ABSTRACT
Coronavirus disease-2019 (COVID-19) pandemic has become a global threat and death tolls are increasing worldwide. The SARS-CoV-2 though shares similarities with SARS-CoV and MERS-CoV, immunopathology of the novel virus is not understood properly. Previous reports from SARS and MERS-CoV documents that preexisting, non-neutralizing or poorly neutralizing antibodies developed as a result of vaccine or infection enhance subsequent infection, a phenomenon called as antibody-dependent enhancement (ADE). Since immunotherapy has been implicated for COVID-19 treatment and vaccine is under development, due consideration has to be provided on ADE to prevent untoward reactions. ADE mitigation strategies like the development of vaccine or immunotherapeutics targeting receptor binding motif can be designed to minimize ADE of SARS-CoV-2 since full-length protein-based approach can lead to ADE as reported in MERS-CoV. The present mini-review aims to address the phenomenon of ADE of SARS-CoV-2 through the lessons learned from SARS-CoV and MERS-CoV and ways to mitigate them so as to develop better vaccines and immunotherapeutics against SARS-CoV-2.
Introduction
Recent pandemic of coronavirus disease-2019 (COVID-19) has been a worldwide problem. COVID-19 caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), a new member of coronavirus even though it shares similarities with SARS-CoV, its pathology, immunology and other aspects of the disease are poorly understood. Similarly, there are several speculations revolving around the source of the virus, its intermediate host, spillover events and zoonotic links.Citation1 Presently, there are no anti-viral drugs and vaccines to prevent and control COVID-19 and hence, supportive therapy is being used to treat patients. The complete lockdown has been followed in several countries to control the rapid spread of SARS-CoV-2. Social distancing, good personal hygiene are recommended to prevent the further spread of SARS-CoV-2 in the community. Symptoms of COVID-19 range from fever, cough, sneezing, dyspnea, headache, myalgia, loss of taste and loss of sense of smell. Asymptomatic cases are higher, hence controlling the spread of the virus becomes a major problem.Citation1,Citation2
The morbidity and mortality rates are increasing day by day worldwide warranting an early development of vaccines and immunotherapies to protect humans from this dreadful disease. In the present situation, plasma therapy seems to be a promising option for the treatment of ill patients. Plasma from convalescent patients possesses a higher titer of antibodies that can be used as prophylactic or as therapeutic treatment for COVID-19. Few reports show promising results on the use of plasma therapy to treat COVID-19 patients but recommend use in the early stage of infection as the end-stage treatment may not prevent mortality.Citation3,Citation4 Use of plasma therapy in patients at an early stage not only prevents infection but also prevents viral shedding.Citation4 Considering the usefulness of plasma therapy and that there are over 10,00,000 recovered patients worldwide who could serve as plasma donors, there is a possibility to curtail this disease employing this therapy.
Vaccines against SARS-CoV-2 will help to prevent the naive population from acquiring COVID-19 disease. Several vaccine platforms such as subunit vaccine, DNA vaccine, mRNA vaccine, and others are under consideration for the development of a safe and protective vaccine against COVID-19. In the near future, there will be an array of vaccines lined up for clinical phase trials since several research groups are working on developing the COVID-19 vaccine.Citation5 To develop a better vaccine and immunotherapeutic agents for the prevention and control of COVID-19, an in-depth understanding of the molecular biology and immunopathology of SARS-CoV-2 is essential. One such important consideration is the antibody-dependent enhancement (ADE) of viruses as seen in Dengue fever.Citation6 ADE occurs due to preexisting, poorly neutralizing or non-neutralizing antibodies that interact with virions and complement components enhancing the subsequent infection.Citation7 ADE has also been documented in other coronaviruses like feline infectious peritonitis (FIP) virus, SARS-CoV and Middle East Respiratory Syndrome Coronavirus (MERS-CoV).Citation8–10 Hence, due consideration regarding ADE is necessary while designing vaccines and immunotherapeutics for SARS-CoV-2. This mini-review addresses the problems associated with ADE of viruses, lessons learned from SARS-CoV and MERS-CoV, hypothesis on ADE of SARS-CoV-2 and ways to mitigate them so as to develop better vaccines and immunotherapeutics against SARS-CoV-2.
Antibody-dependent enhancement of viral infection
Antibody-dependent enhancement is an event where preexisting, non-neutralizing or poorly neutralizing antibodies increase the subsequent viral entry into cells thereby intensifying the infection.Citation7 This is a well-documented phenomenon reported by Hawkes in the year 1964 who showed that there was an increase in infectivity of several arboviruses like Japanese encephalitis virus, West Nile virus, Murray Valley encephalitis virus, and Getah virus under in vitro conditions.Citation11 ADE can also occur due to lesser concentration or lesser affinity of neutralizing antibodies. Increasing the affinity or concentration of neutralizing antibodies can alleviate ADE while increasing the concentration of non-neutralizing antibodies can lead to ADE.Citation12 Under in vivo condition, neutralizing antibodies are defined as the antibodies that can prevent viral entry or its fusion without any additional support.Citation13 Neutralizing antibodies can block the receptor–ligand interaction of virus and simultaneously can network with immunological components leading to clearance of virus from the host.Citation14 However, when these protective antibodies enhance the pathology of the disease, the phenomenon is called ADE.Citation15 In clinical settings, this phenomenon has been reported in several important human viruses like human immunodeficiency virus (HIV), dengue virus, respiratory syncytial virus (RSV), ebola virus, zika virus, influenza virus, etc., and also in veterinary pathogens like foot-and-mouth disease virus (FMDV), porcine reproductive and respiratory syndrome virus (PRRSV), etc.Citation16 Vaccine-induced ADE has been documented in respiratory syncytial virus (RSV), where the formalin inactivated vaccine generated Th2-skewed response and mediated ADE.Citation17 Several factors like affinity, concentration, specificity and isotype of antibodies are responsible for ADE.Citation15
Role of ADE in coronavirus infection
ADE has been reported from the 1980s in feline infectious peritonitis virus (FIPV), an alphacoronavirus highly prevalent among wild and domestic cats. Cats with maternal antibody or received a vaccine against FIP succumbed to virus challenge than did the control animals.Citation8 It was reported that non-neutralizing monoclonal antibodies (mAbs) or diluted neutralizing mAbs against FIPV spike (S) protein showed enhancement of virus infection while protein A treatment of mAbs prevented enhancement under in vitro condition.Citation18 Thus, antibodies against S protein have a role in the development of ADE in FIPV infection. Further, 50% of cats passively immunized with anti-FIPV antibodies developed peritonitis when challenged with the same FIPV serotype.Citation19 This is a contrasting finding as noted in the dengue virus where ADE occurs between different serotypes.
ADE of SARS-CoV
SARS-CoV uses Angiotensin-Converting Enzyme 2 (ACE2) as the receptor for entry into the cells causing respiratory problems. Reports document that SARS-CoV infects immune cells that are devoid of ACE2 and this phenomenon was later linked to ADE.Citation20 Similar to ADE witnessed in FIPV, the higher concentration of anti-spike protein antibodies neutralized the SARS-CoV while diluted anti-spike protein antibodies increased the infectivity of SARS-CoV which is not observed with anti-nucleoprotein antibodies. Thus, spike protein antibodies have a major role in ADE of SARS-CoV.Citation9 Though ADE has been documented in SARS-CoV, studies on the role of ADE in human patients are less. Similarly, care should be provided while using mAbs as a therapeutic agent for CoV treatment. mAbs against epitopes in the receptor binding domain (RBD) in the spike protein of SARS-CoV were found to be protective while mAbs that target other epitopes can lead to ADE. mAb43-3-14 is a SARS-CoV mAb targeting the spike protein epitope at 597 − 603 amino acids and found to mediate ADE in non-human primates.Citation21 Vaccine-associated ADE has been a concern and though vaccine trials in the animal model showed protection, there was Th2 immunopathology with eosinophilic infiltration of the lungs implying hypersensitivity to components of SARS-CoV.Citation22
ADE of MERS-CoV
MERS-CoV uses dipeptidyl peptidase-4 (DPP-4) as the receptor for its entry. It was suggested to evaluate MERS-CoV vaccine targeting full-length spike protein since it has the potential to cause ADE.Citation10 A study conducted on rabbits with MERS-CoV led to the development of non-neutralizing antibodies which caused enhancement of infection during the subsequent challenge. Similarly, passive immunization of naive rabbits with serum from MERS-CoV infected rabbits led to enhanced inflammatory response.Citation23,Citation24 Since ADE has not been studied completely in MERS-CoV infection, investigations on ADE would throw light on the development of a better vaccine.Citation25 There was poor cross-protection between SARS-CoV and MERS-CoV antibodies in animal models and rather there were adverse effects.Citation23,Citation26
A recent study on MERS-CoV showed that the neutralizing antibodies (mAbs) bind to RBD similar to the receptor (DPP-4) binding and this binding causes ADE. Binding of neutralizing antibodies to RBD can cause a conformational change in S protein similar to receptor–virus interaction. A mAb of MERS-CoV, Mersmab1 binds to the RBD, stabilizes it and causes a conformational change in the spike thereby exposing the S2ʹ for proteolytic cleavage similar to DPP-4 receptor.Citation27 Similar reports were observed in SARS-CoV, where S230 (mAb specific to RBD of SARS-CoV) binds to the receptor, stabilizes the RBD and caused a conformational change to expose the S2ʹ.Citation28 Sub-neutralizing or an intermediate dose of mAb can cause ADE. The authors conclude that under in vivo condition, ADE can occur based on the affinity of mAbs and also on the concentration of the antibody. Hence, to mitigate the problem of ADE caused by neutralizing mAbs, mAbs or vaccines can be designed targeting spike protein other than RBD.Citation27
Role of ADE in SARS-CoV-2: what has been learned so far?
Presently there is no proof that ADE occurs in SARS-CoV-2 infection still there are various hypotheses as per the earlier reports of SARS and MERS-CoV and also with few in vitro studies with SARS-CoV-2. The ability of the antibody to neutralize the virus has a role in the production of ADE. Antibodies that bind with higher affinity to RBD of S protein of SARS-CoV can prevent virus-ACE2 binding thus preventing entry of the virus. Higher affinity neutralizing antibodies even at minimal concentration can protect infection and does not undergo ADE.Citation29 Recent finding documents that stronger IgG antibody response against nucleocapsid protein of COVID-19 patients showed a delay in virus clearance while there was an increase in the severity of the infection. Further, it was found that patients with weaker antibody response to nucleocapsid protein had an early clearance of SARS-CoV-2 virus. Hence, antibodies against nucleocapsid protein of SARS-CoV-2 may not be neutralizing. These findings also contributes to the hypothesis of ADE of SARS-CoV-2.Citation30
The IgG antibody bound viruses can be taken up by immune cells like monocytes, macrophages and B cells that possess Fc receptors.Citation9 This uptake increases the virus load instead of clearing the viruses thus causing ADE. ADE is mediated by Fcγ receptor mainly by CD32. A study shows that ADE of SARS-CoV does not use endosomal/lysosomal pathway as used by ACE2 during normal virus transport into the cell.Citation31 Thus, a new cell entry mechanism is utilized during ADE. The CD32a is expressed on alveolar macrophages and once IgG: virus complex interacts with this receptor, there is proinflammatory cytokine release.Citation32 There is a hypothesis that age-related COVID-19 infection may be related to the presence of a higher amount of C-reactive protein (CRP) in adults than children and CD32a is also the receptor for CRP.Citation33,Citation34
ADE in the case of SARS-CoV-2 can occur due to the priming caused by other coronaviruses, leading to development of non-neutralizing or poorly neutralizing antibodies. SARS-CoV-2 had shown 79% identity with SARS-CoV and about 50% with MERS-CoV based on whole-genome analysis while there was 87.99% identity with bat-SL-CoVZC45.Citation35 Based on the S protein amino acid sequences, there was 76% identity between SARS-CoV and SARS-CoV-2.Citation36 The similarity at the amino acid level suggests that there may be cross-reactive epitopes between the two SARS-CoVs. Spike proteins epitopes of SARS-CoV, 447–458 and 789–799 were found to be antigenic and evaluated for the development of vaccines.Citation37 SARS-CoV-2 shares 72.7% similarity with 447–458 and 100% similarity with 789–799 epitopes of SARS-CoV. Other epitopes of SARS-CoV spike protein that do not share similarity with SARS-CoV-2 may be involved in ADE phenomenon.Citation38 Though there are few similar epitopes in the spike proteins of SARS-CoV and SARS-CoV-2, there is no clear understanding regarding the effect of non-neutralizing antibodies generated against SARS-CoV to induce ADE of SARS-CoV-2. As several bat coronaviruses share more similarity with SARS-CoV-2, there may be chances that prior exposure to such viruses can elicit ADE of SARS-CoV-2.Citation38 Surveillance on the bat coronavirus infection in humans can enlighten the possibility of ADE in SARS-CoV-2 due to the priming of other coronaviruses.
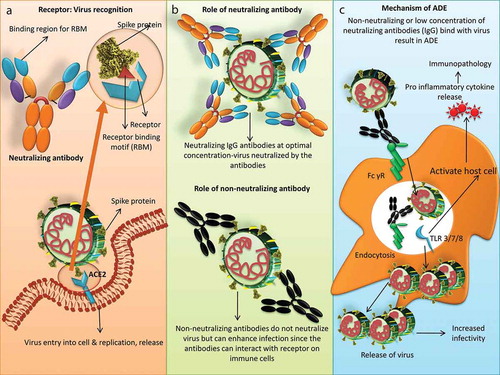
Due consideration on ADE should be provided while utilizing the SARS-CoV plasma or mAbs for COVID-19 treatment. Though SARS-CoV and SARS-CoV-2 employ the same receptor, their binding affinity differs. SARS-CoV-2 binds 10 to 20 times strongly with ACE2 when compared with SARS-CoV indicating that RBD may be different.Citation39 Polyclonal antibodies of SARS-CoV RBD were found to cross-react and prevent the entry of SARS-CoV-2 under in vitro condition.Citation40 Another study reported that SARS-CoV specific mAb, CR3022 could bind to RBD of SARS-CoV-2 but did not compete with ACE2 for its binding.Citation41 Hence, it was concluded that CR3022 bound with a region of the spike protein distal to the RBD recognized by ACE2.Citation42 Contrarily, recent neutralization study documents that cross-neutralization antibody response was poor between SARS-CoV and SARS-CoV-2, indicating the development of non-neutralizing antibody which may have a role in ADE.Citation43 CR3022 though binds strongly with the spike protein yet there was no neutralization of SARS-CoV-2 under in vitro condition. CR3022 should further be investigated for in vivo protection against SARS-CoV-2 in spite of its inability under in vitro condition.Citation42 SARS-CoV-2 RBD immunization in rodent model showed that there was only development of strong neutralizing antibodies against SARS-CoV-2 RBD region and not the ADE.Citation12 Further studies are required for the identification of human mAbs that could aid in the development of a cross-protective vaccine.Citation38 Cocktail of mAbs for COVID-19 treatment could also be an option that showed good response in SARS-CoV and Ebola diseases.Citation44,Citation45 depicts the role of neutralizing and non-neutralizing antibodies in developing ADE of SARS-CoV-2.
Figure 1. Role of neutralizing and non-neutralizing antibodies in viral infection and mechanism of ADE in SARS-CoV-2. (a) Spike protein of SARS-CoV-2 binds with Angiotensin-Converting Enzyme 2 (ACE2) receptor and undergoes replication. ACE2 recognizes receptor binding motif on the spike protein and the same receptor-binding motif (RBM) is recognized by antibodies. (b) Neutralizing antibodies at optimal concentration neutralizes virus while non-neutralizing antibodies can enhance infection. (c) Mechanism of ADE in SARS-CoV-2. Virus-antibody (neutralizing or non-neutralizing) complex bind to Fcγ receptor on the surface immune cells like monocytes or macrophages leading to virus entry without the use of ACE2 receptor. This leads to increased virus replication and release. Virus-antibody binding to FcγR can also induce proinflammatory response. Viral RNA in the endosomes signal through Toll-like receptor 3 (TLR3), TLR7 or TLR8 activating the host cell to release proinflammatory cytokines which leads to immunopathology
Consideration for developing vaccines and immunotherapy for SARS-CoV-2
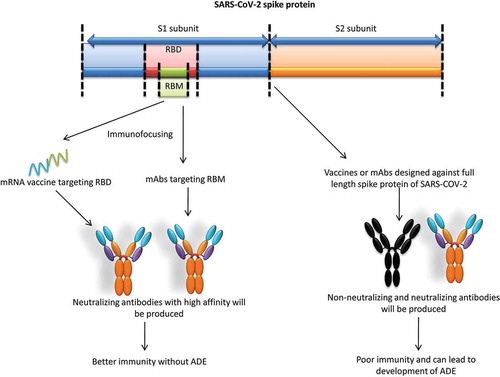
Different approaches can be used to mitigate the problem of ADE in SARS-CoV-2 infection. As the receptor-binding motifs (RBM) in RBD of SARS-CoV-2 produce neutralizing antibodies, regions other than RBM can be protected by glycosylation of these epitopes.Citation25 This can prevent the development of non-neutralizing antibodies. Another approach will be developing a vaccine or immunotherapeutic agent targeting only the neutralizing epitopes to produce a better immune response. This approach is called as immunofocusing and targeting only the epitope in the RBD to produce neutralizing antibodies against SARS-CoV-2 (). As already reported, a vaccine developed using small S1 domain of MERS-CoV elicited better immunity than the full length S protein without the problem of ADE.Citation46 Consideration can also be given on the development of a vaccine based on N protein which was also found to be conserved among SARS and MERS-CoVs.Citation47 The other approach of mRNA vaccine targeting the specific epitope can be developed to produce neutralizing antibodies and already focus on this approach has been provided by vaccine developers to produce a better vaccine.Citation48 Clear understanding of the RBD of both SARS-CoV and SARS-CoV-2 is essential before attempting to develop any cross-protective vaccine. Apart from the choice of antigen target for vaccine development, choice of adjuvant, route of immunization and age at vaccination can also play a role in the development of ADE and immunopathology.Citation46 Vaccine (formalin inactivated) against the measles virus and Respiratory syncytial virus (RSV) that stimulated Th2 response was found to induced ADE. Similarly, studies show that SARS-CoV and MERS-CoV vaccine that stimulates Th1 response protected against infection and also the immunopathology.Citation49,Citation50 Hence, vaccines inducing Th1 immunity can be designed to mitigate the problem of ADE but care should be provided because increased T cell response can also lead to immunopathology.Citation51
Figure 2. Mitigation strategies of ADE in SARS-CoV-2. Targeting full length spike protein can produce antibody-dependent enhancement (ADE) while immunofocusing or targeting the receptor-binding motif (RBM) can elicit high affinity neutralizing antibodies that can prevent ADE
Similarly, mAbs can be engineered to precision, targeting only the neutralizing epitope thereby reducing the possibility of ADE and hence protecting the host from COVID-19. Engineering antibodies to prevent interaction with FcγRs on immune cells can abolish ADE. Methods like mutation in the Fc region can block the interaction of anti-dengue virus antibodies with immune cells.Citation7 Another approach is using nanobodies or single-domain antibodies that contain only one variable domain (VHH, heavy chain variable region) instead of the usual two variable domains as they lack the light chain.Citation52 Nanobodies have various advantages like ease of synthesis, higher target affinity, better tissue penetration, reaches the target site easily due to smaller size and flexibility and importantly due to lack of Fc domain, there would not be a problem of ADE.Citation53,Citation54 Due to the lack of Fc region half-life of these antibodies will be less requiring multiple doses. Nanobodies can further be engineered as per the need and its cell-penetrating ability can be improved by linking these antibodies with cell-penetrating peptides.Citation55 Hence, apart from the choice of the epitope for developing mAbs, the platform of mAb synthesis should also be considered to generate safe and protective therapy without the problem of ADE. Technologies like high thorough put peptide scanning approach, hydrogen-deuterium exchange mass spectrometry and other computational approaches can be employed to identify epitopes associated with ADE and epitopes without ADE can be selected for designing vaccines or mAbs. On a cautionary note, identified epitopes devoid of ADE may not be immunogenic thus can fail in the prime function of protection against infection.Citation56
Conclusions
In the present context, there is no clear understanding of the SARS-CoV-2 virus, starting from the intermediate host to its prevention measures. Hence, the role of ADE in SARS-CoV-2 is not clear but earlier reports on other coronaviruses caution the complications associated with ADE. Hence, due care should be provided before developing vaccine and mAbs for prophylaxis and treatment of COVID-19. In order to achieve a protective and safe vaccine or immunotherapeutic agent, a clear understanding of COVID-19 dynamics is essential so that targets can be selected appropriately. Studies need to be focused on the immunopathology of SARS-CoV-2 to predict the effect of antibodies on subsequent infection and also to develop a universal coronavirus vaccine. To study the immunopathology, the experimental animal model for SARS-CoV-2 needs to be identified and further studies can elucidate the role of ADE. Strategies like targeting RBM of S protein or targeting other proteins that can elicit high affinity neutralizing antibodies can be used for the development of vaccine or immunotherapeutics so as to minimize ADE. To summarize, an immediate but measured approach is essential for the development of vaccines and immunotherapeutics that can safely prevent COVID-19 without untoward problems like ADE.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020:1–7. doi:https://doi.org/10.1080/21645515.2020.1735227.
- Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020. doi:https://doi.org/10.1001/jama.2020.2565.
- Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020;24(1):91. doi:https://doi.org/10.1186/s13054-020-2818-6.
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–60. doi:https://doi.org/10.1038/s41569-020-0360-5.
- Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JI, Gutierrez RA, Gwee SXW, Chua PEY, Yang Q, et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):pii: E623. doi:https://doi.org/10.3390/jcm9030623.
- Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358(6365):929–32. doi:https://doi.org/10.1126/science.aan6836.
- Khandia R, Munjal A, Dhama K, Karthik K, Tiwari R, Malik YS, Singh RK, Chaicumpa W. Modulation of dengue/zika virus pathogenicity by antibody-dependent enhancement and strategies to protect against enhancement in zika virus infection. Front Immunol. 2018;9:597. doi:https://doi.org/10.3389/fimmu.2018.00597.
- Vennema H, de Groot RJ, Harbour DA, Dalderup M, Gruffydd-Jones T, Horzinek MC. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J Virol. 1990;64(3):1407–09. doi:https://doi.org/10.1128/JVI.64.3.1407-1409.1990.
- Wang SF, Tseng SP, Yen CH, Yang JY, Tsao CH, Shen CW, Chen KH, Liu FT, Liu WT, Chen YM, et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451:208–14. doi:https://doi.org/10.1016/j.bbrc.2014.07.090.
- Du L, Tai W, Zhou Y, Jiang S. Vaccines for the prevention against the threat of MERS-CoV. Expert Rev Vaccines. 2016;15(9):1123–34. doi:https://doi.org/10.1586/14760584.2016.1167603.
- Hawkes RA. Enhancement of the infectivity of arboviruses by specific antisera produced in domestic fowls. Aust J Exp Bio Med Sci. 1964;42:465–82. doi:https://doi.org/10.1038/icb.1964.44.
- Quinlan BD, Mou H, Zhang L, Guo Y, He W, Ojha A, Parcells MS, Luo G, Li W, Zhong G, et al. The SARS-CoV-2 receptor-binding domain elicits a potent neutralizing response without antibody-dependent enhancement. bioRxiv Preprint. 2020. doi:https://doi.org/10.1101/2020.04.10.036418.
- DiLillo DJ, Palese P, Wilson PC, Ravetch JV. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest. 2016;126:605–10. doi:https://doi.org/10.1172/JCI84428.
- Yasui F, Kohara M, Kitabatake M, Nishiwaki T, Fujii H, Tateno C, Yoneda M, Morita K, Matsushima K, Koyasu S, et al. Phagocytic cells contribute to the antibody-mediated elimination of pulmonary-infected SARS coronavirus. Virology. 2014;454:157–68. doi:https://doi.org/10.1016/j.virol.2014.02.005.
- Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. 2020. doi:https://doi.org/10.1038/s41577-020-0321-6.
- Takada A, Kawaoka Y. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev Med Virol. 2003;13(6):387–98. doi:https://doi.org/10.1002/rmv.405.
- Ruckwardt TJ, Morabito KM, Graham BS. Immunological lessons from respiratory syncytial virus vaccine development. Immunity. 2019;51(3):429–42. doi:https://doi.org/10.1016/j.immuni.2019.08.007.
- Hohdatsu T, Nakamura M, Ishizuka Y, Yamada H, Koyama H. A study on the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection in feline macrophages by monoclonal antibodies. Arch Virol. 1991;120(3–4):207–17. doi:https://doi.org/10.1007/BF01310476.
- Takano T, Yamada S, Doki T, Hohdatsu T. Pathogenesis of oral type I feline infectious peritonitis virus (FIPV) infection: antibody-dependent enhancement infection of cats with type I FIPV via the oral route. J Vet Med Sci. 2019;81(6):911–15. doi:https://doi.org/10.1292/jvms.18-0702.
- Taylor A, Foo SS, Bruzzone R, Dinh LV, King NJ, Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015;268:340–64. doi:https://doi.org/10.1111/imr.12367.
- Wang Q, Zhang L, Kuwahara K, Li L, Liu Z, Li T, Zhu H, Liu J, Xu Y, Xie J, et al. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis. 2016;2(5):361–76. doi:https://doi.org/10.1021/acsinfecdis.6b00006.
- Tseng CT, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, Peters CJ, Couch RB. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE. 2012;7:e35421. doi:https://doi.org/10.1371/journal.pone.0035421.
- Houser KV, Broadbent AJ, Gretebeck L, Vogel L, Lamirande EW, Sutton T, Bock KW, Minai M, Orandle M, Moore IN, et al. Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody. PLoS Pathog. 2017;13:e1006565. doi:https://doi.org/10.1371/journal.ppat.1006565.
- Smatti MK, Al Thani AA, Yassine HM. Viral-induced enhanced disease illness. Front Microbiol. 2018;9:2991. doi:https://doi.org/10.3389/fmicb.2018.02991.
- Yong CY, Ong HK, Yeap SK, Ho KL, Tan WS. Recent advances in the vaccine development against middle east respiratory syndrome-coronavirus. Front Microbiol. 2019;10:1781. doi:https://doi.org/10.3389/fmicb.2019.01781.
- Padron-Regalado E. Vaccines for SARS-CoV-2: lessons from other coronavirus strains. Infect Dis Ther. 2020. doi:https://doi.org/10.1007/s40121-020-00300-x.
- Wan Y, Shang J, Sun S, Tai W, Chen J, Geng Q, He L, Chen Y, Wu J, Shi Z, et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol. 2020;94:pii: e02015-19.
- Walls AC, Xiong X, Park YJ, Tortorici MA, Snijder J, Quispe J, Cameroni E, Gopal R, Dai M, Lanzavecchia A, et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176(5):1026–1039e15. doi:https://doi.org/10.1016/j.cell.2018.12.028.
- Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe. 2008;4:229–38. doi:https://doi.org/10.1016/j.chom.2008.08.004.
- Tan W, Lu Y, Zhang J, Wang J, Dan Y, Tan Z, He X, Qian X, Sun Q, Hu Q, et al. Viral kinetics and antibody responses in patients with COVID-19. medRxiv. 2020. doi:https://doi.org/10.1101/2020.03.24.20042382.
- Jaume M, Yip MS, Cheung CY, Leung HL, Li PH, Kien F, Dutry I, Callendret B, Escriou N, Altmeyer R, et al. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J. Virol. 2011;85:10582–97. doi:https://doi.org/10.1128/JVI.00671-11.
- Anania JC, Chenoweth AM, Wines BD, Hogarth PM. The human FcγRII (CD32) family of leukocyte FcR in health and disease. Front Immunol. 2019;10:464. doi:https://doi.org/10.3389/fimmu.2019.00464.
- Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The major receptor for C-reactive protein on leukocytes is fcgamma receptor II. J Exp Med. 1999;190:585–90. doi:https://doi.org/10.1084/jem.190.4.585.
- Kadkhoda K. COVID-19: an immunopathological view. mSphere. 2020;5(2):pii: e00344-20. doi:https://doi.org/10.1128/mSphere.00344-20.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74. doi:https://doi.org/10.1016/S0140-6736(20)30251-8.
- Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12(3):pii: E254. doi:https://doi.org/10.3390/v12030254.
- Hua R, Zhou Y, Wang Y, Hua Y, Tong G. Identification of two antigenic epitopes on SARS-CoV spike protein. Biochem Biophys Res Commun. 2004;319:929–35. doi:https://doi.org/10.1016/j.bbrc.2004.05.066.
- Tetro JA. Is COVID-19 receiving ADE from other coronaviruses? Microbes and Infection. 2020;22(2):72–73. doi:https://doi.org/10.1016/j.micinf.2020.02.006.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020. doi:https://doi.org/10.1126/science.abb2507.
- Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613–20. doi:https://doi.org/10.1038/s41423-020-0400-4.
- Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, Lu L, Jiang S, Yang Z, Wu Y, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9(1), 382–85. DOI: https://doi.org/10.1080/22221751.2020.1729069.
- Yuan M, Wu NC, Zhu X, Lee CD, So RTY, Lv H, Mok CKP, Wilson IA. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368(6491):630-33.
- Lv H, Wu NC, Tsang OT, Yuan M, Perera RAPM, Leung WS, So RTY, Chan JMC, Yip GK, Chik TSH Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. Cell Rep. 2020;31(9):107725. doi:https://doi.org/10.1016/j.celrep.2020.107725.
- Elshabrawy HA, Coughlin MM, Baker SC, Prabhakar BS. Human monoclonal antibodies against highly conserved HR1 and HR2 domains of the SARS-CoV spike protein are more broadly neutralizing. PLoS One. 2012;7:e50366. doi:https://doi.org/10.1371/journal.pone.0050366.
- PREVAIL II Writing Group, Multi-National PREVAIL II Study Team,Davey RT Jr, Dodd L, Proschan MA, Neaton J, Neuhaus Nordwall J, Koopmeiners JS, Beigel J, Tierney J, et al. A randomized, controlled trial of ZMapp for ebola virus infection. N Engl J Med. 2016;375:1448–56.
- Du L, Tai W, Yang Y, Zhao G, Zhu Q, Sun S, Liu C, Tao X, Tseng CK, Perlman S, et al. Introduction of neutralizing immunogenicity index to the rational design of MERS coronavirus subunit vaccines. Nat. Commun. 2016;7:13473. doi:https://doi.org/10.1038/ncomms13473.
- Agnihothram S, Gopal R, Yount BL, Donaldson EF, Menachery VD, Graham RL, Scobey TD, Gralinski LE, Denison MR, Zambon M, et al. Evaluation of serologic and antigenic relationships between Middle Eastern respiratory syndrome coronavirus and other coronaviruses to develop vaccine platforms for the rapid response to emerging coronaviruses. J Infect Dis. 2013;209(7):995–1006. doi:https://doi.org/10.1093/infdis/jit609.
- Peeples L. News Feature: avoiding pitfalls in the pursuit of a COVID-19 vaccine. Proc Natl Acad Sci U S A. 2020;117(15):8218–21. doi:https://doi.org/10.1073/pnas.2005456117.
- Li CK, Wu H, Yan H, Ma S, Wang L, Zhang M, Tang X, Temperton NJ, Weiss RA, Brenchley JM, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181(8):5490–500. doi:https://doi.org/10.4049/jimmunol.181.8.5490.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi:https://doi.org/10.12932/AP-200220-0772.
- de Alwis R, Chen S, Gan ES, Ooi EE. Impact of immune enhancement on Covid-19 polyclonal hyperimmune globulin therapy and vaccine development. EBioMedicine. 2020;55:102768. doi:https://doi.org/10.1016/j.ebiom.2020.102768.
- Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–48. doi:https://doi.org/10.1038/363446a0.
- Wilken L, McPherson A. Application of camelid heavy-chain variable domains (VHHs) in prevention and treatment of bacterial and viral infections. Int. Rev. Immunol. 2018;37:69–76. doi:https://doi.org/10.1080/08830185.2017.1397657.
- Chi X, Liu X, Wang C, Zhang X, Ren L, Jin Q, Wang J, Yang W Humanized single domain antibodies neutralize SARS-CoV-2 by targeting spike receptor binding domain. 2020. bioRxiv 2020.04.14.042010; doi: https://doi.org/10.1101/2020.04.14.042010.
- Glab-Ampai K, Chulanetra M, Malik AA, Juntadech T, Thanongsaksrikul J, Srimanote P, Thueng-In K, Sookrung N, Tongtawe P, Chaicumpa W. Human single chain-transbodies that bound to domain-I of non-structural protein 5A (NS5A) of hepatitis C virus. Sci Rep. 2017;7(1):15042. doi:https://doi.org/10.1038/s41598-017-14886-9.
- Eroshenko N, Gill T, Keaveney MK, Church GM, Trevejo JM, Rajaniemi H. Implications of antibody-dependent enhancement of infection for SARS-CoV-2 countermeasures. Nat Biotechnol. 2020 [published online ahead of print, Jun 5];38(7):789–91. doi:https://doi.org/10.1038/s41587-020-0577-1.
