ABSTRACT
Children with onco-hematological diseases are at increased risk of infection. However, this risk can in part be controlled or reduced using currently available vaccines. Despite available evidence, in patients diagnosed with a hematological or oncological disease the vaccination schedule is often inappropriately discontinued. In this study we evaluated whether the diagnosis of an oncological or hematological disease is a determinant of noncompliance with recommended vaccinations.
The study was carried out between March and April 2019. The population was composed of a convenience sample of 228 children cared for in the Pediatric Oncology Department and Pediatric Hematology Department of the Policlinico Giovanni XXIII Pediatric Hospital (Bari, Italy) from 2005 to 2015. Information on the immunization status of the patients was obtained from the Apulia regional immunization database (GIAVA). A post-diagnosis adherence score was calculated.
The vaccination coverage was 87.7% for the DTaP-IPV-Hep B-Hib vaccine (3 doses), 68.7% for the pneumococcal vaccine (3 doses), 75.8% for the MMR vaccine (2 doses) and 75.1% for the varicella vaccine (2 doses). The average age at vaccination was older than that recommended by the National Vaccination Plan. A diagnosis of oncological disease and an older age at enrollment were risk factors for missing vaccinations. These results showed that the overall vaccination status of pediatric onco-hematological patients is suboptimal. Improving provider communication and establishing the hospital as the primary environment for vaccine administration may lead to better vaccination compliance in this group.
Introduction
During the last several decades, the survival time and quality of life of children with onco-hematological diseases has progressively increased, due to therapeutic advances.Citation1 However, most of these patients are immunocompromised, either because of their disease or its treatment, and thus at greater risk of infectious complications. In fact, one of the main effects of chemotherapy is hematological toxicity, which causes a transient immunodeficiency that lasts 6 to 12 months after the end of treatment and affects previously acquired immunity as well.Citation2 A loss of immune protection has also been described after hematopoietic stem cell transplantation (HSCT).Citation3 Depending on the involved components of the immune system, the clinical presentation of these patients can range from asymptomatic to the obvious consequences of severe and invasive infections.Citation4
Certain hematological diseases, such as drepanocytosis, lead to functional asplenia or require splenectomy to control chronic hemolysis, as in hereditary spherocytosis. Following infection with encapsulated bacteria, patients with an anatomical or a functional absence of the spleen are at serious risk of fulminant sepsis, which is associated with a high mortality, especially in preschool children.Citation5,Citation6
Some inherited and acquired hematological diseases require sporadic or recurrent treatment with blood products, which may not be totally free of infective agents. In addition, plasma-derived products such as immunoglobulins, plasma and unwashed red blood cells can reduce the effectiveness of live attenuated vaccines, thus necessitating the adoption of a tailored vaccination schedule and additional doses.Citation7 Moreover, live attenuated vaccines are sometimes involved in the pathogenesis of immune thrombocytopenia.Citation8,Citation9
This increased risk of infection in children with onco-hematological diseases could be eliminated or at least reduced using currently available vaccines.Citation10 Recently, the Italian Primary Immunodeficiencies Network reviewed the evidence regarding vaccination in immunodeficient patients and published a set of recommendations for the vaccination of this population.Citation5 In general, inactivated vaccines are safe and well tolerated whereas live attenuated vaccines should not be given to patients with a positive family history of primary immunodeficiencies until a definitive diagnosis is available.Citation11 In pediatric patients with T-cell immunity disorders, the administration of live attenuated vaccines requires a CD4 level > 1500/mm3 in children <1 year old, 1000/mm3 in children 1–6 years of age and 500/mm3 in those older than 6 years.Citation12 In children with complement deficits, inactivated or purified-antigen-containing vaccines (such as pneumococcal, Haemophilus influenzae and meningococcal vaccines) are strongly recommended, with additional doses, to counter the increased risk of bacterial infection.Citation13
During chemotherapy, there is no contraindication for inactivated vaccines or vaccines containing purified antigens (despite the potential sub-optimal response). Immunization should be carried out only during the low-intensity phase of chemotherapy, when the lymphocyte count is ≥1000/mm3, which allows an adequate response and reduces the risk of adverse effects. Live attenuated vaccines, however, are contraindicated during chemotherapy due to the risk of re-activation of the attenuated virus in the immunodeficient host.Citation14 At the end of chemotherapy, a period of 6–12 months is sufficient for immunological recovery. Revaccination or the administration of a booster dose after 6 months for inactivated vaccines and after 6–12 months for attenuated vaccines is sufficient to elicit a protective titer in almost all patients, without significant side effects.Citation14 The administration of inactivated influenza vaccine is strongly recommended 3 months after the end of chemotherapy because patients are at high risk of complications if they contract influenza.Citation15 Given the high frequency of a loss of immunity after chemotherapy and the high rate of seroconversion with a booster dose or revaccination, it is not necessary to measure the antibody titer before and after revaccination. Patients who have stopped the vaccination schedule to start chemotherapy should continue the schedule starting from the last dose administered.Citation2
After HSCT, an intervening period of 6 months from the interruption of any immunosuppressive therapy is recommended before the administration of inactivated vaccines, while vaccines containing attenuated microorganisms should not be given before 24 months post-HSCT or in patients with graft versus host disease or during immunosuppressive therapy.Citation16
Patients receiving prolonged steroid therapy (prednisone or an equivalent, at a dose of 2 mg/kg/day or a cumulative dose of 20 mg/day) should not receive live attenuated vaccines within 2 weeks in case of a therapy schedule < 14 days, or before 4 weeks in case of longer therapy. Otherwise, there are no restrictions on these vaccines.Citation17 In case of immunosuppressive and biological therapies, live attenuated vaccines are contraindicated during treatment and from 3 to 6 months after its suspension, depending on the drug in question.Citation13,Citation17
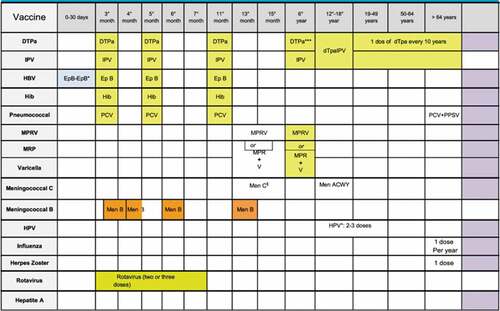
According to the Italian vaccination schedule (), in the first year of life, three doses of DTaP-IPV-Hep B-Hib and pneumococcal conjugate vaccines are administered (at 3, 5 and 11–13 months). In the second year of life, between the 13th and 15th month, the first doses of MMR and varicella vaccine are administered using a tetravalent (MMRV) or trivalent MMR vaccine and a monovalent varicella vaccine; at 15 months, the MenC/ACYW vaccine is also recommended. A booster dose for DTaP and IPV and a second dose of MMR+varicella or MMRV vaccine are recommended in children 5–6 years of age. Finally, since 2014, a meningococcal B vaccine has been available for all newborns; the schedule differs depending on the age at first dose.Citation18
The National Vaccination Prevention Plan (PNPV) 2017–2019 includes a list of the health conditions for which vaccination is indicated because of the increased risk related to infection.Citation18 Despite these recommendations and the available evidence, in pediatric patients with hematological or oncological disease the inappropriate discontinuation of vaccination is not uncommon. This choice may reflect a lack of knowledge about vaccination among physicians, the parents’ or guardian’s fear of adverse effects, the misconception that the disease is a contraindication for vaccination and that vaccination could be a cause of the disease.Citation19
In this study we evaluated pediatric patients with oncological and hematological diseases in terms of their compliance with the immunization program of the National Immunization Schedule. At the time of the study, the vaccination of pediatric onco-hematological patients in Puglia was provided by Vaccination Services while patients were being treated in the hospital.
Methods
This was a retrospective cohort study carried out in March-April 2019. Vaccination coverage was analyzed in two groups of pediatric patients, with oncological and hematological diseases, who were cared for either in the Pediatric Oncology Department or in the Pediatric Hematology Department of the Policlinico Giovanni XXIII Pediatric University Hospital of Bari, Italy. This ~1200-bed hospital is the most important hospital in southern Italy. The study population was drawn from all patients living in Puglia who were cared for in either department during the study period.
At the time of hospitalization, the parents or the guardians of the patients provided informed consent for the use of the children’s data, anonymously collected, for scientific purpose. The list of patients, the diagnosis and the date of the diagnosis were obtained from the hospital database. Since the study involved the use of routinely collected data, the authorization of the Ethics Committee was not requested.
The inclusion criteria were: (1) < 14 years old at the time of diagnosis; (2) a diagnosis of an oncological or hematological disease (International Classification of Diseases, codes 140-–239 and 280–289) and (3) living in Puglia.
The immunization status of the enrolled patients was obtained from the Apulia regional immunization database (GIAVA). The following vaccinations were investigated: 1st, 2nd and 3rd doses of DTaP-IPV-Hep B-Hib and pneumococcal conjugate vaccines, 1st and 2nd doses of the MMR and varicella vaccines, MenC/ACYW conjugate vaccines and the 1st and 2nd boosters of dTaP. The IPV booster was not considered because it was available at a different period from the Puglia Health Trusts. The MenB vaccine was not investigated because the vaccination strategy is relatively new.
For each recruited patient, the following information was recorded: age at the time of the study, age at the time of diagnosis, disease (hematological/oncological), vaccines administered and age at the time of vaccination (days).
The data were entered into a database created using an Excel spreadsheet and analyzed using STATA MP16 software. Continuous variables were expressed as means ± standard deviations, and categorical variables as percentages; 95% confidence intervals (95%CIs) are reported as well. The normality of the continuous variables was assessed using skewness and kurtosis tests. For non-distributed variables it was not possible to establish a normalization model. Continuous variables between groups were compared using the Wilcoxon (non-parametric) rank sum test, and categorical variables between groups using a chi-squared test.
A post-diagnosis adherence score was calculated based on the ratio of vaccine doses administered after disease diagnosis and the number of doses recommended by the Italian Vaccination Plan (according to the age at enrollment). The result was expressed as a percentage.
The relation between the post-diagnosis adherence score and the disease class (oncological vs. hematological), age at enrollment (year), sex (male vs. female) and age at diagnosis (years) was evaluated by multivariate linear regression. The correlation coefficients and 95%CIs were calculated. For all tests, a p-value <0.05 was considered to indicate statistical significance.
Results
Of the 228 patients enrolled in the study, 119 (52.2%) were oncological patients and 109 (47.8%) hematological patients. Immune thrombocytopenia was the most frequent disease within the hematological group (42 patients, 38.5%), and acute lymphoblastic leukemia the most frequent disease within the oncological group (50 cases, 42.0%) ().
Table 1. Distribution of enrolled patients per diagnosis
In the overall group of 228 patients, 56.5% were male. The sex distribution between the hematological and oncological patients did not significantly differ (60/109; 55.1% vs. 69/119; 58.0% respectively; p = .655). The average age of the patients at enrollment was 10.1 ± 3.7 years. There was no statistically significant differences between hematological (10.3 ± 4.0) and oncological (10.0 ± 3.4; p = .870) patients. The distribution of patients by age is presented in . The average age at disease diagnosis was 4.4 ± 3.7 years (range: 0–15), but hematological patients were diagnosed at a significantly younger age than oncological patients: 3.8 ± 3.9 vs.: 4.9 ± 3.5 years (p = .001).
Table 2. Sample distribution per age-group and diseases
The vaccination coverage in each group is reported for each vaccine in . There was no significant difference in vaccination coverage between hematological and oncological patients (p > .05). The exceptions were the 2nd doses of the MMR (83.7% vs. 68.5%; p = .009) and varicella (53.9% vs. 38.1%; p = .020) vaccines.
Table 3. Vaccination coverage of the sample in hematological vs. oncological patients, reported per vaccine. All values are reported in percent
Among the 157 (16.6%; 95%CI = 11.1–23.3%) patients who received a third dose of the pneumococcal vaccine, 26 also received a fourth dose after their diagnosis (hematological disease: 18.9%; 95%CI = 10.7–29.7% vs. oncological disease: 14.5%; 95%CI = 7.7–23.9%; p = .679).
Coverage for the meningococcal ACYW135/C vaccine was 81.1% (95%CI = 75.4–86.0%) and did not significantly differ between hematological (85.3%; 95%CI = 77.3–91.4% and oncological (77.3%; 95%CI = 68.7–84.5%) patients (p = .102). Among the immunized children, 58/185 (31.4%; 95%CI = 24.7–38.6) had been vaccinated after disease diagnosis, on average 4.4 ± 2.5 (range: 2–11) years later. One vaccine dose was repeated in 42 of the remaining 127 (33.1%; 95%CI = 25.0–42.0%) patients; the difference between groups was not significant (hematological disease: 37.5%; 95%CI = 24.9–51.5% vs. oncological disease: 29.6%; 95%CI = 19.3–41.6%; p = .348).
As shown in , there was a significant difference in the age at administration of the 2nd MMR dose, 2nd varicella dose and 1st dTaP booster, as oncological patients were significantly older than hematological patients at the time of these vaccinations (p < .05). No significant differences were observed between groups for the other vaccines under analysis (p > .05).
A comparison between the age at vaccine administration and the gold standard defined by the National Vaccination schedule () showed a significant delay in both groups of patients (p < .05), except for the 2nd dTaP booster in hematological patients.
Table 4. Age (days of life) at the time of vaccine administration in hematological and oncological pediatric patients (mean±SD)) and the gold standard (age, in days) according to the vaccination calendar currently in force in Puglia
The average post-diagnosis adherence score was 67.4 ± 36.3% (range: 0.0–100%). The score was related to both the disease (oncological vs. hematological; correlation coefficient = −0.13; 95%CI = −0.22 to −0.04; p = .004) and the age at enrollment (correlation coefficient = −0.23; 95%CI = −0.04 to −0.01; p = .018). None of the other associations were significant (p > .05; ).
Table 5. Determinants of the post-diagnosis adherence score in a multivariate linear regression model
Discussion
Our results showed that vaccination coverage among pediatric oncological and hematological patients was lower than the recommended level, in particular for vaccines scheduled for children in the second year of life (MMR/MMRV, MenC/ACYW) and 5–6 years of age (DTaP, MMR/MMRV). Missing vaccination was more frequent among oncological patients, perhaps due to the fear of adverse events during chemotherapy. New healthcare tools, such as provider recommendations, may positively influence a caregiver’s decision to restart his or her child’s vaccination. However, guidelines are needed to allow vaccine providers to formulate tailored recommendations.Citation20 In our sample, older age at enrollment increased the risk of missing vaccination. This may have been due to an earlier lack of knowledge among physicians about the risk/benefit ratio of vaccination for onco-hematological patients. Thus, out of a preference for caution, vaccination was likely to be discontinued after the diagnosis. It should be noted that the first official recommendation regarding vaccination for onco-hematological patients became available only in 2014.Citation2
Despite significant improvements in the prevention and management of infections in pediatric patients with hematological or oncological diseases, infectious diseases are still a frequent complication, burdened by high morbidity and mortality in addition to a significant influence on the quality of life and the overall costs of patient management.Citation21 It is therefore not surprising that a diagnosis of onco-hematological disease negatively impacts the patient’s vaccination schedule, causing delays or temporary suspensions.
Data on the efficacy and immunogenicity of vaccinations in patients during and after chemotherapy are limited, but according to available information concerns about the safety of vaccines in this population have not been raised.Citation22 Nonetheless, despite the safety of vaccines, a diagnosis of cancer may pose an obstacle to vaccination.Citation23 In chronically ill patients, vaccine hesitancy may be due to a perception that vaccination is contraindicated rather than to a fear of reactivation of the underlying disease.Citation24 For example, vaccination in hemophilic children requires comprehensive planning, taking into account disease severity, type and route of vaccination, and bleeding risk.Citation25 Disagreement among the various specialists (e.g. oncologist, public health physician and family pediatrician) charged with caring for a complex patient may also arise. A study carried out on 275 chronically ill (type I diabetes mellitus, cystic fibrosis, HIV infection, neurological disease) Italian children demonstrated that a frequent barrier to vaccination was the fear of inducing more serious illness.Citation26 However, the hospital offers a trustworthy setting for the vaccination of patients with chronic or rare diseases, because it is the reference environment and perceived as being safer.Citation27
Our study suggests the need to promote correct vaccination timing in a population already at increased risk of infections and complications, to issue recommendations and to dispel incorrect contraindications.Citation26
The difference in vaccination coverage for the 2nd MMR and varicella doses between hematological and oncological patients can be explained by the fact that both vaccines should be administered between the 5th and 6th year of life; however this is the age at which most oncological pathologies are diagnosed. Intensive chemotherapy protocols contraindicate live attenuated vaccines, which form the basis of the MMR and varicella vaccines, such that vaccination with either one is likely to be delayed. These results are consistent with our finding that oncological patients were vaccinated at a significantly older age than hematological patients. By contrast, the DTaP vaccine is an inactivated and therefore safe vaccine even for patients undergoing chemotherapy, such that a delay in its administration does not seem justified.
A key strength of our study was the large sample, derived from one of the most important hospitals in southern Italy, but there were also several limitations of our analysis. The first was that the Apulian vaccine register GIAVA could not be used to determine the average vaccination age of the general population in our region. It was therefore unclear whether delayed vaccination was a specific problem of the studied groups of patients or a phenomenon common to the entire Apulian pediatric population, such as due to the organization of regional healthcare services. Regardless, our study revealed a significant decline in vaccinations adherence and significant delays in vaccine administration following the diagnosis of hematological or oncological disease. These data are consistent with those in the available literature showing that children who habitually access the Healthcare System have less vaccination coverage than the general population. Therefore, the disease event, instead of reinforcing the importance of being vaccinated, seems to decrease vaccination compliance. A strategy to improve vaccination adherence may be to incorporate the option of vaccination during the control visits for the underlying disease at the reference center.Citation28 Two additional limitations of our study were its monocentric design and our inability to identify the reason for missing or delayed vaccination (fear? Physician’s advice? Other contraindication, such as allergy?).
A study carried out in Australia showed the success of a catch-up strategy based on systematically evaluating the immunization status in children at high risk at the time of hospitalization and then offering vaccination with the missing vaccines during the hospital stay.Citation29 The creation in an Australian cancer center of a service dedicated to the vaccination of patients undergoing autologous transplant has increased vaccination coverage from 86% to 98%.Citation30
Among the factors that undoubtedly affect vaccination compliance is correct information. Many parents fear that vaccination may lead to severe and permanent adverse events or believe that the number of vaccines is excessive.Citation31 Effective communication on the risks of both infectious diseases and, of course, the various vaccines is essential for obtaining informed consent, even from highly skeptical parents or guardians, and thus for a successful vaccination program.Citation3Citation2 According to the same study, the interaction with the health system is the most important factor influencing parents’ consent to their children’s vaccination.Citation3Citation2 Healthcare system operators must also be included in the chain of communication, as they constitute a reference for patients and their parents and are essential in motivating compliance.
Nevertheless, the vaccination of patients with chronic diseases remains a challenge for public health. New studies on the safety and efficacy of vaccination in pediatric patients with serious underlying conditions are needed. The results must be disseminated among physicians in different branches of medicine, as well as among other stakeholders, such as patients’ associations, to support effective partnerships between the public and private sectors. New strategies, such as an in-hospital vaccination clinic, must be also tested with the aim of providing more focussed patient care and avoiding disparate information and approaches on the topic of vaccination from different physicians, which are major risk factors for missing vaccination.
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to declare.
Ethics
Informed consent was obtained from the parents or legal guardians of the paediatric patients in this study. The nature and possible consequences of the studies were fully explained. The privacy rights of all participants were strictly respected.
Acknowledgments
All authors equally contributed to the study idea, to write, revise and validate the paper.
References
- GBD. 2017 childhood cancer collaborators. The global burden of childhood and adolescent cancer in 2017: an analysis of the global burden of disease study 2017. Lancet Oncol. 2019;20:1211–25. doi:10.1016/S1470-2045(19)30339-0.
- Cesaro S, Giacchino M, Fioredda F, Barone A, Battisti L, Bezzio S, Frenos S, De Santis R, Livadiotti S, Marinello S, et al. Guidelines on vaccinations in paediatric haematology and oncology patients. Biomed Res Int. 2014;2014:707691. doi:10.1155/2014/707691.
- Abdel-Azim H, Elshoury A, Mahadeo KM, Parkman R, Kapoor N. Humoral immune reconstitution kinetics after allogeneic hematopoietic stem cell transplantation in children: a maturation block of IgM memory B cells may lead to impaired antibody immune reconstitution. Biol Blood Marrow Transplant. 2017;23:1437–46. doi:10.1016/j.bbmt.2017.05.005.
- Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, Cunningham-Rundles C, Etzioni A, Holland SM, Klein C, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency 2015. J Clin Immunol. 2015;35:696–726. doi:10.1007/s10875-015-0201-1.
- Martire B, Azzari C, Badolato R, Canessa C, Cirillo E, Gallo V, Graziani S, Lorenzini T, Milito C, Panza R, et al. Vaccination in immunocompromised host: recommendations of Italian Primary Immunodeficiency Network Centers (IPINET). Vaccine. 2018;36:3541–54. doi:10.1016/j.vaccine.2018.01.061.
- Luu S, Spelman D, Woolley I. Post-splenectomy sepsis: preventative strategies, challenges, and solutions. Infect Drug Resist. 2019;12:2839–51. doi:10.2147/IDR.S179902.
- Gallo G, Mel R, Ros E, Filia A Guida alle controindicazioni alle vaccinazioni. Quinta edizione -Febbraio 2018. Allegato al parere del Consiglio Superiore di Sanità del 23 marzo. 2018
- Cecinati V, Principi N, Brescia L, Giordano P, Esposito S. Vaccine administration and the development of immune thrombocytopenic purpura in children. Rev Hum Vaccin Immunother. 2013;9:1158–62. doi:10.4161/hv.23601.
- Del Vecchio GC, De Santis A, Giordano P, Amendola G, Baronci C, Del Principe D, Nobili B, Jankovic M, Ramenghi U, Russo G, et al. AIEOP ITP study group.Management of acute childhood idiopathic thrombocytopenic purpura according to AIEOP consensus guidelines: assessment of Italian experience. Acta Haematol. 2008;119:1–7. doi:10.1159/000112837.
- Acquafredda S, Tafuri S. “My son can not attend the school because 5 classmates are unvaccinated”. On the question of compulsory vaccinations and the risk for immune-compromised children into the schools: the case of paediatric cancer patients. Hum Vaccin Immunother. 2019;15:643–44. doi:10.1080/21645515.2018.1537757.
- Medical Advisory Committee of the Immune Deficiency Foundation; Shearer WT, Fleisher TA, Buckley RH, Ballas Z, Ballow M, Blaese RM, Bonilla FA, Conley ME, Cunningham-Rundles C, Filipovich AH, et al. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol. 2014;133:961–66. doi:10.1016/j.jaci.2013.11.043.
- Pickering LK, Baker CJ, Kimberlin DW, Long SS Red book: 2012 report of the committee on infectious diseases. American Academy of Pediatrics. 2012.
- Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H, et al. IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2013;2014(58):309–18.
- Patel SR, Ortín M, Cohen BJ, Borrow R, Irving D, Sheldon J, Heath PT. Revaccination of children after completion of standard chemotherapy for acute leukemia. Clin Infect Dis. 2007;44:635–42. doi:10.1086/511636.
- Goossen GM, Kremer LC, van de Wetering MD. Influenza vaccination in children being treated with chemotherapy for cancer. Cochrane Database Syst Rev. 2009;15(2):CD006484.
- Hilgendorf I, Freund M, Jilg W, Einsele H, Gea-Banacloche J, Greinix H, Halter J, Lawitschka A, Wolff D, Meisel R. Vaccination of allogeneic haematopoietic stem cell transplant recipients: report from the international consensus conference on clinical practice in chronic GVHD. Vaccine. 2011;29:2825–33. doi:10.1016/j.vaccine.2011.02.018.
- American Academy of Pediatrics. Committee on Infectious Diseases, Kimberlin DW, Brady MT, Jackson MA, Long SS. Red BOOK: 2015 report of the committee on infectious diseases. American Academy of Pediatrics; 2015
- Ministero della Salute. Piano Nazionale Prevenzione Vaccinale 2017-2019. Gennaio 2017. [accessed 2020 Jul 22]. http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf
- Paterson P, Meurice F, Stanberry LR, Glismann S, Rosenthal SL, Larson HJ. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700–06. doi:10.1016/j.vaccine.2016.10.042.
- Warner EL, Vaca Lopez PL, Kepka D, Mann K, Kaddas HK, Fair D, Fluchel M, Knackstedt ED, Pannier ST, Martel L, et al. Influence of provider recommendations to restart vaccines after childhood cancer on caregiver intention to vaccinate. J Cancer Surviv. May 26 2020. doi:10.1007/s11764-020-00890-y.
- Bailey LC, Reilly AF, Rheingold SR. Infections in pediatric patients with hematologic malignancies. Semin Hematol. 2009;46:313–2. DaleyMF, Beaty BL, Barrow J, Pearson K, Crane LA, Berman S, Kempe A. Missed opportunities for influenza vaccination in children with chronic medical conditions. Arch Pediatr Adolesc Med 2005;159:986-981. doi:10.1053/j.seminhematol.2009.03.010.
- Santoro N, Colombini A, Silvestri D, Grassi M, Giordano P, Parasole R, Barisone E, Caruso R, Conter V, Valsecchi MG, et al. Screening for coagulopathy and identification of children with acute lymphoblastic leukemia at a higher risk of symptomatic venous thrombosis: an AIEOP experience. J Pediatr Hematol Oncol. 2013;35:348–55. doi:10.1097/MPH.0b013e31828dc614.
- Pandolfi E, Carloni E, Marino MG, Ciofi Degli Atti ML, Gesualdo F, Romano M, Giannattasio A, Guarino A, Carloni R, Borgia P, et al. Immunization coverage and timeliness of vaccination in Italian children with chronic disease. Vaccine. 2012;30:5172–78. doi:10.1016/j.vaccine.2011.02.099.
- Santagostino E, Riva A, Cesaro S, Esposito S, Matino D, Mazzucchelli RI, Molinari AC, Mura R, Notarangelo LD, Tagliaferri A, et al. Consensus statements on vaccination in patients with haemophilia-results from the Italian Haemophilia and vaccinations (HEVA) project. Haemophilia. 2019;25:656–67. doi:10.1111/hae.13756.
- Gallone MS, Martino C, Quarto M, Tafuri S. Active offer of vaccinations during hospitalization improves coverage among splenectomized patients: an Italian experience. Am J Infect Control. 2017;45:e87–e89. doi:10.1016/j.ajic.2017.02.039.
- Gallone MS, Infantino V, Ferorelli D, Stefanizzi P, De Nitto S, Tafuri S. Vaccination coverage in patients affected by chronic diseases: a 2014 cross-sectional study among subjects hospitalized at bari policlinico general hospital. Am J Infect Control. 2018;46:e9–e11. doi:10.1016/j.ajic.2017.10.004.
- Elia S, Perrett K, Newall F. Providing opportunistic immunisations for at-risk inpatients in a tertiary paediatric hospital. J Spec Pediatr Nurs. 2017;22(1). doi:10.1111/jspn.12167.
- Teh BW, Joyce T, Slavin MA, Thursky KA, Worth LJ. Impact of a dedicated post-transplant vaccination service at an Australian cancer centre. Bone Marrow Transplant. 2017;52:1681–83. doi:10.1038/bmt.2017.195.
- Offit PA, Quarles J, Gerber MA, Hackett CJ, Marcuse EK, Kollman TR, Gellin BG, Landry S. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics. 2002;109:124–29. doi:10.1542/peds.109.1.124.
- Tafuri S, Gallone MS, Cappelli MG, Martinelli D, Prato R, Germinario C. Addressing the anti-vaccination movement and the role of HCWs. Vaccine. 2014;32:4860–65. doi:10.1016/j.vaccine.2013.11.006.
- Haley MC, Pickering LK. How to communicate with vaccine-hesitant parents. Pediatrics. 2011;127(Suppl 1):S127–33. doi:10.1542/peds.2010-1722S.