand and acceptance considering vaccination effectiveness, existing treatment facilities, and cost burden to prepare for the possible introduction of a dengue vaccine in Bangladesh. To quantitatively analyze individual benefit and demand for dengue vaccine and treatment, we employ contingent valuation (CV), a well-established method for assessing respondent’ monetary valuations in terms of WTP or WTA.Citation25 To date, several studies have assessed WTP for the dengue vaccine, showing acceptance for the future vaccine in relation to countries’ economic and demographic characteristics: Palanca-Tan et al.Citation26 in Metro Manila (Philippines); Hadsoemarto et al.Citation27 in Bandung (Indonesia); Harapan et al.Citation28 in Aceh (Indonesia); Lee et al.Citation29 in Vietnam, Thailand and Colombia; Godoi et al.Citation30 in Brazil; Yeo et al.Citation31 in Penang (Malaysia); Dhiman et al.Citation32 in Nepal; Lam et al.Citation33 in Philippines; and Zheng et al.Citation34 in ten countries in Asia and Latin America. Besides, treatments as well as medical services have been studied by researchers.Citation35,Citation36 Moreover, to evaluate individual as well as country risks from dengue disease, case-control (CC) and test negative (TN) studies have been conducted by Pang et al.,Citation37 Gibson et al.Citation38 and Nealon et al.Citation39
In countries other than the ones mentioned above, it is important to assess the WTA and WTP values for vaccines (with varying levels of effectiveness) and treatment policies. Bangladesh is one such country; to date, no study like this has been conducted for Bangladesh. Thus, this study will evaluate the current situation in Bangladesh to provide relevant information to the government, the public, and manufacturers to develop guidelines for a future dengue vaccine.
Methodology
Study variable
To evaluate vaccine acceptance and its accompanying factors, we used two key variables, willingness-to-accept (WTA) and willingness-to-pay (WTP), throughout our study.
WTA
To evaluate whether one accepts vaccination (treatment), we defined WTA. WTA is estimated based on the respondent’s response (“Yes” or “No”) to the direct question of whether they would accept the vaccination. WTA is represented by the percentage of respondents who answered “Yes” to the question of whether they would accept the vaccination.
WTP
In this study, we measured arguably the most important factor, WTP, to understand and show how the public would perceive a future dengue vaccine. WTP is the maximum estimated price at or below which an individual would be willing to purchase a good or service, and in this case, a dengue vaccine. The iterative bidding method was applied to find the hypothetical rather than actual WTP, followed by the CV method. The mean WTP is calculated by summing the maximum bidding prices, and the median WTP is calculated by assessing the price at which half of the respondents would agree to purchase the vaccine. We conduct an analysis for both the mean and median WTP using parametric and non-parametric estimation.
Study design
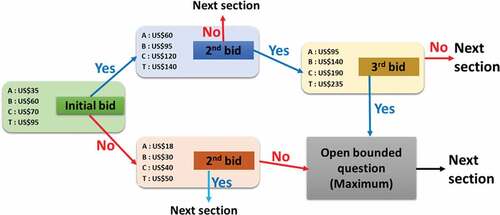
To assess individual demand, feasibility, and WTP for hypothetical dengue fever vaccines and treatment, we administered a set of survey questionnaires. A cross-sectional contingent valuation (CV) method with dichotomous choice via a bidding game approach was adopted to quantify the WTP of each respondent for three hypothetical dengue vaccines (Vaccines A, B, and C) and for ex-post treatment. The effectiveness, or probability of acquiring perfect immunity, was set for each vaccine as 0.65 (65%), 0.80 (80%), and 0.95 (95%). We fixed the lowest effectiveness (65%) based on earlier clinical trial results that have previously been presented by Hadinegoro et al.Citation40 for the case study of Latin America and Asian countries (65.6% effectiveness). Then, we assumed two more effectiveness for future dengue vaccine. Ex-post treatment was defined as medical care given to a person infected by the dengue virus. We presumed four (five) pre-assigned bidding prices () for the following provisions: Vaccine A, Vaccine B, Vaccine C, and Treatment T, where four are pre-assigned fixed bidding prices and one is an open-ended price. Before conducting the survey, we performed an online pilot test in which 230 respondents participated. The respondents were contacted individually. Subsequently, we finalized the interview-based questionnaire, bidding price, and effectiveness via an open-ended group discussion.
Figure 2. Double-bounded dichotomous choice and bidding game approach presented for elicitation of WTP amount. Since there was no previous data about the cost of the dengue vaccine available in Bangladesh, the starting bid (A-US$ 35, B-US$ 60, C-US$ 70, and T-US$ 95) was established by referring to some previous studies and tests online. Here, No indicates an unwillingness to pay and Yes indicates WTP
The main survey was performed over 10 days from July 13–22, 2019, by ten interviewers (enumerators) in ten different locations. The respondents were questioned face-to-face by trained enumerators, who possessed undergraduate and master`s (MS) degrees from local universities. The enumerators were provided with day-long trainings that included detailed explanations about dengue disease, questionnaire techniques, and the role of enumerators. They were also given appropriate compensation for their work on the survey. All people above the age of 17 years old were considered as survey respondents.
Population, sites selection, and data collection
The survey involved personal interviews (the subjects were selected randomly using social connections) in ten different locations: the capital (Dhaka), cities, and villages in Bangladesh. The ten sites were selected ()) in accordance with the goals of the research. For example, we chose to include both urban and rural areas as well as those with low and high levels of previous dengue transmission. Respondents were eligible to participate in the interview-based survey if they had been residing in one of the respective places for more than 2 years. Furthermore, as much as possible, enumerators randomly selected respondents from different social backgrounds in terms of age, social status, education, income, and residence. To conduct the survey, enumerators visited respondents at their home, workplace, hospital, educational institute, and on the street. We did this to provide an in-depth, comprehensive picture for acceptance and WTP for the dengue vaccine (treatment). Four of the ten enumerators were deployed to the capital (Dhaka), while six went to other districts comprising urban areas and villages.
Capital
Dhaka is the capital city, in the center of Bangladesh. It is also the largest and most densely populated cityCitation41 in the world; its population was 18.2 millionCitation42 in 2016. Annually, dengue cases are reported across the country, but 94% of cases between 2000 and 2017 were from Dhaka,Citation11,Citation12 which is classified as a highly epidemic region. For this reason, we appointed four enumerators to conduct the census in four locations in Dhaka: Jatrabari, Khilgao, Mirpur, and Lalbagh/Azimpur.
Urban areas and villages
Six enumerators randomly selected respondents in urban areas and villages in six of the 64 total districts in Bangladesh: Mymensingh, Bogra, Pabna, Kushtia, Magura, and Feni. In terms of the geographic distribution of the study areas throughout the country, Mymensingh is in the north; both Bogra and Pabna are in the northwest; Kushtia and Magura are in the southwest; and Feni is in the southeast.
Survey questionnaire
The survey performed through face-to-face interviews by trained enumerators, with each interview lasting 15–20 minutes. The survey’s structured questionnaire consisted of four sections. The first section included socio-economic demographic questions on age, gender, occupation, education, marital status, income, health, and other personal characteristics and also asked questions about previous experience with dengue. The second section probed respondents’ dengue fever knowledge and prevention practices. The third section inquired about the current vaccination situation and the possibility of future vaccine acceptance.
Finally, section four provided some fundamental information about the three hypothetical dengue vaccines (A, B, and C) including vaccine effectiveness, number of doses, and side effects. This section also described ex-post treatment, which included taking medications or receiving medical care as an ultimate provision following dengue infection. The costs of the treatment included any hospitalizations, drugs, and follow-up visits or examinations.
The fourth section also constructed the CV scenario, asking the WTP question to assess dengue vaccine acceptance after offering three provisions (A, B, and C) and treatment (T). For example, the WTP question was worded as follows: “Would you be willing to pay US$ 40 per dose for a dengue vaccine?” For respondents who were not willing to pay any amount for the vaccine or treatment at the specified prices, an additional set of question probed their reasons for refusal. The last section of the questionnaire inquired about respondents’ independent choices to take any provisions from A, B, C, and T.
The final form of the survey questionnaire, which was in English, was translated into the Bangla language. Results from the pilot program and discussion period were not considered in this analysis. The total number of respondents was 3,251. In SI (supplementary information), the questionnaire is included in both languages.
CV method
We used the contingent valuation (CV) method to estimate the WTP for a future dengue vaccine and for dengue treatment facilities in Bangladesh. In general, in a CV study, the WTP question has two folds: a dichotomous choice (DC) question and an open-ended question. Questions in the DC format were simple “yes/no” questions about whether respondents would purchase the vaccine (treatment) at a given price. At every bidding stage, if the respondent said “yes,” the next higher price was offered in the next choice and if they said “no” to the lower price given. To conclude, an open-ended question probed the respondents’ maximum WTP amount for the dengue vaccine (treatment).
This survey employed four classes of CV tests. The first three tests pertained to the three types of vaccination based on effectiveness (Vaccine, 65%; B, 80%; and C, 95%) and the final test pertained to treatment burden. The bidding process with the CV method detailed in demonstrated the double-bounded dichotomous choice by asking for two or three bids, followed by an open-ended question that requested the maximum WTP for each provision.
Statistical analysis
Assuming that there are three vaccines with their associated levels of effectiveness and one treatment, a result was considered to be statistically significant if the P-value was smaller than 0.05. ANOVA was used to analyze respondents’ characteristics; descriptive and inferential statistics have been stated as percentages and means and include standard deviations where applicable. ANOVA is a form of statistical hypothesis testing used in the study of survey data; it is calculated from the null hypothesis and the sample. The test outcome is said to be statistically significant presuming the truth of the null hypothesis. This study also shows both parametric and non-parametric estimates of the mean WTP and WTA for three dengue vaccines based solely on the sample survey data. All statistical data were analyzed using visual c++ and MS-excel tools.
Results
Willingness-to-accept
Socio-demographic profile
summarizes socio-economic demographic characteristics. Among respondents, the mean age was 32.07 years, and 44.9% of them were 21–30 years old. We had slightly more male respondents (61.7%). The majority were living in the capital city of Dhaka (51.3%), were married (54.4%), and had a household monthly family-accumulated income of US$181–US$355 (42.3%). Respondents with no formal or primary education were 16.1%’ those who passed secondary level (6–10) were 13.7%; those with some college-level education were 25.1%; those who completed university or above were 45.2%. Around 37.5% of respondents mentioned that they had experienced dengue fever themselves or that family members or friends had suffered from it. There were a few respondents (3.2%) who reported that someone they knew had died from dengue.
Table 1. Respondents’ socio-demographic profile of dengue vaccine acceptance (n=3251)
Dengue disease knowledge and prevention practices
presents respondents’ knowledge about ten areas where dengue fever is prevalent, and each item has the options of “yes,” “no,” or “don’t know” for an answer. Almost all people reported knowing that Aedes mosquitoes (89.5%) are responsible for dengue fever and that the disease can be fatal (88.7%). Around half of the respondents were knowledgeable about when Aedes mosquitos are active (55.5%) and their breeding places (47.7%). Many respondents either responded incorrectly or did not know the answer to the remainder of the six questions.
Table 2. Respondents’ socio-demographic profile of dengue vaccine acceptance (n=3251)
also displays the dengue prevention behaviors practiced by individuals in response to five-scale questions: never, occasionally, sometimes, often, and always. The majority of people reported engaging in the following actions: regularly cleaning (question #1); buying insect repellent (question #2); and using bed/window nets (question #3). However, most people also reported that they did not limit outdoor activities during the early morning and late evening hours, which would help them avoid being bitten by mosquitos (question #4).
Vaccination attitude and acceptance
Our summarized results show that the respondents’ attitudes toward the reliability of vaccination in preventing disease were relatively optimistic (). Also, the majority of people reported that they would trust a registered vaccine in Bangladesh approved by the Ministry of Health (MOH). Furthermore, respondents were most likely to accept a 100% safe and fully protective dengue vaccine that was provided free by the government. Unfortunately, most respondents were either unsatisfied or unfamiliar with the dengue treatment options in their own area. Finally, this study discovered that 55.8% of respondents claimed to be informed about dengue disease as a result of information in newspapers and on television; 22.3% of the respondents learned about it from friends or family members; and the remaining respondents obtained the information from social media (18.7%).
Socio-demographic relationships with dengue knowledge and prevention practices
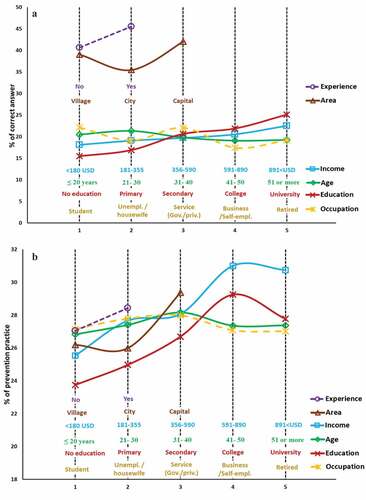
Notably, dengue knowledge and engaging in prevention practices were associated with individual socio-demographic characteristics. The proportion of correct answers for dengue knowledge (score) and prevention practices (score) by respondent characteristics is depicted in . As expected, respondents with previous dengue experience obtained a higher proportion of correct answers with respect to dengue knowledge and prevention practices (purple). The relationship between dengue knowledge and education/income presented with an increasing tendency ()). However, in the relationship to prevention practices ()), higher income and education levels showed fluctuating tendencies. The respondents’ age and occupation variables were not significant. Comparing the villages, cities, and capital, the respondents living in the capital (Dhaka) had higher dengue knowledge and more regularly engaged in preventive practices. Overall, it can be concluded that respondents who reported higher education and income, lived in the capital region, and had previous dengue experience were more likely to have knowledge about dengue and engage in prevention practices.
Willingness to pay
Respondent’s characteristics: socioeconomics, knowledge, and attitude
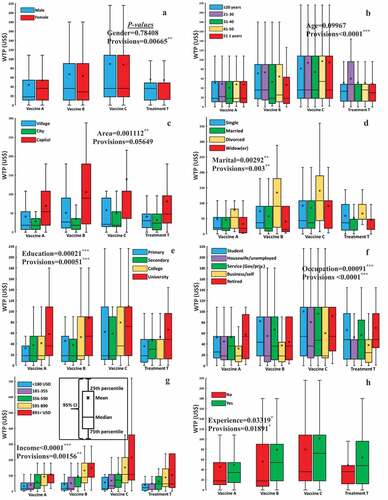
displays synopses of the three vaccine-effectiveness and treatment scenarios. It also shows the statistical summary of WTP (sample mean, median, 25th and 75th percentiles, and 95% confidence interval) in relation to sociodemographic characteristics of respondents for a three-dose vaccine and treatment. The summarized results showed that the respondents were more likely to pay for vaccine C; in other words, the WTP for vaccine C was higher compared with other provisions. Moreover, the respondents with higher education level, divorced, government or private sector employee or students, upper-income family, lived in the capital and previous dengue experienced were, with statistical significance, more likely to pay for vaccine and treatment. On the other hand, the following respondents had a lower WTP: those residing in small cities, those with only a secondary-level education; those who were unemployed/housewives or self-employed in a small business; and those who were without previous dengue experience.
Figure 4. Statistics of WTP of respondents, classified by socio-demographic characteristics: (A) Gender, (B) age, (C) area, (D) marital status, (E) education, (F) occupation, (G) income, and (H) dengue experience. Values with *, **, and *** refer to statistical significance with a level 5%, 1%, and 0.1%, respectively
Meanwhile, a two-way ANOVA was conducted to examine the effect of respondents’ socio-demographic characteristics, knowledge, prevention practices, and vaccine attitudes on mean WTP amount. There were statistically significant interactions in all groups except in the gender and age groups (see SI/Table SI1–SI4). Regarding the ANOVA results in SI, it is notable that when respondents had more dengue knowledge, prevention practices, and more positive vaccine attitudes, it had a significant effect on their WTP amount.
Socio-demographic cross analysis
shows the summary of the statistical analysis (p-values) of the cross-relation between respondents’ demographic characteristics (area, income, education, occupation, experience, and age) influencing the average WTP of vaccines A, B, and C. We can confirm that there were some combinations in which not only one (highlighted by blue) but both factors (highlighted by green) were more significant than 5% We plotted the respective two-directional bar chart in that shows the mean WTP amount based on two-factor significance (highlighted by green in ). Respondents those who lived in the capital and had higher income or described themselves as “students” in terms of current social status (perhaps their family background was upper-class) had quite a high WTP for any vaccine: A, B, and C. Interestingly, respondents having previous dengue experience with younger people (younger than 30 years) offered a higher WTP for vaccine B.
Figure 5. Bar chart of mean WTP for respondents’ groups categorized according to two attributes; (A) vaccine A for occupation vs. area, (B) vaccine A for income vs. area, (C) vaccine B for occupation vs. area, (D) vaccine B for age vs. experience, (E) vaccine C for occupation vs. area and (F) vaccine C for
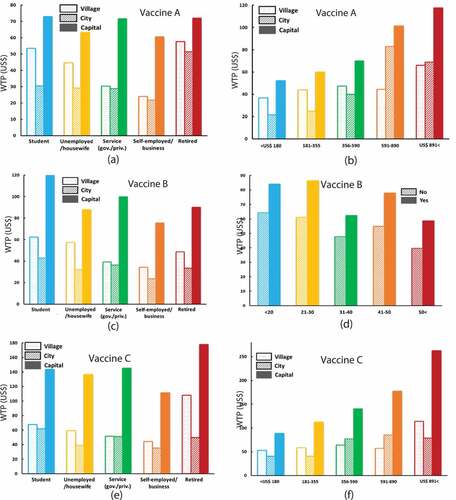
Table 3. ANOVA analysis (p-value) of multiple factors of respondents’ socio-demographic conditions on willingness to pay for vaccines A, B, and C. Values with *, **, and *** refer to statistical significance with a level 5%, 1%, and 0.1%, respectively. Green, blue, and white colors mean that both factors were significant, only one factor was significant, and none were significant, respectively
Cost-effectiveness analysis
) shows the results for the WTA and the mean WTP for each provision (A, B, C, and T) with some statistical information at a glance. The percentage of respondents who accepted the dengue vaccines was estimated at 41.4%, 30.5%, and 54.8% for vaccines A, B, and C, respectively. The added value, 71.2% (represented by the thin, dashed gray line), indicates the percentage of respondents selecting any preemptive vaccination(s) when presuming the WTA setting. Vaccine C, which is the most effective and has the highest cost, is most favored. This is followed by Vaccine A, which is the least effective with the lowest cost. Vaccine B is least favored. In accordance with basic economic theory, more individuals accepted vaccines for lower cost and reliability (effectiveness) and for higher cost and reliability. This may be because the two vaccines have extreme specifications in terms of quality (high or low) and cost (high or low), and both vaccines will meet the differing preferences of differing consumers. In contrast, a vaccine with intermediate specifications (Vaccine B in this case) shows less preference in comparison. In this sense, the result obtained here seems reasonable.
Figure 6. (A) Statistical summaries of respondents’ mean WTP, WTA for vaccines A, B, and C, and treatment T. (B) Histogram of respondents’ WTP for hypothetical vaccinations indicating willingness to accept the vaccine
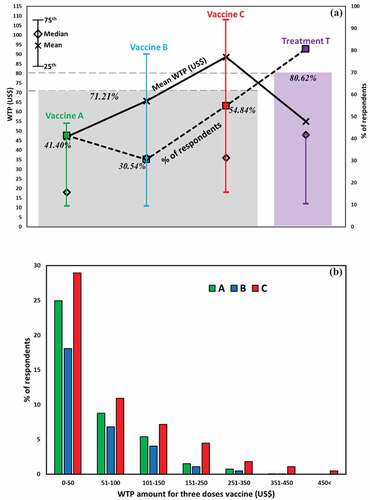
Another notable finding is that there was a gap between the mean and median WTP for Vaccine C that was much larger than the one between mean and median WTP for Treatment. This may be because the decisions of several affluent people pulled up the average WTP for Vaccine C. By contrast, less affluent or even poor people would choose Treatment, deflating both the average and median WTP at the same time.
Individual demand analysis (WTP)
Observed WTPs for vaccinations differed from one respondent to another. ) shows the distribution of cumulative acceptance of percentages below which each of the vaccinations is purchased. As discussed in the previous section, Vaccination C has a flatter tail than does Vaccines A or B. Many people are attracted to Vaccine C because of its high reliability. However, most people (i.e., those who are not affluent) would not be able to afford it. Affluent people, on the other hand, would not be deterred by the surplus and could afford the high cost for Vaccine C.
The result shown here has serious implications for the real world. Considering the average income in Bangladesh, a surplus of US$80–100 for a vaccination would not really be affordable for the average person on the street, although the observed, average WTP is equivalent to this value. This result suggests that governmental intervention in the form of a subsidy program would be needed to protect the population from the risk of dengue.
Cost-effective implementation in relation to the epidemic model
In recent years, the so-called vaccination game (e.g.,Citation43–45), a mathematical epidemic model such as SIR that is juxtaposed with evolutionary game theory has been introduced to quantitatively predict the final sizes of epidemics, preemptive vaccination coverage, and the total social cost. These exercises lend quantitative information as evidence for building up public health policy. It would be meaningful and informative if the results of this study could be connected to the predictive results from vaccination game models.
Let us consider two vaccination models by Kuga et al.Citation43 and Kabir et al.Citation45 The first modelCitation43 describes the social dynamics that result from an individual deciding whether to commit to a vaccination in advance of a single epidemic season. It then relies on SIR dynamics to reproduce how a disease spreads through the population. At the end of a one season, an individual is allowed to refresh his or her strategy for the next season and either obtain the vaccination or not depending on what happened in the most recent season. If an individual is fortunate and others cooperatively commit to vaccination, he or she might be able to be a herd immunity free rider, paying neither the disease cost nor the vaccination cost but still being protected from infection. In such a case, he or she becomes a non-vaccinator in the next season. Considering all of these mechanisms and social dynamics, the model quantifies how many people commit to vaccination (vaccination coverage) at social equilibrium after repeating many seasons.
On the other hand, the second modelCitation45 focuses on how vaccination coverage evolves and a disease spreads in one single season, in which an individual would be pushed to vaccinate depending on the size of the epidemic, vaccination coverage, and vaccination cost. These results would differ from those of the previous model.
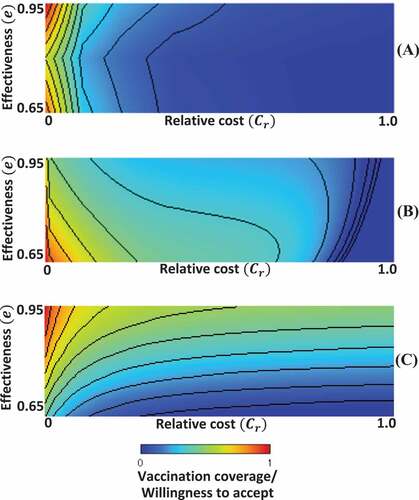
compares two models as a heat map of vaccination coverage drawn on the two-dimensional plane of relative vaccination cost (Cr) versus vaccine effectiveness (e). ) is redrawn from , in which the acceptance of each of the three effectiveness levels (e = 0.65, 0.80, and 0.95) was shown, which is interpreted as vaccination coverage. The observed WTP is interpreted as the vaccination cost. To identify the relative vaccination cost (Cr), or the normalized vaccination cost, we presumed the maximal “cost burden” reported by Ref.Citation46 Panels (b) and (c) resulted from the two aforementioned models, presuming the relative vaccination cost as the fraction of vaccination cost to the disease cost [Citation43,Citation45]. The heat maps that result from those two models, originally ranging from and from
, are partially focused to draw panels (b) and (c), respectively.
Figure 7. 2D phase diagram of vaccination coverage/willingness to accept (WTP); (A) survey results (B) epidemic vaccination game model with repeated seasonsCitation43 and (C) single-season epidemic model.Citation45
Discussion
This study successfully assessed the acceptance (WTA) and WTP value for three hypothetical dengue vaccines (Vaccines A, 65% effectiveness; B, 80% effectiveness, and C, 95% effectiveness) in the capital city and in rural areas in Bangladesh. Our results indicated that a reasonable fraction of our sample (71.2%, see ) was willing to accept the dengue vaccines, suggesting that implementation is feasible. The percentages of respondents that accepted (WTA) the dengue vaccines were associated with the levels of effectiveness: 41.4% (A), 30.6% (B), and 54.8% (C), respectively. Our results, in terms of WTA, are similar to those of recent community-based studies in Aceh (77.3%)Citation47 and Bandung (94.6%)Citation27 Indonesia; the Philippines (75%);Citation48 Colombia (88.6%);Citation49 and Vietnam (77.3%).Citation50 Furthermore, most respondents who refused to accept any vaccines reported that they would prefer to have free vaccinations from the government or an insurance company (61.6%). Around, 15.1%, 12.7%, and 10.6% of respondents reported that they would prefer more prevention practices and scientific evidence or that they could not afford the vaccine, respectively.
The adjusted mean estimated WTP for the hypothetical dengue vaccines A, B, and C were US$ 47.00 (US$ 15.70/dose), US$ 66.00 (US$ 22.00/dose), and US$89.00 (US$ 29.7/dose), respectively, when respondents in Bangladesh were presented with the hypothetical three-dose vaccines. The mean estimated WTP amount for the dengue vaccines in this study (US$15.70, US$ 22.00, and US$ 29.70 per dose) can be compared to those from the Philippines (US$ 27–32/dose);Citation25 Malaysia (US$ 28.26/dose);Citation30 Colombia (US$ 30.45);Citation29 and Vietnam (US$ 24.46/dose).Citation29 These seem comparable, given the similar GDPs of the countries in 2018: Bangladesh (274.03 billion); Philippines (330.85 billion); Malaysia (354.35 billion); Colombia (279.62 billion); and Vietnam (241.27 billion). On the other hand, the estimated WTP amount obtained in this study was lower than Thailand’s (US$ 47.26)Citation29 and higher than Indonesia’s (US$ 1.94–4).Citation27,Citation28 We should be careful, however, about comparing our findings with those of other countries, since we introduced more than one hypothetical vaccine (in terms of effectiveness) unlike the previous studies mentioned above, and our CV settings were different.
From the standpoint of public health, the three most important issues in the current discussion are dengue-related knowledge, prevention practices, and vaccine attitudes. One of the things we found in the survey is that one’s level of knowledge about dengue is positively correlated with one’s acceptance of preemptive vaccination and with one’s WTP. Thus, any governmental program would absolutely need to enlighten people that dengue is a communicable disease. In other words, it would need to strongly emphasize that dengue is not like influenza. It is a resident (human)-to-vector infection that can cause deadly epidemics. This approach would increase the chance that the introduction of a new vaccine would be socially acceptable. Also, the huge gap between the average and median WPT for Vaccine C implies that a governmental intervention is strongly needed. This could be realized, for instance, through a subsidy program to incentivize the average person to obtain the vaccination.
The increase in dengue disease in Bangladesh along with the lack of available vaccines is likely to motivate the government to construct advanced medical facilities to treat the disease. This study’s estimated WTP for treatment provides valuable information about how much money people are willing to pay for treatment, including all medical and non-medically related costs. The estimated WTP amount suggests an economic burden (not disease cost) of dengue fever that depends upon human decisions, a country’s healthcare system, and one’s social status. The government of Bangladesh is planning a feasibility study into importing Dengvaxia against the dengue virus from Sanofi.Citation51 The proportion of the private economic burden of dengue fever was highest in the low-income group and lowest in the high-income group. Thus, the authority will introduce either free/subsidize vaccines or privately available costly vaccines.
In this study, we found that 80.6% of respondents were supportive of accepting dengue fever treatment as an ex-post provision. As an alternative provision to vaccination (mutually exclusive), only 35.0% agreed to obtain treatment. Respondents who refused to accept treatment (69.4%) mostly expressed that they wanted to receive free treatment from the government or insurance companies. Another 18.6% and 10.2% were willing to naturally recover or had an insufficient budget, respectively. Nevertheless, 47.9% of respondents in this study mentioned that they were not satisfied with the current dengue treatment in their respective area.
The mean estimated WTP for treatment in this study was US$ 55.0, while the median WTP was US$ 48.0. Similar to vaccine cases, the estimated WTP was associated with an individual`s social-demographic characteristics, dengue knowledge, prevention practices, and vaccine attitudes. Those with higher education levels, student or pensioner status, upper income status, a residence in the capital, and previous dengue experience expressed that they would pay high prices for treatment. Hence, this study sheds light on how much of the population could be helped if the government were to subsidize dengue treatment costs and how decision-makers might allocate subsidies across different socio-demographic groups. Moreover, this study also provides manufacturers and private medical clinics with a more comprehensive picture of people`s perceptions of dengue fever treatment.
An interesting finding is that the most favored among the four provisions was Treatment T. This can be explained as follows. People recognize that a vaccination is a preemptive provision, while a treatment is an ex-post one. Thus, people may want to avoid committing to a vaccination because of its cost, risks, and inconvenience. However, people also perceive the threat posed by dengue fever, and if only one of the four preferences is allowed (see Figure SI4), they will opt for the best way to protect themselves, which is Vaccine C. In contrast, if they are allowed to choose any of the four options, they do not choose preemptive vaccinations but opt for the ex-post treatment instead. Ex-post treatment incurs the burden of cost, risk, and cumbersomeness only when a person is infected, which is not a guaranteed outcome. Therefore, people might prefer to delay the timing of their decision, which allows for the possibility of avoiding any cost burden until the end of the epidemic season. An additional explanation to be noted is that people might assume, over the course of the questionnaire, that ex-post treatment is not an either-or provision (in the way that committing to a vaccination is), but that it is rather inevitable and is a provision with no alternatives. If this is the case, most people would respond that they “favor” treatment vis-à-vis vaccinations. If this is the case, it is worth questioning whether the results for treatment should be treated in the same manner as those for preemptive vaccinations.
Several limitations of this study should be acknowledged. First, our study did not consider any specific dengue vaccines because no dengue vaccine is officially available in Bangladesh. This study was designed to explore demand as a WTP value for three hypothetical vaccines. Second, the participants were randomly recruited using social connection; the heterogeneous sampling from the population may have resulted in under or overrepresentation of certain groups. Also, the representation of confirmed cases was relatively low that could be considered as a limitation. Third, due to the retrospective data collection scheme, there could be some recall bias. Finally, it is also possible that some participants may have overvalued or biased due to massive media coverage and pro-or anti-vaccine sentiment.
First of all, as a general tendency, the decrease in vaccination coverage that takes place with increasing cost is fully shared by both the field survey and the model predictions, which is not surprising. Secondly and more importantly, we must note that the tendency suggested by the field survey that higher vaccination coverage would be associated with either higher or lower vaccine effectiveness cannot be reproduced in either of the two models. Interestingly, though, the first model considers repeating epidemic seasons and shows a higher (lower) vaccination coverage with lower (higher) effectiveness, whereas the second one considers just a single season, and the time-evolution shows the inverse tendency. It suggests a lower (higher) vaccination coverage with a lower (higher) effectiveness. One justification for this is that the setting of repeating seasons in the first model allows individuals to learn about the best strategy after reflecting on what happened in the previous season. Thus, when the vaccination becomes more reliable, people have more faith in their ability to free ride on herd immunity while incurring no vaccination costs, as a result of a strong social dilemma situation. On the other hand, in the context of the dynamics of one single season, people behave in a more myopic way, not directly considering the possibility of free riding and instead focusing on how many people are being infected and vaccinated right now. To this end, they favor committing to vaccination with an increase in its reliability.
Obviously, the respondents in the present survey were asked about their own actions and preferences irrespective of what others are doing. Thus, these respondents could not have imagined the possibility of free riding on herd immunity, at all. Also, we should note that the reasons for the relatively higher vaccination coverages being observed in our survey’s higher and lower effectiveness settings differ from the reasons for the higher vaccination coverages predicted in panel (b) and (c). More importantly, any of the previous models (relying on an analytical approach) presumed a homogeneous population approaching a threshold of obtaining the vaccination, despite the fact that noise effects somehow reproduced individual variance. The reality might be quite different from what is suggested by the current survey results, which sheds some light on directions for forthcoming studies.
Conclusion
In this study, we aimed to quantitively estimate people`s preferences and the mean WTP amount for dengue vaccines. We conducted an interview-based questionnaire survey and employed a contingent valuation method with a bidding game approach. Our statistical analyzes suggest that higher WTP amounts were associated with respondents’ social characteristics and knowledge about dengue. Additionally, this study sheds light on some private economic benefits as well as people’s perceptions of dengue disease, vaccines, and treatments. Our results suggest that a private market for the dengue vaccine may exist in Bangladesh. Even when vaccines were costly, there was some demand for vaccines in private markets. For lower-income groups with lower WTPs, decision-makers could implement a subsidization policy along with nationwide campaigns to enlighten the population about dengue risks. Finally, our results also found some potential cost-effectiveness relationships for the vaccine WTAs compared to the results of the vaccination game models. These findings could inform the direction of new modeling efforts over the next few years.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (2.6 MB)Acknowledgments
This study was partially supported by Grant-in-Aid for Scientific Research from JSPS, Japan, KAKENHI (Grant No. JP 18K18924, JP 19KK0262), the Support Center for Advanced Telecommunications Technology (SCAT), the Research Foundation, the I-O DATA Foundation, and the Foundation for the Fusion of Science and Technology (FOST Foundation) through awards to Professor Tanimoto. We would like to express our gratitude to all of these organizations.
Supplementary materials
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2020.1796424.
References
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–07. doi:10.1038/nature12060.
- Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue’ a systematic analysis. Lancet Infect Dis. 2016;16(8):935–41. doi:10.1016/S1473-3099(16)00146-8.
- Ferrar J, Hotez PJ, Junghanss T, Kang G, Lalloo D, White NJ. Monson`s tropical infectious diseases. ELSEVIER; 2014.
- World Health Organization. Comprehensive guideline for prevention and control of dengue and dengue haemorrhagic fever. Geneva; 2011.
- WHO, Regional strategic plan for integrated neglected tropical diseases control in South-East Asia Region, 2012-2016, SEA-CD-250 (2012).
- Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760. doi:10.1371/journal.pntd.0001760.
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomized, controlled phase 2b trial. Lancet. 2012;380(9853):1559–67. doi:10.1016/S0140-6736(12)61428-7.
- Bhatia R, Dash A, Sunyoto T. Changing epidemiology of dengue in South-East Asia. WHO-SEAJPH. 2013;2(1):23–27. doi:10.4103/2224-3151.115830.
- Messina JP, Brady OJ, Scott TW. Global spread of dengue virus types: mapping the 70 years history. Trends Microbiol. 2014;22(3):138–46. doi:10.1016/j.tim.2013.12.011.
- Sharmin S, Viennet E, Glass K, Harley D. The emergence of dengue in Bangladesh: epidemiology, challenges, and future disease risk. Trans R Soc Trop Med Hyg. 2015;109(10):619–27. doi:10.1093/trstmh/trv067.
- Mutsuddy P, Jhora ST, Shamsuzzaman AKM, Kaisar SMG, Khan MNA. Dengue situation in Bangladesh: an epidemiological shift in terms of morbidity and mortality, Hindawi. CJIDMM. 2019;3516284:1–12.
- Sharmin S, Glass K, Viennet E, Harley D. Geostatistical mapping of the seasonal spread of under-reported dengue cases in Bangladesh. PLoS, Negl Trop Dis. 2018;12(11):e0006947. doi:10.1371/journal.pntd.0006947.
- DeRoeck D, Deen J, Clemens JD. Policymakers views on dengue fever/dengue haemorrhagic fever and the need for dengue vaccines in four Southeast Asian countries. Vaccine. 2003;22(1):121–29. doi:10.1016/S0264-410X(03)00533-4.
- Vannice KS, Durbin A, Hombach J. Status of vaccine research and development of vaccines for dengue. Vaccine. 2016;34(26):2934–38. doi:10.1016/j.vaccine.2015.12.073.
- Wichmann O, Vannice K, Asturias EJ, de Albuquerque Luna EJ, Longini I, Lopez AL. Live-attenuated tetravalent dengue vaccines: the needs and challenges of post-licensure evaluation of vaccine safety and effectiveness. Vaccine. 2017;35(42):5535–42. doi:10.1016/j.vaccine.2017.08.066.
- Sanofi’s dengue vaccine approved in 11 countries. Reuters. 2016. [accessed 2017 August 13].
- East S. World’s first dengue fever vaccine launched in the Philippines. CNN. [accessed 2016 October 17].
- Zachri E, Planasari S. Dengue fever vaccine available in Indonesia. WIB, [accessed 2016 October 17].
- Thomas SJ, Yoon IK. A review of Dengvaxia®: development to deployment. Hum Vacc Imm. 2019;15(10):2295–314. doi:10.1080/21645515.2019.1658503.
- Silveira LTC, Tura B, Santos M. Systematic review of dengue vaccine efficacy. BMC Infect Dis. 2019;19(750). doi:10.1186/s12879-019-4369-5.
- World Health Organization, Regional office for the Western Pacific. Dengue situation update 2020. 2020. http://iris.wpro.who.int/handle/10665.1/14461
- Dengue vaccine: WHO position paper – September 2018. Weekly Epidemiological Record. 2018 September 7;93(36):457–76.
- Tricou V, Sáez-Llorens X, Yu D, Rivera L, Jimeno J, Villarreal AC, Dato E, de Suman OS, Montenegro N, DeAntonio R, et al. Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2-17 years: a randomized, placebo-controlled, phase 2 trial. LANCET. 2020;395(10234):1434–43. doi:10.1016/S0140-6736(20)30556-0.
- Biswal S, Borja-Tabora C, Martinez Vargas L, Velásquez H, Theresa Alera M, Sierra V, Johana Rodriguez-Arenales E, Yu D, Wickramasinghe VP, Duarte Moreira E, et al. Efficacy of a tetravalent dengue vaccine in healthy children aged 4-16 years: a randomized, placebo-controlled, phase 3 trial. LANCET. 2020;395(10234):1423–33. doi:10.1016/S0140-6736(20)30414-1.
- Harapan H, Fajar JK, Sasmono RT, Kuch U. Dengue vaccine acceptance and willingness to pay. Hum Vaccin Immunother. 2017;13(4):786–90. doi:10.1080/21645515.2016.1259045.
- Palanca-Tan R. The demand for a dengue vaccine: a contingent valuation survey in Metro Manila. Vaccine. 2008;26(7):914–23. doi:10.1016/j.vaccine.2007.12.011.
- Hadsoemarto PF, Castro MC. Public acceptance and willingness-to-pay for a future dengue vaccine: a community-based survey in Bandung, Indonesia. PloS Negl Trop Dis. 2013;7(9):e2427. doi:10.1371/journal.pntd.0002427.
- Harapan H, Anwar S, Bustamam A, Radiansyah A, Angraini P, Fasli R, Salwiyadi S, Bastian RA, Oktiviyari A, Akmal I, et al. Willingness to pay for a dengue vaccine and its associated determinants in Indonesia: a community-based, cross-sectional survey in Aceh. Acta Trop. 2017;166:249–56. doi:10.1016/j.actatropica.2016.11.035.
- Lee J-S, Mogasale V, Lim JK, Carabali M, Sirivichayakul C, Anh DD, Lee K-S, Thiem VD, Limkittikul K, Tho LH, et al. A multi-country study of the household willingness-to-pay for dengue vaccines: household surveys in Vietnam, Thailand, and Colombia. PloS Negl Trop Dis. 2015;9(6):e0003810. doi:10.1371/journal.pntd.0003810.
- Godoi IP, Santos AS, Reis EA, Lemos LLP, Brandão CMR, Alvares J, Acurcio FA, Godman B, Guerra Júnior AA. Consumer willingness to pay for dengue vaccine (CYD-TDV, Dengvaxia(R)) bin Brazil; implications for future pricing considerations. Front Pharmacol. 2017;8:41. doi:10.3389/fphar.2017.00041.
- Yeo HY, Shafie AA. The acceptance and willingness to pay (WTP) for hypothetical dengue vaccine in Penang, Malaysia: a contingent valuation study. BMC Cost Eff Res Allo. 2018;16(60).
- Dhiman M, Aryal KK, Dhimal ML, Gautam I, Sing SP, Bhusal CL, Kuch U. Knowledge, attitude, and practice regarding dengue fever among the healthy population of highland and lowland communities in central Nepal. Plos One. 2014;9(7):e102028. doi:10.1371/journal.pone.0102028.
- Lam H, Ku GM, Wu D, Cheng KJG, Rivera A, Tumanan-Mendoza B, Alejandria M. Cost-effectiveness analysis of dengue vaccination in the Philippines. Int J Infect Dis. 2016;45:421. doi:10.1016/j.ijid.2016.02.897.
- Zeng W, Halasa-Rappel YA, Baurin N, Coudeville L, Shepard D. Cost-effectiveness of dengue vaccination in ten endemic countries. Vaccine. 2018;36(3):413–20. doi:10.1016/j.vaccine.2017.11.064.
- Okanurak K, Sornmani S, Mas-ngammueng R, Sitaputra P, Krachangsang S, Limsomboon J. Treatment seeking behavior of DHF patients in Thailand. Southeast Asian J Trop Med Pub Health. 1997;28:351–58.
- Tran BX, Nguyen NK, Nguyen LP, Nguyen CT, Nong VM, Nguyen LH. Preference and willingness to pay for traditional medicine services in rural ethnic minority community in Vietnam. BMC Complement Altern Med. 2016;16(1):48. doi:10.1186/s12906-016-1010-7.
- Pang J, Salim A, Lee VJ, Hibbert ML, Chia KS, Leo YS, Lye DC. Diabetes with hypertension as risk factors for adult dengue hemorrhagic fever in a predominantly dengue serotype 2 epidemic: a case control study. PLoS Negl Trop Dis. 2012;6(5):e1641. doi:10.1371/journal.pntd.0001641.
- Gibson G, Souza-Santos R, Honório NA, Pacheco AG, Moraes MO, Kubelka C, Brasil P, Cruz O, Carvalho MS. Conditions of the household and peridomicile and severe dengue: a case control in Brazil. Infect Ecol Epidemiol. 2014;4:1–7.
- Nealon J, Lim WY, Moureau A, Linus Lojikip S, Junus S, Kumar S, Nachiappan JP, Devi Sekaran S, Radigue C, Cowling BJ, et al. Feasibility of case-control and test negative designs to evaluate dengue vaccine effectiveness in Malaysia. Vaccine. 2019;37(39):5891–98. doi:10.1016/j.vaccine.2019.07.083.
- Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Hj Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373(13):1195–206. 26214039. doi:10.1056/NEJMoa1506223.
- Population & Housing Census-2011 (PDF). Bangladesh Bureau of Statistics. p. 41. Archived from the original (PDF) on 8 December 2015, [accessed December 2015 15].
- Dhaka Population. World population review. October 2018. [accessed March 2019 18].
- Kuga K, Tanimoto J. Impact of imperfect vaccination and defense against contagion on vaccination behavior in complex networks. Jstate. 2018;113402(11).
- Kabir KMA, Kuga K, Tanimoto J. Effect of information spreading to suppress the disease contagion on the epidemic vaccination game. Cha. Sol Frac. 2019;119:180–87. doi:10.1016/j.chaos.2018.12.023.
- Kabir KMA, Tanimoto J. Dynamical behaviors for vaccination can suppress infectious disease - a game theoretical approach, Cha. Sol Frac. 2019;123:229–39. doi:10.1016/j.chaos.2019.04.010.
- Lee JS, Mogasale V, Lim JK, Carabali M, Lee K-S, Sirivichayakul C, Dang DA, Palencia-Florez DC, Nguyen THA, Riewpaiboon A. A multi-country study of the economic burden of dengue fever: vietnam, Thailand, and Colombia. PLoS Negl Trop Dis. 2017;11(10):e0006037. doi:10.1371/journal.pntd.0006037.
- Harapan H, Anwar S, Setiawan AM, Sasmono R. Aceh dengue study. Dengue vaccine acceptance and associated factors in Indonesia: a community based cross-sectional survey in Aceh. Vaccine. 2016;34(32):3670–75. doi:10.1016/j.vaccine.2016.05.026.
- Palanca-Tan R. The demand for a dengue vaccine: a contingent valuation survey in Metro Manila. Vaccine. 2006;26(7):914–23. doi:10.1016/j.vaccine.2007.12.011.
- Bracho-Churio YT, Martinez-Vega RA, Rodriguez-Morales AJ, Diaz-Quijano RG, Luna-Gonzalez ML, Diaz-Quijano FA. Determinants of felt demand for dengue vaccines in the North Caribbean region of Colombia. Ann Clin Microbiol Antimicrob. 2017;16(1):38. doi:10.1186/s12941-017-0213-1.
- Nguyen LH, Tran BX, Do CD, Hoang CL, Nguyen TP, Dang TT, Thu Vu G, Tran TT, Latkin CA, Ho CS, et al. Feasibility and willingness to pay for dengue vaccine in the thread of dengue fever outbreaks in Vietnam. Patient Preference Adherence. 2018;12:1917–26. doi:10.2147/PPA.S178444.
- The daily star, Dengue Vaccine: Relief turns into worry. 2017. https://www.thedailystar.net/frontpage/dengue-vaccine-relief-turns-worry-1507933