ABSTRACT
Introduction
Globally, pneumococcal disease represents a significant burden. South Korea implemented the 7-valent pneumococcal conjugate vaccine (PCV7) in 2003, replaced with the 10-valent (PCV10) and 13-valent (PCV13) vaccine in 2010. In 2014, both vaccines were introduced in the national immunization program (NIP) for infants with 3 primary doses and one booster dose We performed a cost-effectiveness evaluation to elucidate which vaccine may be expected to provide greater impact if included in a NIP.
Methodology
Using an established model, we estimated the impact of introducing either PCV13 or PCV10 into the South Korean NIP in 2015. Vaccine impact was based on historic observed impact of PCV13 from 2010 to 2015 in Korea given high uptake of PCV13, and PCV10 impact was estimated based on experiences in countries using PCV10. Incidence and costs for all ages and including invasive pneumococcal disease, pneumonia, and acute otitis media were derived from the literature and Health Insurance Review and Assessment database.
Results
In the base-case, over 5-years PCV13 was estimated to avert 550,000 more cases of pneumococcal disease compared to PCV10, driven by broader serotype coverage and less replacement due to serotypes 3 and 19A. This translated to a cost-savings of $47.4 million USD despite PCV13’s higher cost. Sensitivity analysis found incremental cost-effectiveness ratios (ICERs) ranged from cost-saving to $7,300 USD per quality-adjusted life year (QALY).
Conclusion
A NIP using PCV13 was estimated to have a more substantial public health impact and be cost-saving compared to a program with PCV10 due to broader serotype coverage.
Introduction
Streptococcus pneumoniae (S. pneumoniae) is a gram-positive bacterium with more than 90 serotypes associated with diseases such as acute otitis media (AOM), pneumonia, and invasive pneumococcal diseases (IPD), such as bacteremia and meningitis. Although IPD is more severe and has a greater chance of leading to mortality, pneumonia and AOM represent a significant portion of the burden of disease and associated medical costs.Citation1,Citation2
Since the early 2000s, pneumococcal conjugate vaccines (PCVs) containing 7 (PCV7, Prevnar/Prevenar®, Wyeth Lederle Vaccines S.A.), 10 (PCV10, Synflorix®, GlaxoSmithKline Biologicals S.A.), and 13 serotypes (PCV13, Prevnar 13®, Wyeth/Pfizer Vaccines) have been developed and implemented around the world for use in routine infant immunization programs. PCV10 contains antigens for original seven serotypes in PCV7 (4, 6B, 9 V, 14, 18 C, 19 F, and 23 F) plus provides protection against 1, 5 and 7 F, while PCV13 contains the serotypes in PCV10 plus serotypes 3, 6A, and 19A (Supplementary Material S1). The dissemination and implementation of these vaccines into national immunization programs (NIPs) have substantially reduced the burden of vaccine-type pneumococcal disease in both vaccinated children, as well as adults from their impact on reducing transmission of pneumococcal carriage.Citation3,Citation4
The prevalence of serotypes causing diseases varies over time, regions and age. In South Korea, from 1996 to 2005, S. pneumoniae was the most common cause of invasive bacterial disease in children aged 3 months to 4 years.Citation5 PCV7 was first approved in June 2002 in South Korea and was used voluntarily in the private sector in a 3 + 1 schedule with 3 priming doses in the first year of life and a booster at one year. Before and during the private use of PCV7, IPD isolates from numerous hospitals in Korea from 1996 to 2008, serotypes 19 F (9.8%), 23 F (8.3%), 19A (7.8%), 6A (7.5%), 3 (7.3%), 9 V (6.5%), 6B (6.2%), 14 (4.9%), 1 (3.9%), 11A (3.9%) and 4 (3.1%) represented 69.2% of all isolates.Citation6
After this private use, from 2006 to 2010 invasive isolates from 8 centers found a reduction in PCV7 serotypes isolates (4, 6B, 9 V, 14, 18 C, 19 F, 23 F, and cross-reactive 6A) from 62.5% to 21.4%.Citation7 Similarly, there was an increase in disease caused by non-vaccine serotypes 3, 6A, and 19A from 18.8% to 42.9%.Citation7
In March 2010, PCV10 and PCV13 were approved in Korea and gradually replaced the PCV7 also as an optional use in private sector. After this introduction, IPD cases collected from January 2011 to December 2013 across 25 hospitals in Korea found that the proportion of serotype 19A decreased from 37.5% in 2011 to 22.2% of all isolates in 2013.Citation8 Nasopharyngeal aspirates from children diagnosed with AOM from seven centers reported serotype 19A (22.4%), serogroups 11 (14.7%), and 15 (13.5%) as the most common S. pneumoniae serotypes in 2011 to 2012,Citation7,Citation9 and nasopharyngeal aspirates from infants and children with respiratory symptoms from a single center reported serotypes 19A (14.0%), 23A (12.8%), 15B/C (10.7%), 11A (10.1%), 6 C (7.8%), and 6A (6.3%) as the most common serotypes in 2010–2015.Citation10 Overall, PCV proportion decreased after the use of the vaccine but still 19A was reported as the most predominant serotype.Citation8 Even though PCV13 and PCV10 were only used in the private setting, a nationwide survey of immunization revealed that 83.4% of infants younger than 2 received one or more doses of PCV and 70.4% received all 4 doses.Citation10 In May 2014, the first Korean pediatric pneumococcal NIP was implemented with a 3 + 1 schedule in infants and both PCV10 and PCV13 were included as a physician choice; however, 88.6% of infants are vaccinated with PCV13.Citation11
Although a recent cost-effectiveness study by Zhang et al. estimated that PCV10 would be cost-saving compared to PCV13 in Korea,Citation12 it did not capture the full spectrum of health outcomes associated with introducing PCVs. Notably, Zhang et al. did not account for population-level indirect effects in older age groups due to reductions in carriage circulation, therefore imply that both PCVs would induce the same herd protection and have no impact on the results.
The objective of our study is to expand upon this body of evidence and estimate the clinical and economic impact of introducing a PCV13 or PCV10 NIP in Korea, while considering the full population-level impact of pneumococcal vaccination.
Methods
Model structure
We adapted a previously published epidemiologic forecasting model to estimate the impact of introducing either a PCV13 or PCV10 NIP in South Korea.Citation13,Citation14 Briefly, owing to the uncertainty of translating clinical effectiveness data into real-world population-based vaccine impact, this model leverages the real world observed trends in individual serotypes to estimate the continued impact of either PCV13 or PCV10 in the Korean NIP.
Age- and serotype-specific incidence for each of the 13-vaccine serotypes included in PCV13 are modeled independently for each vaccine under investigation (Supplementary Material S2). Non-PCV13 serotypes are grouped together given low incidence rates of individual serotypes and for model simplicity. Historical behavior of IPD is captured as the best fit trend line to observed increases or decreases of each serotype in each age group in the presence or absence of vaccine pressure. Each IPD incidence trend line is assumed to begin at the point where that serotype is being covered by vaccine in the specific country under investigation. These trend lines are then assumed to continue for each in the presence or absence of vaccine pressure when PCV13 or PCV10 is being forecasted, depending on the serotype trend lines being evaluated. Therefore, this methodology captures vaccine pressure and its observed effects in surveillance data. For example, as PCV10 has shown cross-reactivity between serotype 19 F (contained in the vaccine) and serotype 19A in case control studies,Citation15,Citation16 therefore using surveillance data would inherently capture this serotype behavior under real-world vaccine pressure. In addition, under vaccine pressure, modeled incidence rates can climb due to non-vaccine type serotype replacement. However, modeled incidence rates are capped to never surpass pre-PCV incidence levels despite some countries observing potentially higher rates of disease after serotype replacement in certain age groups.Citation17
The benefit of this methodology is that it can capture changing serotype dynamics which has not been possible in models using “steady state” frameworks in the past.Citation12,Citation18,Citation19 These “steady state” models assume that serotype epidemiology is either constant, or has reached an equilibrium, which has shown not to be the case in numerous countries using PCVs given the consistency of serotype replacement of non-vaccine serotypes. One proposed approach to capture these dynamics is a transmission dynamic model; however, these models are extremely complex requiring numerous assumptions and have been shown to produce comparable results as static models.Citation20 In contrast, the methodology used in this study also allows a more simplified approach and dataset than alternative transmission dynamic models.Citation21,Citation22
Incidence rates of noninvasive disease are based on a proportional change relative to the forecasted changes in IPD. This is based on the assumption that increases or decreases in circulating carriage would equally be likely to cause IPD as noninvasive outcomes such as pneumococcal AOM or pneumococcal pneumonia. This methodology has been used in the pneumococcal modeling space both in a static forecasting framework similar to this model,Citation23 as well as in a dynamic modeling framework.Citation24,Citation25 This methodology could be considered conservative as it does not consider the changes in all-cause pneumonia or AOM given the complexities of disentangling the etiology of disease and relative impact of each PCV on these outcomes.
Incidence rates of invasive and noninvasive disease are then used to calculate the number of cases of diseases expected with each vaccination program each year, and for each case a disutility, cost, and risk of mortality is estimated to derive the overall impact of each vaccine as well as the incremental cost-effectiveness ratio (ICER). All costs and outcomes are discounted at a rate of 5% and ICER was calculated over a 5-year time horizon in the base case.
Epidemiologic parameters
Incidence of IPD across all ages was derived from the Korean Center for Disease Control (KCDC) from 2005 through 2015 and is summarized in .Citation26 As this data is not serotype specific, incidence for each age group was weighted based on circulating serotypes reported in the literature. IPD cases in both infants and adults were assumed to be either pneumococcal meningitis, or pneumococcal bacteremia based on historical rates of disease.Citation5,Citation7,Citation8,Citation26,Citation27 Patients experiencing a case of pneumococcal meningitis also carry a risk of long-term sequelae from either epilepsy or hearing loss at a rate of 7% and 13%, respectively.Citation28,Citation29
Table 1. Epidemiologic inputs used in cost-effectiveness analysis
Incidence of pneumococcal pneumonia was also derived from KCDC reporting from 2005 through 2014 for all age groups.Citation26 In Korea the majority of pneumonia cases are treated in the inpatient setting, and given there is limited evidence on the number of outpatient cases, we assumed all pneumococcal pneumonias to be inpatient cases based on clinical practice in Korea.Citation30 Rates of all-cause AOM were derived from a recent cost-effectiveness study and were only considered for 0-<2 and 2–4 year olds.Citation12 However, because these AOM rates were assumed pre-PCV, these were adjusted from 2010 to 2015 to reflect a small decline year on year to reflect increasing PCV use. This was based on data from the United States where a 3 + 1 PCV13 schedule was used.Citation31 However, because our model only considers pneumococcal AOM, we only considered relative rates of change for those 39.7% of AOM cases are assumed to be caused by the S. pneumoniae bacteria.Citation9 No consideration was made for AOM cases caused by other pathogens for either vaccine.
Both IPD and pneumococcal pneumonia cases were assumed to carry an age-specific risk of mortality based on published case fatality rates.Citation32–36 No risk of mortality was considered for AOM cases.
Economic parameters
Costs included vaccine acquisition and direct medical costs associated with disease outcomes. Costs associated with each outcome were derived from the 2018 Health Insurance Review and Assessment (HIRA) database based on Korean Standard Classification of Diseases (KCD) code: pneumococcal bacteremia (A403), pneumococcal meningitis (G001), pneumococcal pneumonia (J13), and otitis media (H66).Citation37 For otitis media and pneumonia, outpatient cost was used for mild cases and inpatient cost for moderate and severe cases. Additional costs related to sequelae from meningitis were not included to avoid double counting. Costs are reported in US Dollars in .
Table 2. Cost, utility and case fatality parameters used in cost-effectiveness analysis
Both PCV13 and PCV10 were assumed to be given in a 3 + 1 schedule at a price of 56 USD and 48 USD per dose in 2018, respectively, based on the Korean NIP program implemented by KCDC. In addition, vaccine administration fee of 17 USD was considered.Citation38
Utility decrements were applied for each disease outcome. Annual decrements of 0.0079 and 0.0232 were assumed for bacteremia and meningitis, respectively.Citation39 Meningitis-related sequelae utility decrements were considered for neurological impairment (0.40) and hearing loss (0.20).Citation40,Citation41 AOM, and pneumococcal pneumonia carried decrements of 0.005 and 0.006, respectively.Citation42 Both costs and outcomes were discounted at a rate of 5%.
Base case assumptions
In the base case analysis, incidence of pneumococcal disease is forecasted based on circulating disease with either a PCV13 only or PCV10 only immunization program. In the base case, impact of PCV13 is estimated based on the trends observed from 2010 through 2015 in Korea while PCV10 trends are assumed based on historic trends observed in Finland and applied to the baseline Korean epidemiology.Citation17
The base case also assumes that any change in serotype epidemiology between the two vaccines would take at least one year given a sizable portion of the infant population would need to be vaccinated with one vaccine or another. Indirect effects for pneumococcal pneumonia were also included in the base case analysis and results were estimated over a 5 year time horizon as serotype trends become less reliable over longer time periods given changes in vaccination rates and antibiotic resistance.
Sensitivity analysis
Given South Korea has not used a single vaccine in its NIP, sensitivity analyses were undertaken leveraging serotype behavior trends from several different countries to test uncertainties around future serotype behavior. For PCV13, trends from the United States were used given that it also uses a 3 + 1 schedule similar to South KoreaCitation43 and the United Kingdom was used given its higher level of serotype placement observed compared to other PCV13 countries.Citation44 No country currently uses a 3 + 1 PCV10 schedule in a full NIP with robust surveillance, so we also leveraged data from the Netherlands to determine an alternative trajectory of disease with PCV10.Citation45,Citation46
Additional sensitivity analyses were undertaken varying the time horizon (10 and 20 years) and the percent of AOM that is assumed to be caused by S. pneumoniae (25%, 50%). Additionally, because South Korea does not use a single NIP, we varied the time before the serotype incidence trends would diverge in sensitivity analysis. This was varied by assuming the vaccines would produce the same benefit for 1- or 2-years post implementation given that previous cohorts of infants would have been largely protected by PCV13 given its widespread use.
One-way sensitivity analyses were also undertaken by varying the remainder of model variables by 95% Confidence intervals where available, or by ± 10% around the base case value. Probabilistic sensitivity analysis was also undertaken using a second-order Monte Carlo simulation with 1,000 iterations in which all parameters were varied. Maximum disease incidence reemergence limits, time to disease reemergence, costs, and all-cause mortality were drawn from a gamma distribution. All other parameters were drawn from a beta distribution.
Results
Base case
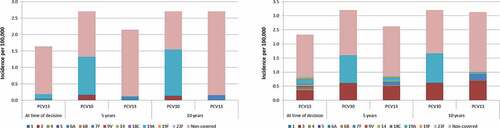
At the year of modeled vaccine introduction, 11% of all IPD cases in 0-<2 year olds (1.64 per 100,000) and 38% of IPD cases in 65+ year olds (2.33 per 100,000) were estimated to be caused by PCV13 serotypes, with the remaining disease caused by non-PCV13 serotypes (). Because vaccine-type disease and overall incidence of IPD are low in South Korea the model assumes that disease has reached somewhat of an equilibrium, allowing minimal additional reductions in vaccine-type disease. Therefore, both PCV13 and PCV10 were predicted to cause serotype replacement and have a marginal net increase of 0.51 cases and 1.06 cases of IPD per 100,000 in 0-<2 year olds 5-years following NIP implementation, respectively. These rates, however, are still lower than pre-PCV incidence, highlighting an overall reduction in disease since introduction of PCVs. The higher level of replacement with PCV10, however, was driven by serotypes 3 and 19A, which were estimated to form most of the disease burden in the PCV10 arm. Non-PCV13 type disease increased in the PCV13 arm in all age groups but net changes were less significant than in the PCV10 arm due to broader protection against serotype 3 and 19A. This was consistent in the 65+ age group where the incidence of IPD increased by 0.29 cases per 100,000 in the PCV13 arm, and by 0.87 cases per 100,000 in the PCV10 arm. In contrast to 0–2 year olds, there was a steady increase in serotype 3 in both the PCV13 and PCV10 groups.
Figure 1. Incidence of IPD today and in 5 and 10 years from now given a PCV10 and PCV13 vaccination policy in individuals aged 0 to 2 years and ≥65 years
presents the cumulative number of cases of pneumococcal disease, costs, and deaths associated with implementing either a PCV10 3 + 1 or PCV13 3 + 1 NIP over 5 years in South Korea. Based on forecasted serotype trends, model estimates determined that a PCV13 only NIP in South Korea would result in 556,379 fewer cases of pneumococcal disease compared to a PCV10 only NIP. Based on these cases, there would also be a reduction in over 2,000 pneumococcal deaths with PCV13 compared to PCV10. Despite an additional 56 USD million USD investment cost in a PCV13 only NIP, overall use of PCV13 would result in a net-savings of 47 USD million USD due to less disease cases. This resulted in a PCV13 only NIP being a cost-saving strategy compared to a PCV10 only NIP over a 5-year time horizon.
Table 3. Prospective impact of a PCV13 or PCV10 NIP over 5 years
Sensitivity analysis
In scenario analysis varying trend line assumptions (), PCV13 remained cost-saving except when Netherlands trends were used for PCV10 and United Kingdom trends were used for PCV13; however, PCV13 remained highly cost-effective at 15,210 USD per quality-adjusted life year (QALY) (<1xGDP per capita). When lengthening the model time horizon and varying the percent of AOM cases that are caused by S. pneumoniae, PCV13 remained cost-saving across all scenarios. When assuming the two vaccines would produce an equivalent effect for 2 years, the ICER increased to 7,373 USD per QALY.
Table 4. Scenario analyses of a PCV13 NIP compared with a PCV10 NIP in South Korea
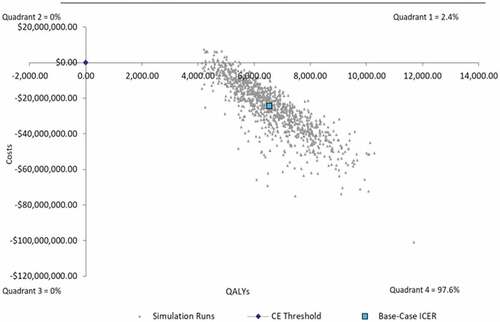
The remainder of one-way sensitivity analyses remained cost-saving when varying upper and lower bound values by 10% in one-way sensitivity analysis (). In 1,000 simulations in probabilistic sensitivity analysis around the base case, 97.6% of simulations remained cost-saving, with 2.4% of simulations remaining cost-effective under 5,000 USD per QALY ().
Figure 2. One-way sensitivity analysis: PCV13 versus PCV10 incremental cost per quality-adjusted life-year
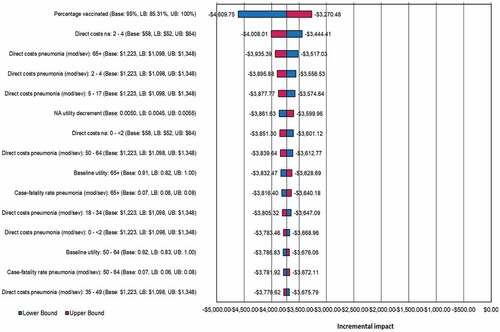
Figure 3. Probabilistic sensitivity analysis for Base Case Analysis
Discussion
The objective of this modeling exercise was to add to the existing literature on PCVs in South Korea by estimating the national impact of using either a 3 + 1 PCV13 or 3 + 1 PCV10 NIP rather than the current NIP where both vaccines are used. Our model estimated that a single source NIP with PCV13 would be cost-saving compared to a program with PCV10, preventing over 550,000 cases of pneumococcal disease and 2,000 associated deaths. Despite the higher investment cost, PCV13 was determined to be a cost-saving strategy in the base case analysis and was robust to numerous different sensitivity analyses. In one scenario where the two vaccines were assumed to produce short-term comparable effects based on the routine use of both vaccines in clinical practice in South Korea, the ICER was highest in this study at 7,373 USD per QALY. However, recent evidence from Belgium where routine practice changed from PCV13 to PCV10 found that serotype dynamics have rapidly changed, resulting in a 10 fold increase in 19A cases in 0–2 year olds; therefore, our base-case results are likely justified and may even be considered conservative.Citation47,Citation48 Results of this exercise highlight the importance of providing broad PCV coverage, which will be important to reconsider as higher valency PCVs become available.Citation49 Higher-valent PCVs may add even more clinical and economic benefit by preventing more disease burden due to additional serotypes in their formulations. Thus, increased serotype protection by the highest-valent PCV available can drive direct health-care cost-savings and improve cost-effectiveness results, which was demonstrated in this analysis. The impact of switching from PCV13 to a higher valency PCV in the NIP will need to be considered to maintain the broadest coverage considering local epidemiology both in terms of direct protection, as well as overall population impact through indirect effects. For example, local assessment of the most prevalent serotypes and a PCV’s potential to reduce the total pneumococcal disease burden which might increase without the broadest coverage due to serotype replacement.
Our findings are consistent with numerous previously published models looking at the cost-effectiveness of PCV13 versus PCV10.Citation13,Citation14,Citation18,Citation50-52 While there is a body of evidence, including a recent study in South Korea, found PCV10 to be cost-saving compared to PCV13,Citation12,Citation53-55 these analyses are driven primarily by a supposed impact of PCV10 on AOM including AOM-associated tube replacement caused by non-typeable haemophilus influenzae (NTHi). However, this benefit was based on evidence from an investigational 11-valent vaccine rather than from the PCV10 vaccine,Citation56 and there have not been any real-world population-based studies to demonstrate any statistically significant effect of PCV10 on AOM caused by NTHi. In contrast, there are several studies that have suggested PCV13 prevents AOM cases caused by NTHi given the likelihood for PCV13 to avert more severe early onset vaccine-type pneumococcal otitis that then reducing late-stage non-pneumococcal pathogens.Citation57–59 However, considering this contrasting evidence, our model conservatively did not include any benefits for non-pneumococcal pathogens.
Our study differs from the recent publication by Zhang et al. (2018) for other important reasons aside from excluding NTHi.Citation12 First, we leveraged real-world population-level surveillance data to model serotype dynamics and vaccine impact across all ages. This methodology captures nuances of vaccine introduction such as uptake, indirect effects, and serotype replacement, and by including sensitivity analyses using different experiences with both vaccines, various potential serotype trajectories could be explored. This is specifically important given the assumption made by Zhang et al. that PCV10 provides cross-protection against serotype 19A based on case-control studies undertaken in Finland and Brazil.Citation15,Citation16 However, in both countries surveillance data have since shown increases in 19A disease, which now represents a significant proportion of pneumococcal disease in both countriesCitation60,Citation61 and there have been several recent studies pointing to limited cross-protection from PCV10 against 19A.Citation62,Citation63 Furthermore, recent real-world evidence has emerged from Belgium where 18 months following a change from PCV13 to PCV10 there was a 10 fold increase in cases of 19A in children 0-<2 years olds 47. Second, our model includes the indirect effects afforded by both vaccines reducing vaccine-type disease in unvaccinated age groups as well as the impact on serotype replacement. There is a significant body of evidence demonstrating the effect of PCVs on unvaccinated age groups for both PCV10 and PCV13,Citation4,Citation64 demonstrating that the broader serotype coverage afforded by PCV13 results in less serotype replacement in older ages. Therefore, by ignoring this important impact, much of the value of pneumococcal vaccination is excluded. Finally, Zhang et al. (2018) assume that PCV13 provides 0.0% vaccine efficacy (VE) for PCV13 against disease caused by serotype 3. Like serotype 19A, our model estimates impact of PCV13 on serotype 3 based on real-world surveillance changes. In children, our model estimated that serotype 3 remained at low levels given limited observed cases in South Korea. This is consistent with a recent meta-analysis that found PCV13 to have a positive statistically significant direct effect against serotype 3.Citation65 In contrast, our model found serotype 3 disease in adults to increase in both the PCV13 and PCV10 arm, highlighting potentially limited indirect effect of PCV13 against serotype 3. This is consistent with a clinical trial comparing PCV7 and PCV13 which found no efficacy against serotype 3 carriage.Citation66
Despite the strengths of our modeling exercise, there are several important limitations. First, our model assumes that serotypes move in a constant direction based on historical behaviors. While these trend lines are based on up to 8 years of vaccine history in most cases, changes in population dynamics and antibiotic use could possibly alter the trajectories of specific serotypes. We attempted to mitigate for these uncertainties by varying trend lines from several different countries to try to capture different serotype behaviors, under which scenarios our results remained consistent; however, these results should be updated as more years of surveillance become available to reflect changes in both vaccine and non-vaccine serotypes.
A second limitation in our study is the lack of nationwide surveillance system for pneumococcal disease in South Korea. Our study relied on time-specific, hospital-based reports for serotype-specific incidence data; most of which were based on confirmed cultured cases. Therefore, our study and the incidence reports used to generate model estimates, likely underestimate the true burden of pneumococcal disease in South Korea. Further research is necessary to fully evaluate the burden of invasive and noninvasive pneumococcal disease outcomes.
Finally, our model assumes a constant proportion of AOM cases are caused by S. pneumoniae and does not make any consideration for AOM cases caused by other pathogens such as NTHi. As already discussed, evidence has suggested PCVs may reduce noninvasive disease caused by other pathogens, but this was deemed outside the scope of our study. As these vaccines are used however, the proportion of disease caused by each pathogen would change and therefore the total proportion of disease impacted by PCVs would alter over time. The model presented is limited in that it assumes the proportion of AOM and pneumonia caused by S. pneumoniae is static, which may over or underestimate the impact of PCVs. However, data have demonstrated that there is a strong correlation between changes in IPD and noninvasive pneumococcal disease outcomes,Citation67 and that there is a multiplicative relationship between these outcomes.Citation21,Citation23,Citation24 So, while the overall proportion of disease may change, our model captures the relative transmission dynamics between serotypes, so the overall conclusions should remain robust when choosing PCV13 versus PCV10. In addition, one-way sensitivity analysis found that the proportion of noninvasive disease caused by S. pneumoniae was not a variable that significantly impacted the results of our analysis ().
Some parts of the benefit of vaccination program are difficult to capture and evaluate due to lack of reliable information. One of the vaccination benefits is reduction of antibiotic-resistant pneumococcus and change of antibiotic-resistant serotypes. The vaccine efficacy change and the potential herd effect from decreased antibiotic resistance is difficult to be assessed; however, a recent systematic review found consistent reductions in antimicrobial resistance of S. pneumoniae isolates following the introduction of PCVs in NIPs.Citation68 As resistance is considered as a risk factor for infections, antibiotic-resistance reduction could have additional benefits not measured in the current analysis and this should be considered in future research on the impact of PCVs in South Korea.
Conclusion
This model represents the first population-based analysis to estimate the population-level impact of implementing either a PCV10 or PCV13 program in South Korea, taking into consideration all serotype dynamics and indirect effects. While net vaccine acquisition cost may be lower with PCV10 compared to PCV13, when implementing NIPs it is integral to consider the impact of vaccines on total vaccine-preventable disease and their value for money.
Contributors
HYK, ESK, SML, SBP, HJK, and MW conceived and designed the analysis; HYK, ESK, SML, SBP, HJK, and MW collected the data; HYK, ESK, SML, SBP, HJK, and MW analyzed and/or interpreted the results; HYK, ESK, SML, MW wrote the first draft; HYK, ESK, SML, SBP, HJK, and MW critically reviewed the content and approved the final version.
Data sharing
Data used for the analyses are available on request.
Disclosure of potential conflicts of interest
HY Kim and ES Kang are employees of Pfizer Ltd. and M Wasserman is an employee of Pfizer Inc. SB Park, SM Lee, and HJ Kim are no longer an employee of Pfizer Ltd. but were when this study was conducted.
Transparency
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplemental Material
Download MS Word (91.9 KB)Supplementary material
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2020.1796426..
Additional information
Funding
References
- Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29(18):3398–412. doi:10.1016/j.vaccine.2011.02.088.
- De Wals P, Petit G, Erickson LJ, Guay M, Tam T, Law B, Framarin A. Benefits and costs of immunization of children with pneumococcal conjugate vaccine in Canada. Vaccine. 2003;21(25–26):3757–64. doi:10.1016/S0264-410X(03)00361-X.
- Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, Lukšić I, Nair H, McAllister DA, Campbell H. Burden of Streptococcus pneumoniae and haemophilus influenzae type B disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Global Health. 2018 e744-e757;6(7):e744–e757. doi:10.1016/S2214-109X(18)30247-X.
- Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, McCarthy ND, Petrou S. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Global Health. 2017;5(1):e51–e59. doi:10.1016/S2214-109X(16)30306-0.
- Lee J-H, Cho HK, Kim K-H, Kim CH, Kim DS, Kim KN, Cha S-H, Oh SH, Hur JK, Kang JH. Etiology of invasive bacterial infections in immunocompetent children in Korea (1996-2005): a retrospective multicenter study. J Korean Med Sci. 2011;26(2):174–83. doi:10.3346/jkms.2011.26.2.174.
- Lee S, Bae S, Lee K-J, Yu J-Y, Kang Y. Changes in serotype prevalence and antimicrobial resistance among invasive Streptococcus pneumoniae isolates in Korea, 1996–2008. J Med Microbiol. 2013;62(8):1204–10. doi:10.1099/jmm.0.058164-0.
- Cho EY, Lee H, Choi EH, Kim Y-J, Eun BW, Cho YK, Kim Y-K, Jo DS, Lee HS, Lee J, et al. Serotype distribution and antibiotic resistance of Streptococcus pneumoniae isolated from invasive infections after optional use of the 7-valent conjugate vaccine in Korea, 2006–2010. Diagn Microbiol Infect Dis. 2014;78(4):481–86. doi:10.1016/j.diagmicrobio.2013.12.016.
- Cho EY, Choi EH, Kang JH, Kim K-H, Kim DS, Kim Y-J, Ahn YM, Eun BW, Oh SH, Cha S-H, et al. Early changes in the serotype distribution of invasive pneumococcal isolates from children after the introduction of extended-valent pneumococcal conjugate vaccines in Korea, 2011-2013. J Korean Med Sci. 2016;31(7):1082–88. doi:10.3346/jkms.2016.31.7.1082.
- Han SB, Kim J-H, Kang JH, Ma SH, Kim CS, Kim K-H, Kim HM, Choi YY. Recent epidemiology of Streptococcus pneumoniae in nasopharynxes of Korean children with acute otitis media. J Infect Chemother. 2017;23(3):136–41. doi:10.1016/j.jiac.2016.10.006.
- Lee JK, Yun KW, Choi EH, Kim SJ, Lee SY, Lee HJ. Changes in the serotype distribution among antibiotic resistant carriage streptococcus pneumoniae isolates in children after the introduction of the extended-valency Pneumococcal conjugate vaccine. J Korean Med Sci. 2017;32(9):1431–39. doi:10.3346/jkms.2017.32.9.1431.
- IQVIA. IQVIA national sales audit. 2018.
- Zhang X-H, Leeuwenkamp O, Oh K-B, Lee YE, Kim C-M. Cost-effectiveness analysis of infant pneumococcal vaccination with PHiD-CV in Korea. Hum Vaccin Immunother. 2018;14(1):85–94. doi:10.1080/21645515.2017.1362513.
- Wilson M, Wasserman M, Jadavi T, Postma M, Breton MC, Peloquin F, et al. Clinical and economic impact of a potential switch from 13-valent to 10-valent pneumococcal conjugate infant vaccination in Canada. Infect Dis Ther. 2018;7(3):353–71.
- Wasserman M, Palacios MG, Grajales AG, Baez/Revueltas FB, Wilson M, McDade C, Farkouh R. Modeling the sustained use of the 13-valent pneumococcal conjugate vaccine compared to switching to the 10-valent vaccine in Mexico. Hum Vaccin Immunother. 2019;15(3):560–69.
- Domingues CMAS, Verani JR, Montenegro Renoiner EI. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med. 2014;2(6):464–71. doi:10.1016/S2213-2600(14)70060-8.
- Jokinen J, Rinta-Kokko H, Siira L, Palmu AA, Virtanen MJ, Nohynek H, Virolainen-Julkunen A, Toropainen M, Nuorti JP. Impact of ten-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in Finnish children – a population-based study. PLoS One. 2015;10(3):e0120290. doi:10.1371/journal.pone.0120290.
- National Institute for Health and Welfare Incidence of invasive pneumococcal disease in Finland. 2016; https://www.thl.fi/en/web/thlfi-en/research-and-expertwork/projects-and-programmes/monitoring-the-population-effectiveness-of-pneumococcal-conjugate-vaccination-in-the-finnish-national-vaccination-programme/incidence-of-invasive-pneumococcal-disease-in-finland.
- Strutton DR, Farkouh RA, Earnshaw SR, Hwang S, Theidel U, Kontodimas S, Klok R, Papanicolaou S. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine: Germany, Greece, and The Netherlands. J Infect. 2012;64(1):54–67. doi:10.1016/j.jinf.2011.10.015.
- Kulpeng W, Leelahavarong P, Rattanavipapong W, Sornsrivichai V, Baggett HC, Meeyai A, Punpanich W, Teerawattananon Y. Cost-utility analysis of 10- and 13-valent pneumococcal conjugate vaccines: protection at what price in the Thai context? Vaccine. 2013;31(26):2839–47. doi:10.1016/j.vaccine.2013.03.047.
- Debicki D, Ferko N, Demarteau N, Gallivan S, Bauch C, Anonychuk A, Mantovani L, Capri S, Chou C-Y, Standaert B. Comparison of detailed and succinct cohort modelling approaches in a multi-regional evaluation of cervical cancer vaccination. Vaccine. 2008;26:F16–F28. doi:10.1016/j.vaccine.2008.02.040.
- Wasserman M, Lucas A, Jones D, Wilson M, Hilton B, Vyse A, Madhava H, Brogan A, Slack M, Farkouh R. Dynamic transmission modelling to address infant pneumococcal conjugate vaccine schedule modifications in the UK. Epidemiol Infect. 2018;146(14):1797–806. doi:10.1017/S095026881800198X.
- Wu DB, Chang CJ, Huang YC, Wen YW, Wu CL, Fann CS. Cost-effectiveness analysis of pneumococcal conjugate vaccine in Taiwan: a transmission dynamic modeling approach. Value Health. 2012;15(1 Suppl):S15–19. doi:10.1016/j.jval.2011.11.013.
- Thorrington D, van Rossum L, Knol M, de Melker H, Rümke H, Hak E, van Hoek AJ. Impact and cost-effectiveness of different vaccination strategies to reduce the burden of pneumococcal disease among elderly in the Netherlands. PloS One. 2018;13(2):e0192640. doi:10.1371/journal.pone.0192640.
- van Hoek AJ, Choi YH, Trotter C, Miller E, Jit M. The cost-effectiveness of a 13-valent pneumococcal conjugate vaccination for infants in England. Vaccine. 2012;30(50):7205–13. doi:10.1016/j.vaccine.2012.10.017.
- Lucas AWM, Wasserman M, Jones D, Vyse A, Slack M, Hilton B, Madhava H, Farkouh R Dynamic transmission modeling of pneumococcal conjugate vaccine and potential dosing reduction in The United Kingdom. International Society of Pneumococci and Pneumococcal Diseases 2018, Melbourne (Australia); 2018.
- Prevention KCfDCa. Epidemiology and prevention of vaccine-preventable disease. Osong 2017;255–58.
- Song JH, Jung SI, Ko KS, Kim NY, Son JS, Chang H-H, Ki HK, Oh WS, Suh JY, Peck KR. High prevalence of antimicrobial resistance among clinical streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother. 2004;48(6):2101–07. doi:10.1128/AAC.48.6.2101-2107.2004.
- Pomeroy SL, Holmes SJ, Dodge PR, Feigin RD. Seizures and other neurologic sequelae of bacterial meningitis in children. N Engl J Med. 1990;323(24):1651–57. doi:10.1056/NEJM199012133232402.
- Mclntyre PB, Berkey CS, King SM, et al.Dexamethasone as adjunctive therapy in bacterial meningitis: a meta-analysis of randomized clinical trials since 1988. Jama. 1997;278(11):925–31. doi:10.1001/jama.1997.03550110063038.
- Chong YP, Jung K-S, Lee KH, Kim M-N, Moon SM, Park S, Hur J, Kim D-M, Jeon MH, Woo JH. The bacterial etiology of community-acquired pneumonia in Korea: a nationwide prospective multicenter study. Infect Chemother. 2010;42(6):397–403. doi:10.3947/ic.2010.42.6.397.
- Marom T, Tan A, Wilkinson GS, Pierson KS, Freeman JL, Chonmaitree T. Trends in otitis media-related health care use in the United States, 2001-2011. JAMA Pediatr. 2014;168(1):68–75. doi:10.1001/jamapediatrics.2013.3924.
- Song JY, Choi JY, Lee JS, Bae I-G, Kim YK, Sohn JW, Jo YM, Choi WS, Lee J, Park KH. Clinical and economic burden of invasive pneumococcal disease in adults: a multicenter hospital-based study. BMC Infect Dis. 2013;13(1):202. doi:10.1186/1471-2334-13-202.
- Choi E-H, Lee H-J. Clinical outcome of invasive infections by penicillin-resistant streptococcus pneumoniae in Korean children. Clin Infect Dis. 1998;26(6):1346–54. doi:10.1086/516340.
- Woo JH, Kang JM, Kim YS, Shin WS, Ryu JH, et al. A prospective multicenter study of community-acquired pneumonia in adults with emphasis on bacterial etiology. Korean J Infect Dis. 2001;33(1):1–7.
- Hoshi S-I, Kondo M, Okubo I. Economic evaluation of vaccination programme of 13-valent pneumococcal conjugate vaccine to the birth cohort in Japan. Vaccine. 2013;31(25):2762–71. doi:10.1016/j.vaccine.2013.03.052.
- (KCDC). KCfDCP. A research report for pneumococcal disease burden and serotype analysis. 2007.
- Service HIRaA. Health insurance review and assessment service database (2017 data). HIRA. 2017.
- (KCDC). KCfDC. Vaccine administration fee based on regulation on the entrustment of vaccination services. Announcement No. 2018-232. 2018.
- Bennett JE, Sumner W, Downs SM, Jaffe DM. Parents’ utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med. 2000;154:43–48.
- Morrow A, De Wals P, Petit G, Guay M, Erickson LJ. The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol. 2007;18(2):121–27. doi:10.1155/2007/713576.
- Cheng AK, Niparko JK. Cost-utility of the cochlear implant in adults: a meta-analysis. Arch Otolaryngol Head Neck Surg. 1999;125(11):1214–18. doi:10.1001/archotol.125.11.1214.
- Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–14. doi:10.1016/j.vaccine.2004.05.003.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–09. doi:10.1016/S1473-3099(14)71081-3.
- Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51. doi:10.1016/S1473-3099(18)30052-5.
- Knol MJ, Berbers GA, Bootsma H, van der Ende A, Kaaijk P, et al. 7.9 Pneumococcal disease. In: The national immunisation programme in the Netherlands surveillance and developments in 2015-2016. RIVM Report 2016-0141; 2016 [Accessed 2017 Feb 26]. http://www.rivm.nl/bibliotheek/rapporten/2016-0141.pdf
- Knol MJ, de Melker HE, Sanders EAM, van der Ende A Incidence of IPD in the Netherlands up to five years after introduction of PCV10. 2016.
- Desmet S, Verhaegen J, Van Ranst M, Peetermans W, Lagrou K. Switch in a childhood pneumococcal vaccination programme from PCV13 to PCV10: a defendable approach? Lancet Infect Dis. 2018;18(8):830–31. doi:10.1016/S1473-3099(18)30346-3.
- Wasserman MD, Sings HL, Wilson MR, Postma MJ, Breton M-C, McDade C, Farkouh RA. Re-analysis of modeling a switch from a 13-valent to 10-valent pneumococcal conjugate vaccine in Canada: leveraging real-world experience from Belgium. Infect Dis Ther. 2019;8(1):1–3.
- Weinberger DM, Shapiro ED. Prevention of pneumococcal infections in adults using conjugate vaccines: no easy answers. Clin Infect Dis. 2019:50–51.
- Wu DB, Roberts C, Lee VW, Hong L-W, Tan KK, Mak V, Lee KKC. Cost-effectiveness analysis of infant universal routine pneumococcal vaccination in Malaysia and Hong Kong. Hum Vaccin Immunother. 2016;12(2):403–16. doi:10.1080/21645515.2015.1067351.
- Suaya JA, Ohno T, Hilton B, Farkouh R, Hagiwara Y, Isturiz R, Arguedas A. Cost-effectiveness analysis of 13-valent versus 10-valent pneumococcal conjugate vaccines as part of routine infant pneumococcal vaccination program in Japan. Jpn J Pediatr. 2015;68(6):1197–217.
- Klok RM, Lindkvist RM, Ekelund M, Farkouh RA, Strutton DR. Cost-effectiveness of a 10- versus 13-valent pneumococcal conjugate vaccine in Denmark and Sweden. Clin Ther. 2013;35(2):119–34. doi:10.1016/j.clinthera.2012.12.006.
- Shiragami M, Mizukami A, Leeuwenkamp O, Mrkvan T, Delgleize E, Kurono Y, Iwata S. Cost-effectiveness evaluation of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein Dconjugate vaccine and 13-valent pneumococcal vaccine in Japanese children. Infect Dis Ther. 2015;4(1):93–112.
- Delgleize E, Leeuwenkamp O, Theodorou E, Van de Velde N. Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: a comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model. BMJ Open. 2016;6(11):e010776. doi:10.1136/bmjopen-2015-010776.
- Castiglia P, Pradelli L, Castagna S, Freguglia V, Palù G, Esposito S. Overall effectiveness of pneumococcal conjugate vaccines: an economic analysis of PHiD-CV and PCV-13 in the immunization of infants in Italy. Hum Vaccin Immunother. 2017;13:2307–15.
- Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both streptococcus pneumoniae and non-typable haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367(9512):740–48. doi:10.1016/S0140-6736(06)68304-9.
- Lewnard JA, Givon-Lavi N, Weinberger DM, Lipsitch M, Dagan R. Pan-serotype reduction in progression of streptococcus pneumoniae to otitis media after rollout of pneumococcal conjugate vaccines. Clin Infect Dis. 2017;65(11):1853–61. doi:10.1093/cid/cix673.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Impact of widespread introduction of pneumococcal conjugate vaccines on pneumococcal and nonpneumococcal otitis media. Clin Infect Dis. 2016;63(5):611–18. doi:10.1093/cid/ciw347.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Near-elimination of otitis media caused by 13-valent pneumococcal conjugate vaccine (PCV) serotypes in southern Israel shortly after sequential introduction of 7-valent/13-valent PCV. Clin Infect Dis. 2014;59(12):1724–32. doi:10.1093/cid/ciu683.
- Isturiz R, Sings HL, Hilton B, Arguedas A, Reinert -R-R, Jodar L. Streptococcus pneumoniae serotype 19A: worldwide epidemiology. Expert Rev Vaccines. 2017;16(10):1007–27. doi:10.1080/14760584.2017.1362339.
- Agudelo CI, DeAntonio R, Castañeda E. Streptococcus pneumoniae serotype 19A in Latin America and the Caribbean 2010–2015: a systematic review and a time series analysis. Vaccine. 2018;36(32):4861–74. doi:10.1016/j.vaccine.2018.06.068.
- Rinta-Kokko H, Palmu AA, Auranen K, Nuorti JP, Toropainen M, Siira L, Virtanen MJ, Nohynek H, Jokinen J. Long-term impact of 10-valent pneumococcal conjugate vaccination on invasive pneumococcal disease among children in Finland. Vaccine. 2018;36(15):1934–40. doi:10.1016/j.vaccine.2018.03.001.
- Richter L, Schmid D, Kanitz EE, Zwazl I, Pöllabauer E, Jasinska J, Burgmann H, Kundi M, Wiedermann U. Invasive pneumococcal diseases in children and adults before and after introduction of the 10-valent pneumococcal conjugate vaccine into the Austrian national immunization program. PloS One. 2019;14(1):e0210081. doi:10.1371/journal.pone.0210081.
- Hanquet G, Krizova P, Valentiner-Branth P, Ladhani SN, Nuorti JP, Lepoutre A, et al. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax. 2019;74(5):473–82.
- Sings HL, De Wals P, Gessner BD, Isturiz R, Laferriere C, McLaughlin JM, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against invasive disease caused by serotype 3 in children: a systematic review and meta-analysis of observational studies. Clin Infect Dis. 2019;68(12):2135–43.
- Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013;57(7):952–62. doi:10.1093/cid/cit428.
- Thorrington D, Andrews N, Stowe J, Miller E, van Hoek AJ. Elucidating the impact of the pneumococcal conjugate vaccine programme on pneumonia, sepsis and otitis media hospital admissions in England using a composite control. BMC Med. 2018;16(1):13. doi:10.1186/s12916-018-1004-z.
- Tin Tin Htar M, van den Biggelaar AH, Sings H, Ferreira G, Moffatt M, Hall-Murray C, Verstraeten T, Gessner BD, Schmitt H-J, Jodar L. The impact of routine childhood immunisation with higher-valent pneumococcal conjugate vaccines on antimicrobial-resistant pneumococcal diseases and carriage: a systematic literature review. Expert Rev Vaccines. 2019;18(10):1069–89. doi:10.1080/14760584.2019.1676155.
