ABSTRACT
Given the availability of an effective and safe vaccine, the World Health Organization (WHO) declared that global measles eradication is achievable, and measles elimination goals have since been established as interim steps toward eradication. As part of a strategy to maintain elimination, the Pan American Health Organization (PAHO) and WHO stipulate a minimum annual reporting rate of discarded non-measles cases of ≥2 per 100,000 population, in order to ensure sensitive surveillance and adequate investigative effort. With its effective vaccination program, the United States in 2000 was among the first countries to verify elimination, although subsequently, it has not routinely reported discarded rates. We estimated MLI investigation rates among insured individuals during 2010–2017, using data from the MarketScan® databases. We defined “MLI investigations” as measles serologic testing within 5 days following diagnostic codes for measles-compatible symptoms and conditions. We provide a rationale for pre-specifying three subgroups for analysis: children aged ≤15 years; males aged 16–22 years excluding data from summer months; and males aged ≥23 years. MLI investigation rates ranged from 6.6─26.4 per 100,000, remaining stable over time except during the 2015 measles outbreaks when rates increased, particularly among young children. In addition to high vaccine uptake, measles elimination requires ongoing vigilance by clinicians and high-quality, case-based surveillance. Estimated rates of MLI investigations in this U.S. population suggesting that the quality of measles surveillance is sufficiently sensitive to detect endemic measles circulation if it were to be occurring.
Introduction
Measles is a highly contagious disease defined by a prodrome of fever, malaise, cough, coryza, and conjunctivitis, followed by a maculopapular erythematous rash.Citation1 Historically, measles was responsible for millions of annual deaths worldwide, but with availability of effective and safe vaccines, the World Health Organization (WHO) declared global measles eradication to be achievable, and measles elimination goals were established as interim steps toward eradication. With its effective vaccination program, the United States in 2000 was among the first countries to verify elimination, and in 2016, and the WHO Region of the Americas (Pan American Health Organization, PAHO) was certified as having eliminated measles.Citation2–4 However, that status can be tenuous:Citation5 due in part to the success of the vaccination program and receding memories of measles, a growing reluctance to vaccinate resulted in 2019 to the largest number of measles cases in more than 25 years,Citation6,Citation7 and, since many U.S. clinicians had never seen measles, some may have overlooked it as an etiology of febrile rash illnesses.
Measles surveillance in the United States involves a complex public-private partnership. Initial consideration of measles, diagnostic activity, and notification of public health authorities are all functions of private clinicians and allied sources of disease reporting. This partnership determines the performance of the surveillance system: clinician vigilance and immediate notification of suspected cases are critical for management, and to enable public health authorities to implement control measures for all measles cases in a timely manner, thereby containing spread. While there are a number of attributes that are critical to good surveillance (that should be routinely evaluated to identify gaps in the quality),Citation8 in the context of elimination, sensitivity is particularly important. Sensitivity and investigative effort are crucial for elimination to be achieved and maintained, and to assure that the absence of reported measles cases reflects the absence of measles itself.
PAHO developed a strategy for evaluating the sensitivity of polio surveillance, a necessary component of the global effort to eradicate polio.Citation9–13 Polio is characterized by acute flaccid paralysis (AFP), but other conditions can also cause this clinical syndrome, at a relatively stable rate. If a surveillance system is sensitive, it should identify and investigate a substantial portion of these expected AFP cases, whether or not polio itself is circulating. PAHO set a minimum AFP investigation rate as a measure of investigative effort – an indicator for establishing the sensitivity of polio surveillance.
An analogous approach has been used to assess the sensitivity of measles surveillance. The WHO surveillance manual stipulates that a suspected case with fever and maculopapular rash, or in whom a health-care worker suspects measles should be investigated and classified as confirmed or discarded.Citation8 The list of etiologies for febrile rash illnesses is long, and measles-like illnesses (MLI) are ubiquitous. Laboratory assessment is generally crucial for investigating MLI (i.e., for clinical diagnosis and public health confirmation of measles), with measles immunoglobulin M (IgM) antibody testing conducted on a serum specimen upon initial presentation being a commonly used diagnostic and confirmatory test. With sensitive measles surveillance, one would expect a substantial number of MLIs to be identified and investigated, regardless of the epidemiology of measles itself. All WHO Regions including PAHO now recommend that surveillance programs identify at least 2 non-measles discarded cases per 100,000 population (of all ages) annually as an indicator to assure sensitivity.Citation4,Citation8 Importantly, because of the variability in measles surveillance systems by country (differences in the capacity to conduct elimination-standard surveillance, ascertainment of suspected cases, laboratory testing algorithms), a degree of variability on how this indicator is estimated is expected.Citation14
We have previously used the rate of measles IgM testing as a way to assess investigative effort for detecting MLI in the United States.Citation15 Monitoring the rate of measles investigations is challenging, particularly in countries with complex, decentralized medical care systems like the United States, where private clinicians rely on a variety of public and private laboratories for measles diagnostics. Although clinicians should immediately notify public health authorities regarding suspected measles (before laboratory confirmation), many may not do so, and they would be unlikely to notify once the diagnosis has been ruled out. During the 1990s, the United States compiled quarterly data from state health departments on measles investigations that were confirmed or ruled-out. However, this activity did not capture much of the diagnostic effort occurring in the private sector,Citation16 and it required substantial effort and was not sustainable. Administrative databases can serve as an effective and efficient source for analyzing trends in large, nationwide populations.Citation17–21 Here we describe the use of an administrative database to estimate the annual rate of MLI investigations being conducted among insured individuals in the United States.
Methods
Overview
We developed an algorithm to estimate MLI investigations conducted in the United States using data from large, administrative databases of healthcare encounters and payments. We defined “MLI investigations” as procedure codes for measles serologic testing conducted in conjunction with (i.e., within 0 to 5 days after) diagnostic codes for measles-compatible symptoms and conditions; the 5-day range provided flexibility (i.e., delayed blood draws or code entry). Notably, there is no procedure code for measles IgM testing alone: the procedure code for measles serologic testing also includes measles immunoglobulin G (IgG) testing, which is only used rarely for diagnosis since that requires testing of paired sera (i.e., acute and convalescent). IgG testing (conducted on one serum specimen) is, however, frequently used to screen for immunity to measles. To be conservative and avoid including false-positive MLI investigations (i.e., measles IgG testing for immunity screening coincidentally conducted within 5 days following measles-compatible symptoms and conditions), we added other restrictions to our algorithm (specifics described below).
Data source and study population
We conducted a retrospective cohort study using data from the IBM Marketscan® Databases (IBM Watson Health, Ann Arbor, MI).Citation18,Citation22 These data derive from the healthcare claims of employees and their beneficiaries covered by employer-sponsored insurance, from all states. We accessed data using MarketScan’s online dynamic tool, Treatment Pathways version 4.0. This tool includes approximately 100 million MarketScan enrollees for whom outpatient pharmaceutical claims data are available. The study population consisted of MarketScan Treatment Pathways enrollees from January 2010 to December 2017. Enrollees are assigned a de-identified unique number, which allows linkage of claims over time. Demographic data, clinical characteristics, medical procedures and laboratory services, and pharmaceutical treatment data are available for inpatient and outpatient services. This analysis only included de-identified data; it was therefore deemed not to be human subjects research by the Centers for Disease Control and Prevention (CDC), and it did not require CDC’s Institutional Review Board review.
Algorithm and algorithm definitions
As introduced above, we defined MLI investigations as procedure codes for measles serologic testing (Current Procedural Terminology [CPT] code 86765), conducted in conjunction with diagnostic codes for measles-compatible symptoms and conditions (both found in the same databases). These diagnostic codes, in turn, consisted of any International Classification of Disease, 9th or 10th Revisions, Clinical Modification (ICD-9/10) codes that were either consistent with the measles clinical case definition (e.g., fever, rash, cough, coryza, or conjunctivitis), or were used in association with actual measles diagnostic codes (i.e., in the same medical encounters: see Supplemental Table I).
Furthermore, as noted above, there is no procedure code for measles IgM testing alone; the CPT code for measles serologic testing includes both IgM and IgG testing (used for diagnosis and to test immunity, respectively) and does not distinguish them. The portion of 86765 CPT codes reflecting immunity screening may be increased in some situations, even when conducted in conjunction with ICD-9/10 codes for measles-compatible symptoms and conditions (i.e., the two codes were not connected; their timing was a mere coincidence). Such coincidences are most likely to occur in persons undergoing frequent measles immunity screening: pregnant women and those initiating college or health care employment. Therefore, we prespecified groups to exclude from analysis: we excluded MLI investigations involving females aged ≥16 years (since their serologic testing could reflect occupational or pregnancy screening), and males aged 16–22 years (typical ages for college or workforce entry) during the months June through August (for pre-matriculation and pre-employment immunity screening). We were left with three subgroups: children aged ≤15 years, males aged 16–22 years excluding the months June through August, and males aged ≥23 years, which we refer to as children, young adult males, and older adult males, respectively, throughout the paper.
To check our assumptions, we evaluated MLI investigations for signals suggesting misclassification, i.e., false-positives: based on the epidemiology of non-measles rash illnesses, we assumed that true MLI investigations could vary somewhat by age or calendar year but not much by sex, and we assumed that true MLI investigations should not be particularly elevated during late spring and summer months when most measles immunity testing is conducted. As expected, we found that among children, MLI investigation rates did not vary much by sex or during summer month, that among adults, rates in females exceeded those in males, and that rates were increased among young adult males during summer months (Supplement, Supplemental Figure 1). The patterns for children, young adult males, and older adult males, seemed stable and reliable.
Analysis
For each of the three subgroups, we calculated the annual rate of MLI investigations as the number of people with ≥1 MLI investigation divided by 100,000 people enrolled in MarketScan for any period during that calendar year. For each subgroup, the person-time denominator was adjusted for exclusions: for children, it included all person-time, for young adult males, it included person-time for males only (excluding June through August), and for older adult males, it included person-time for males only. Finally, to evaluate how public awareness during an outbreak could influence MLI investigation rates, we used the Google Trends tool to assess the volume of searches for “measles” and CDC webpage view data to determine the number of unique visitors to CDC measles informational sites.
Results
Between 2010 and 2017, an annual average of 7.2 million children (range, 4.9–9.2 million), 1.9 million young adult males (range, 1.4–2.4 million), and 12.1 million older adult males (range, 8.6–15.2 million) were included in the analysis (see subgroup definitions in Methods). The aggregate number of MLI investigations for all three subgroups during the study interval was 23,565, with an average annual number of 590 (range, 342–761), 312 (range, 155–416), and 2,043 (range, 1,083–2,539) for the children, young adult males, and older adult males subgroups, respectively. Demographic characteristics of these three study groups and those with MLI investigations are shown in for 2017, the most recent year of available data.
Table 1. Demographic and clinical characteristics of individuals with MLI investigations, MarketScan 2017a
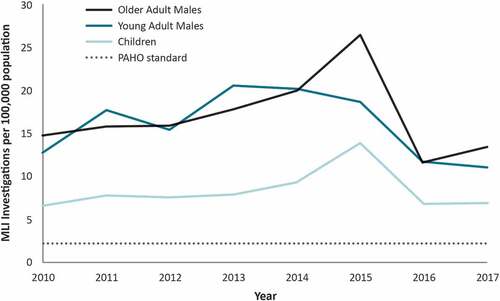
Between 2010 and 2017, the annual rate of MLI investigations per 100,000 ranged from 6.6 to 13.9 (median, 7.7) for children, 11.2 to 20.6 (median, 16.5) for young adult males, and 11.8 to 26.4 (median, 15.9) among older adult males (). Rates were stable over time within each of the study groups except for peaks during 2015 among children and older adult males, when rates were 1.8 and 1.6 times higher than the average rate for the other study period years, respectively.
Figure 1. Measles-like Illness (MLI) investigations by Age Group and Year, MarketScan 2010–2017a
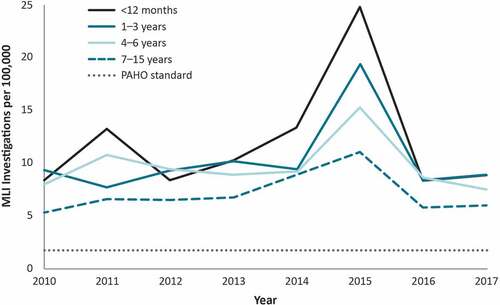
We evaluated age-specific rates of MLI investigations per 100,000 among children aged ≤15 years during our study interval (). Median rates were lowest in children aged 7–15 years and highest in infants aged <12 months (6.5 vs. 9.5, respectively). These rates were stable across time, with a peak in 2015 in all age groups that was particularly pronounced for infants aged <12 months.
Figure 2. Measles-like illness (MLI) investigations for people aged ≤15 years by year, MarketScan 2010–2017
In late 2014 through early 2015 there were several measles outbreaks in the United States, including a large outbreak associated with exposures at Disney theme parks that garnered significant media coverage.Citation23,Citation24 Heightened media attention to measles outbreaks may increase clinician awareness and influence MLI investigation rates. To evaluate whether the 2015 rate peak from our analysis was associated with an increase in attention to measles, we examined data from Google Trends and CDC measles webpage views. Google Trends analyzes the popularity of specified search queries by timeframe and CDC webpage view data tracks the number of unique hits on CDC’s informational websites.Citation25,Citation26 Both data sources exhibited pronounced spikes in measles-related website searches and visits to the CDC measles webpages spanning January and February of 2015 (CDC unpublished dataCitation26), precisely when MLI rates peaked (Supplemental Figure 2).
Discussion
The results from our analyses suggest that there is substantial measles investigative effort in the United States. The estimated rate of MLI investigations ranged from 6.6 to 26.6 per 100,000 during 2010–2017. Although our definition of MLI investigation differs from the WHO and PAHO standard, and might not be directly comparable, our MLI investigation estimates exceeded the minimum threshold of 2 per 100,000 proposed by the WHO and PAHO, suggesting measles surveillance in the US is robust.Citation8,Citation27 However, the number of MLI investigations in a given year provides additional information regarding the adequacy of measles surveillance. From 2010 to 2017, we estimated a total of 23,565 MLI investigations conducted among our three subgroups (average annual number of 2,946). During the same time period, there were a total of 1,589 confirmed measles cases reported (a ratio of almost 15 to 1), derived from the subgroups in our administrative database population, but also from all other sources.Citation7,Citation28 Our analysis therefore suggests that during 2010–2017, there were numerous MLI investigations conducted for every confirmed measles case. It thus seems unlikely that many cases could have escaped detection had measles virus been circulating during this period. Global public health authorities might wish to consider the ratio of MLI investigations to confirmed measles cases as an additional index for assessing the adequacy of measles surveillance.
We found a relationship between the number of MLI investigations and actual measles circulation that perhaps was mediated by patterns of measles awareness. Measles remains uncommon in the United States and most clinicians have not seen cases for some time. The median rate of MLI investigations that we detected was highest during 2015, when a large, high-profile measles outbreak also occurred.Citation22,Citation23 The increase in MLI investigations was much greater during this period than the number of confirmed outbreak-associated measles cases, suggesting that clinicians were casting a “wider net” and that measles investigative effort had been heightened. Results from our analysis of Google Trends and CDC webpage views demonstrated peaks in web activity for measles that were concurrent with the peak in MLI investigation rates seen in our analysis. This temporal association suggests that clinician awareness of measles activity and risk may influence diagnostic behavior, or perhaps that frequent media references, triggered by outbreaks, remind clinicians to re-include measles in their differential diagnoses for rash illnesses irrespective of measles activity. The qualitative and quantitative relationships between disease activity, clinician awareness, and diagnostic behavior are important issues that are rife with opportunity for additional study.
MLI investigation rates have been assessed in prior U.S. studies.Citation15,Citation16,Citation29-31 In a compilation of surveillance and laboratory data collected between 1986 and 1999 from 86 international and domestic surveillance programs, MLI investigation rates were found to vary substantially by site (from 0.1–22.6 per 100,000).Citation15 In 2006, Averhoff et al., reported data from a national measles survey of public health departments and found that a substantial number of measles investigations were conducted, with the majority of departments reporting having conducted at least 1 investigation each year.Citation31 Kolasa et al., evaluated surveillance data from 5 participating public health departments and found that annual investigation rates varied across sites and years and ranged from 0.33 and 14.11 investigations/100,000 population.Citation29 Finally, Nordin et al., used data from a 300,000 member managed care organization, including billing codes, electronic medical records, and laboratory requisitions to calculate an annual rate of 4.5 investigations/100,000 population.Citation30 Despite differences in approach, our algorithm produced similar investigation rates to these previous studies.
There is no single “true” rate of non-measles febrile rash illnesses across settings. Syndromic surveillance indicators such as AFP or MLI are important evaluation and management tools and are not intended to capture all AFP or MLI cases or to accurately assess the rates of these syndromes. The differential diagnosis for MLIs is broad and includes a number of febrile rash illnesses (parvovirus, herpesvirus, Zika, dengue, and enteroviral infections) that can co-circulate with measles, each with its own epidemiology varying by environment (e.g., geography, season) and hosts of interest (e.g., age). Furthermore, MLIs, including measles, exhibit variable severity that leads to variation in healthcare seeking behaviors and thus, disease severity affects surveillance.Citation32
Given the broad differential diagnosis for MLIs, clinicians and commercial laboratories play a vital role in measles surveillance by assuring that suspected cases are diagnosed accurately and specifically. Like other healthcare providers, laboratories are mandated to report cases that are laboratory-confirmed, but laboratories are particularly well-suited for public health surveillance given the volume of testing being conducted at these facilities and their use of automated electronic data reporting systems.Citation33 Indeed, laboratories that use electronic systems have been shown to be more reliable at reporting than those using other types of reporting sources.Citation34 On the other hand, laboratories are not routinely set up to allow for the health department-to-clinician feedback that is essential to good surveillance (regarding completeness of reporting, timely notification as soon as measles is suspected, the importance of measles genotype testing, etc.).
This study had limitations. MarketScan databases are a convenience sample and include individuals with private, employer-based health insurance only; we cannot be certain that our results are generalizable to populations without insurance, with public-based insurance, or with limited access to healthcare. However, approximately 50% of the U.S. population had employer-sponsored insurance during our study interval,Citation35 and the MarketScan databases are well-populated, with an average of 100 million enrollees during 2010–2017.Citation18 There is no specific reason to expect that measles investigative effort by clinicians caring for this large, diverse population, in a large, diverse, range of healthcare settings, would be fundamentally unrepresentative. Even without generalizing to the remaining U.S. population, it is still useful and notable to document that investigative effort appears to be adequate in this substantial portion of the total population.
Also, we were unable to validate the administrative claims with medical record reviews. Administrative databases contain coding errors that may influence our data; indeed, in 2015, ICD-9 codes were replaced with ICD-10 codes, which may partially explain why rates were lower in all groups during 2016–2017 than in prior years.
Our algorithm has limitations too. Some limitations may have resulted in false-negative results (i.e., missed MLI investigations): our methods could not capture measles molecular testing (i.e., real-time RT–PCR), diagnostic testing conducted by public health laboratories, or measles-compatible conditions that remained untested due to clear alternative diagnoses. These missed MLI investigations would have led to an underestimation of investigative efforts. More important to highlight are those limitations that might have resulted in false-positive results and thereby inflated our estimates. Since our algorithm for MLI investigations consisted of serologic testing conducted in conjunction with a measles-compatible symptoms and conditions, the likelihood of such false-positive results would be increased if the definitions in our algorithm were too inclusive for serologic testing and/or for symptoms and conditions.
Regarding serologic testing, we attempted to reduce false-positive results by excluding the tests most likely to represent measles immunity screening. Having no codes to specifically exclude women undergoing screening during pregnancy and adults undergoing screening for healthcare employment, we excluded based on sex, age, and calendar month – methods that were inefficient and not comprehensive. Aside from excluding useful information, our algorithm probably included some false-positive results, possibly among male healthcare workers who underwent immunity screening during non-summer months. Such false-positive results may have inflated our MLI investigation estimates.
Regarding measles-compatible symptoms and conditions, we used a broad definition, with symptoms not very specifically associated with measles. These included codes for fever, rash, cough, coryza, or conjunctivitis (components of the clinical case definition for measles), as well as codes that most frequently accompanied the diagnostic code for measles itself. When linked specifically to codes for measles serologic testing, this expansive list probably helped avoid a large excess of false-positive results, since most of the testing was probably conducted to rule-out measles, i.e., as MLI investigations.
All in all, while we believe that our algorithm provided a reasonable approximation of MLI investigative effort, we assume that false-positive results were least likely among children. Nonetheless, we pursued the other two subgroups since we expected them to be instructive and to provide complementary information. The limitations in our methods need to be considered in the context of MLI investigations as surveillance indicators and as evaluation and management tools: the number of MLI investigations that was theoretically warranted in our study population during the study interval is unknowable. However, in view of the many-fold excess of MLI investigations to confirmed measles cases during that interval and the apparent responsiveness of investigative behavior to actual measles activity and awareness, our results suggest that surveillance is adequate to find measles cases when the illness circulates in the United States.
We were able to develop an algorithm of diagnostic and procedural codes to estimate MLI investigations in the United States using a large administrative database, containing relevant data for a substantial portion of its population. Many countries do not have such platforms and others have excellent public health infrastructures and well-developed methods for evaluating the quality of their measles surveillance. Regardless, administrative databases provide opportunities to efficiently evaluate large populations for outcomes applicable to surveillance, without the need for significant additional resources – countries that do have such platforms could repurpose them creatively to evaluate surveillance, i.e., as an adjunct to their other routine evaluation regimes.
Given the rising rates of measles in 2019, with many countries experiencing sizable measles outbreaks due to vaccine hesitancy and related phenomena,Citation36 establishing and maintaining adequate measles surveillance is essential for prevention and control of the disease. Adequate surveillance includes clinician awareness and vigilance regarding suspected measles cases, with appropriate investigation and testing. Evaluating the rate of MLI investigations can play an important role in assuring the quality of surveillance.Citation36 Our evaluation suggests that investigative behavior by clinicians in the United States is responsive to increases in measles circulation. Perhaps even more reassuring, it suggests that clinicians also remain vigilant during periods of relative measles quiescence.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Financial support
There were no sources of financial support.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services.
Supplemental Material
Download Zip (2.3 MB)Acknowledgments
Sarah Poser who analyzed the data for CDC website hits. Thanks to Mark Papania for reviewing and providing expert feedback and Mary Ann Hall for technical editing.
Supplemental Material
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2020.1798712.
References
- Strebel PM, Papania MJ, Gastanaduy PA, Goodson JL. Measles vaccines (Chapter 37). In: Plotkin S, Orenstein W, Offit P, Edwards KM, editors. Philadelphia (PA): Vaccines. Elsevier Inc; 2018. p. 579–618.
- Patel M, Lee AD, Redd SB, Clemmons NS, McNall RJ, Cohn AC, Gastañaduy PA. Increase in measles cases - United States, January 1-April 26, 2019. MMWR Morb Mortal Wkly Rep. 2019;68:402–04. doi:10.15585/mmwr.mm6817e1.
- Orenstein WA, Cairns L, Hinman A, Nkowane B, Olive JM, Reingold AL. Measles and Rubella global strategic plan 2012–2020 midterm review report: background and summary. Vaccine. 2018;36(Suppl 1):A35–a42. doi:10.1016/j.vaccine.2017.10.065.
- Pan American Health Organization. Region of the Americas is declared free of measles. Washington (DC): Pan American Health Organization; 2016. [accessed 2019 Mar 20]. http://www.paho.org/hq/index.php?option=com_content&view=article&id=12528&Itemid=1926&lang=en
- Health and Human Services Press Office. With end of New York outbreak, United States keeps measles elimination status. 2019 Oct 4. [accessed 2020 Jan 15]. https://www.hhs.gov/about/news/2019/10/04/end-new-york-outbreak-united-states-keeps-measles-elimination-status.html
- Patel M, Lee AD, Clemmons NS, Redd SB, Poser S, Blog D, Zucker JR, Leung J, Link-Gelles R, Pham H, et al. National update on measles cases and outbreaks — United States, January 1–October 1, 2019. MMWR Morb Mortal Wkly Rep. 2019;68:893–96.
- Clemmons NS, Wallace GS, Patel M, Gastanaduy PA. Incidence of measles in the United States, 2001–2015. Jama. 2017;318:1279–81. doi:10.1001/jama.2017.9984.
- WHO. Measles. Vaccine-preventable diseases surveillance standards. 15 Oct 2018. [accessed 2019 Mar 21]. https://www.who.int/immunization/monitoring_surveillance/burden/vpd/WHO_SurveillanceVaccinePreventable_11_Measles_R2.pdf
- Organization PAH. XXIV Meeting of the Technical Advisory Group (TAG) on Vaccine-preventable Diseases, Panama City, Panama; 2017 Jul 12–14. Washington, DC: PAHO.
- de Quadros CA, Hersh BS, Olive JM, Andrus JK, da Silveira CM, Carrasco PA. Eradication of wild poliovirus from the Americas: acute flaccid paralysis surveillance, 1988–1995. J Infect Dis. 1997;175(Suppl 1):S37–42. doi:10.1093/infdis/175.Supplement_1.S37.
- Andrus JK, de Quadros CA, Olive JM. The surveillance challenge: final stages of eradication of poliomyelitis in the Americas. MMWR CDC Surveill Summ. 1992;41:21–26.
- Patel JC, Diop OM, Gardner T, Chavan S, Jorba J, Wassilak SGF, Ahmed J, Snider CJ. Surveillance to track progress toward polio eradication - worldwide, 2017–2018. MMWR Morb Mortal Wkly Rep. 2019;68:312–18. doi:10.15585/mmwr.mm6813a4.
- Pan American Health Organization. Strategies for the certification of the eradication of wild poliovirus transmission in the Americas. Expanded program on immunization, Pan American Health Organization. Bull Pan Am Health Organ. 1993;27:287–96.
- Patel MK, Gibson R, Cohen A, Dumolard L, Gacic-Dobo M. Global landscape of measles and rubella surveillance. Vaccine. 2018;36:7385–92. doi:10.1016/j.vaccine.2018.10.007.
- Harpaz R, Papania MJ. Can a minimum rate of investigation of measleslike illnesses serve as a standard for evaluating measles surveillance? J Infect Dis. 2004;189(Suppl 1):S204–9. doi:10.1086/378776.
- Harpaz R, Papania MJ, Fujii KE, Redd SB, Wharton ME, Redd SC, Gindler J. Lessons learned from establishing and evaluating indicators of the quality of measles surveillance in the United States, 1996–1998. J Infect Dis. 2004;189(Suppl 1):S196–203. doi:10.1086/381127.
- Birkhead GS, Klompas M, Shah NR. Uses of electronic health records for public health surveillance to advance public health. Annu Rev Public Health. 2015;36:345–59. doi:10.1146/annurev-publhealth-031914-122747.
- Quint JB. Health research data for the real world: the MarketScan databases. Truven Health Analytics White Paper. 2015.
- Grosse SD, Boulet SL, Amendah DD, Oyeku SO. Administrative data sets and health services research on hemoglobinopathies: a review of the literature. Am J Prev Med. 2010;38:S557–67. doi:10.1016/j.amepre.2009.12.015.
- Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario. Determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–16.
- Yu AY, Holodinsky JK, Zerna C, Svenson LW, Jette N, Quan H, Hill MD. Use and utility of administrative health data for stroke research and surveillance. Stroke. 2016;47:1946–52. doi:10.1161/STROKEAHA.116.012390.
- Shoffstall AJ, Gaebler JA, Kreher NC, Niecko T, Douglas D, Strong TV, Miller JL, Stafford DE, Butler MG. The high direct medical costs of Prader-Willi syndrome. J Pediatr. 2016;175:137–43. doi:10.1016/j.jpeds.2016.05.018.
- CDC. Measles cases and outbreaks. [accessed 2019 Mar 28]. https://www.cdc.gov/measles/cases-outbreaks.html
- Zipprich J, Winter K, Hacker J, Xia D, Watt J, Harriman K. Measles outbreak–California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64:153–54.
- Google Trends. 2019.
- CDC Digital Media Metrics.
- Castillo-Solorzano C, Reef SE, Morice A, Andrus JK, Ruiz Matus C, Tambini G, Gross-Galiano S. Guidelines for the documentation and verification of measles, rubella, and congenital rubella syndrome elimination in the region of the Americas. J Infect Dis. 2011;204(Suppl 2):S683–9. doi:10.1093/infdis/jir471.
- Lee AD, Clemmons NS, Patel M, Gastanaduy PA. International importations of measles virus into the United States during the post-elimination era, 2001–2016. J Infect Dis. 2018:1616–23.
- Kolasa M, Alexopoulos N, Diaz P, Kellachan J, Lowrey MJ, Shelton B, Harpaz R. Measles surveillance in five major US cities: Chicago, Houston, Los Angeles, Miami, and New York. J Infect Dis. 2004;189(Suppl 1):S216–21. doi:10.1086/377717.
- Nordin JD, Harpaz R, Harper P, Rush W. Syndromic surveillance for measleslike illnesses in a managed care setting. J Infect Dis. 2004;189(Suppl 1):S222–6. doi:10.1086/378775.
- Averhoff F, Zucker J, Vellozzi C, Redd S, Woodfill C, Waterman S, Baggs J, Weinberg M, Rodriquez-Lainz A, Carrion V, et al. Adequacy of surveillance to detect endemic rubella transmission in the United States. Clin Infect Dis. 2006;43(Suppl 3):S151–7. doi:10.1086/505948.
- Ewert DP, Westman S, Frederick PD, Waterman SH. Measles reporting completeness during a community-wide epidemic in inner-city Los Angeles. Public Health Rep. 1995;110:161–65.
- Roush S, Birkhead G, Koo D, Cobb A, Fleming D. Mandatory reporting of diseases and conditions by health care professionals and laboratories. JAMA. 1999;282:164–70.
- Effler P, Ching-Lee M, Bogard A, Ieong MC, Nekomoto T, Jernigan D. Statewide system of electronic notifiable disease reporting from clinical laboratories: comparing automated reporting with conventional methods. JAMA. 1999;282:1845–50. doi:10.1001/jama.282.19.1845.
- Berchick ER, Hood E, Barnett JC. Health insurance coverage in the United States: 2017. Current Population Reports. Washington (DC); 2018. P. 60–264.
- World Health Organization. New measles surveillance data for 2019. 2019 Apr 15. [accessed 2019 Nov 4]. https://www.who.int/immunization/newsroom/measles-data-2019/en/
