ABSTRACT
Meningococcal serogroups A and C cause significant numbers of cases in China. The Sanofi Pasteur meningococcal polysaccharide A + C vaccine (Men-AC) was licensed in China in 1995. Immunogenicity and safety of a single dose of Men-AC against a similar marketed vaccine, the Lanzhou Institute serogroups A and C vaccine (Lanzhou-AC), were evaluated in children 2 to 6 y of age. Antibody titers were determined before and on Day 30 after vaccination using a serum bactericidal assay using baby rabbit complement (SBA-BR). Immunogenicity endpoints included rates of seroconversion (postvaccination antibody titers ≥4-fold higher) and seroprotection (postvaccination titers ≥1:8). Unsolicited systemic adverse events (AEs) within 30 minutes after vaccination, solicited injection site and systemic reactions between Days 0 and 7, unsolicited non-serious AEs within 30 d, and serious adverse events (SAEs) throughout were recorded. Seroconversion rates against serogroups A and C were 97.0% (95% confidence interval [CI], 94.5–98.6) and 94.7% (95% CI, 91.6–97.0), respectively, in the Men-AC group and 97.7% (95% CI, 95.4–99.1) and 94.8% (95% CI, 91.7–97.0), respectively, in the Lanzhou-AC group, while seroprotection rates were 98.0% (95% CI, 95.8–99.3) and 97.0% (95% CI, 94.5–98.6), respectively, in the Men-AC group and 99.0% (95% CI, 97.2–99.8) and 96.8% (95% CI, 94.1–98.4), respectively, in the Lanzhou-AC group. Non-inferiority of Men-AC with regard to immunogenicity was demonstrated since the lower bounds of the 95% CIs of the differences in rates between the two groups were > −5% for both serogroups. Both vaccines were well tolerated.
Introduction
Invasive meningococcal disease (IMD) is caused by Neisseria meningitidis, a Gram-negative aerobic diplococcus, and mainly presents itself in the forms of meningitis and septicemia, the latter of which can range from uncomplicated bacteremia to sepsis leading to organ failure and death.Citation1 Rapidly fatal in approximately half of those affected if untreated, in up to 20% of survivors, it can result in sequelae such as disability, brain damage, and auditory symptoms; the fatality rate of IMD is 8–15% even with early diagnosis and immediate treatment.Citation2 Twelve meningococcal serogroups have been identified, but only six (A, B, C, W, X, and Y) cause IMD globally.Citation2–4
Prior to the introduction of the group A meningococcal polysaccharide-tetanus toxoid conjugate vaccine (PsA-TT; MenAfriVac) in the meningitis belt that extends across sub-Saharan Africa, 1.2 million cases of IMD were reported by the World Health Organization (WHO) each year and 135,000 deaths were attributed to this disease annually.Citation2,Citation5 Prior to the 1990s, IMD showed high prevalence in China, with Shanghai being a high-incidence zone with peak incidence as high as 434 cases per 100,000 population in 1967.Citation6–8 Incidence of IMD caused by serogroup A declined significantly following the universal introduction of serogroup A (MenA) polysaccharide vaccines in China in the 1980s.Citation9 Since then, the incidence has been as low as 0.09 cases per 100,000 population (0.60 cases per 100,000 population in children younger than 1 y of age) from 2005 to 2010.Citation10 A subsequent systematic review and meta-analysis estimated the incidence of IMD in China to be 1.84 cases per 100,000 population and the associated mortality to be 0.33 per 100,000 population between 2005 and 2015.Citation11 In the last century, serogroup A was responsible for up to 95% of cases of meningococcal disease in China, whereas serogroups B and C caused only sporadic cases.Citation12–15 However, following reports of increased incidence of disease caused by serogroup C strains in Anhui province and in Hefei between 2003 and 2005,Citation12,Citation16 serogroups A and C polysaccharide vaccine have been routinely used nationwide in China since 2006.Citation8,Citation17 The vaccination of children in China with a meningococcal vaccine is important not only because of the documented higher incidence in this age group but also because many of them live in regions where meningococcal infections are epidemic.
The meningococcal polysaccharide A + C vaccine (Men-AC) produced by Sanofi Pasteur (Meningo A + C®) is a lyophilized, heat-stable suspension of equal parts of purified polysaccharides derived from serogroups A (strain A4 Branham) and C (strain C2241 Gotschlich) of N. meningitidis. Although licensed since 1995 in China and effectively used in the Chinese population aged 2 y and older, Men-AC has not been directly compared in children 2 to 6 y of age with a similar vaccine that has been licensed in the Chinese market. This phase IV, randomized, controlled, blind-observer, non-inferiority study was conducted as required by the China State Food and Drug Administration (SFDA) in healthy Chinese participants 2 to 6 y of age to assess safety and immunogenicity of a single dose of Men-AC with the intent to support license renewal of the Men-AC in China.
Materials and methods
This phase IV, randomized, controlled, blind-observer, non-inferiority study was conducted at the Jiangsu Provincial Center for Disease Prevention and Control in Jiangsu Province, China. The trial protocol was approved by the Independent Ethics Committee of Jiangsu Provincial Center for Disease Prevention and Control prior to the start of the trial. A parent or legal guardian for each participant provided written informed consent before initiation of any study-related procedures. The study has been registered at ClinicalTrials.gov (NCT01430611).
Participants
Eligible participants were 2 to 6 y of age and had documentation of immunization against meningococcal disease according to the national immunization Expanded Program on Immunization (EPI) schedule, which included two doses with meningococcal group A polysaccharide vaccine between 6 and 18 months of age. Exclusion criteria included: receipt of any vaccine in the 4 weeks preceding the trial vaccination or planned receipt of any vaccine in the 4 weeks following the trial vaccination (except influenza vaccine); previous vaccination against meningococcal disease within the 12 months prior to trial vaccination; previous vaccination with any meningococcal conjugate vaccine; receipt of immune globulins, blood, or blood-derived products in the 3 months prior to enrollment; congenital or acquired immunodeficiency, or receipt of immunosuppressive therapy, or long-term systemic corticosteroid therapy; history of confirmed meningococcal infection; being at high risk for meningococcal disease during the trial, including increased susceptibility, anatomic or functional asplenia, or exposure to persons at high risk for exposure; systemic hypersensitivity to vaccine components; thrombocytopenia, bleeding disorder, or receipt of anticoagulants in the 3 weeks prior to enrollment; or chronic illness that would interfere with trial conduct or completion. Temporary exclusions by which participation could be delayed until resolution of the condition included acute illness or infection on the day of the vaccination or febrile illness (defined by the SFDA as axillary temperature ≥37.1°C); or receipt of antibiotic therapy within 72 hours before blood sampling.
Vaccines
Each 0.5-mL dose of Men-AC (batch G1089-1) contained 50 μg each of N. meningitidis group A and group C polysaccharides. Each 0.5-mL dose of the comparator vaccine, meningococcal (groups A and C) polysaccharide vaccine manufactured by Lanzhou Institute of Biological Products (Lanzhou-AC; Meng Ling Kang®; batch 201011121[1–2]) contained 50 μg each of N. meningitidis group A and group C polysaccharides. Both vaccines were provided as freeze-dried powders with solvent for resuspension.
Study design
Participants were randomly assigned in a 1:1 ratio to receive either Men-AC or Lanzhou-AC. Participants received a single dose of study vaccine on Day 0 that was administered subcutaneously in the anterolateral aspect of the upper arm. Blood samples for immunogenicity testing were collected prevaccination on Day 0 and postvaccination at Day 30 (window, +7 d).
Endpoints
The primary endpoint was the rate of seroconversion for each vaccine, defined as a ≥ 4-fold increase between pre-vaccination antibody titers against meningococcal serogroups A and C and post-vaccination titers measured 30 d after vaccine administration using the 2,3,5-triphenyltetrazolium chloride (TTC) serum bactericidal assay (SBA) using baby rabbit complement (TTC SBA-BR), which measures antibody-mediated, complement-dependent killing of target bacteria.Citation18–20 Secondary endpoints included antibody titers against meningococcal serogroups A and C and rates of seroprotection, defined as the percentage of participants with postvaccination titers ≥1:8 for both serogroups. Safety outcomes included the occurrence, intensity, and relationship to vaccination of any unsolicited systemic adverse events (AEs) reported within 30 minutes after vaccination and the occurrence, time to onset, duration, and intensity of solicited injection site reactions, solicited systemic reactions, adverse reactions (ARs), unsolicited AEs, and serious AEs (SAEs) occurring from Day 0 through Day 30 after vaccination. Definitions of terms and descriptions of intensity scales related to solicited injection site and systemic reactions are provided in , respectively. Although the definitions and intensity scale classifications for injection site erythema, injection site swelling, and fever used by both the China SFDA and the sponsor (Sanofi Pasteur) are listed and were used in the statistical analysis, the reported results were based only on the China SFDA definitions and intensity scale classifications. The same definitions and intensity scale classifications were used for both vaccines.
Table 1. Terminology, definitions, and intensity scales of solicited injection site reactions
Table 2. Terminology, definitions, and intensity scales of solicited systemic reactions
Table 3. Seroconversion rates (per-protocol analysis set)a.
Table 4. Seroprotection rates (per-protocol analysis set)a.
Table 5. GMTs and GMTRs (per-protocol analysis set)
Table 6. Summary of solicited AEs per China SFDA definitions and intensity scale classifications (safety analysis set)
Table 7. Summary of unsolicited AEs and ARs per China SFDA definitions and intensity scale classifications (safety analysis set)
Serologic evaluations
Antibody titers were determined using the TTC SBA-BR. Bacteria, baby rabbit complement, TTC, and serially diluted sera are incubated together in microtiter plates and covered with an agar overlay. The endpoint titer was determined by the reciprocal serum dilution yielding ≥50% killing compared with the mean of the complement control wells containing no serum. Sera with titration <1:2 (dilution) were considered to be negative. Serological tests were performed at the China National Institutes for Food and Drug Control (NIFDC) laboratory.
Statistical analysis
A total of 300 evaluable participants per group were calculated to provide 80% power in a one-sided test for non-inferiority at a significance level alpha of 0.025 and a 5% clinical non-inferiority margin when the underlying seroconversion rates were expected to be 97% in each group for both serogroups A and C (calculated using the Farrington and Manning formula). Additionally, a sample size of 300 subjects vaccinated in Group 1 would allow 95% probability of detecting an AE with a true incidence rate of 1%. A total sample size of 333 per group assumed a 10% drop-out/unevaluable rate.
The hypotheses tested for the primary (seroconversion) and secondary (seroprotection) endpoints were that 30 d after a single dose, Men-AC would be inferior to Lanzhou-AC in terms of seroconversion and seroprotection rates for serogroups A and C. To test the two hypotheses, two-sided 95% confidence intervals (CIs) were constructed around the difference between the percentages of participants with postvaccination titers against both serogroups that were ≥4-fold higher than the prevaccination titers (seroconversion) and around the difference between the percentages of participants with postvaccination titers ≥1:8 against both serogroups (seroprotection). The inferiority assumption was rejected for each serogroup if the lower bound of the two-sided 95% CI was > −5%, using a one-sided Type I error rate of 0.025 and a non-inferiority margin of 5%. If the null hypothesis was rejected for both serogroups while testing the two hypotheses, it was concluded that Men-AC was non-inferior to Lanzhou-AC with regard to both seroconversion and seroprotection. The Wilson score method without continuity correction was used for the calculation of the CI of the difference of percentages between groups. For safety endpoints, two-sided 95% CIs for the percentages of participants with an event were calculated using the exact binomial method (Clopper-Pearson method). No imputation for missing data was performed. Statistical analyses were performed using Statistical Analysis Software (SAS®) version 9.2 (SAS Institute, Cary, NC, USA).
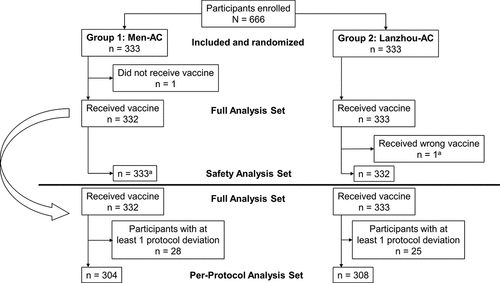
The safety analysis set included all participants who received the study vaccine and for whom safety data were available ().
The full analysis set (FAS) comprised all participants who received the study vaccine and had a postvaccination serology result. The per-protocol analysis set (PPAS) consisted of all participants who received the study vaccine and complied with all protocol-specified requirements and procedures.
Results
Participants
This study was conducted between August 23, 2011 (first participant enrolled) and October 12, 2011 (last participant visit). Of 666 participants who enrolled in the study, 333 each were randomly assigned to receive either Men-AC or Lanzhou-AC (). Of these, 315 (94.6%) participants who received Men-AC and 318 (95.5%) participants who received Lanzhou-AC completed the study. One participant who was randomly assigned to receive Lanzhou-AC received Men-AC and was therefore included in the Men-AC safety analysis set. The mean (standard deviation [SD]) age was 3.2 (1.1) y and 58.3% of the participants were male. Demographic characteristics were similar between vaccine groups.
Immunogenicity
For serogroups A and C, the lower bound of the 95% CI around the difference in seroconversion rates at 30 d postvaccination was > −5%, demonstrating non-inferiority of the Men-AC vaccine compared with the Lanzhou-AC vaccine ().
Similarly, non-inferiority was also demonstrated with regard to seroprotection rates for both serogroups ().
Results are shown for the per-protocol analysis set; results for the full analysis set were similar. Although not a primary or secondary endpoint, the percentage of participants with SBR-BR titers ≥1:128 was determined since titers ≥1:128 have been shown to correlate well with protection.Citation21 For serogroup A, the percentages of participants with titers ≥1:128 did not differ appreciably between vaccine groups both before (8/304 [2.6%] in the Men-AC group versus 9/308 [2.9%] in the Lanzhou-AC group; median titer in both groups: 1.0) and 30 d after vaccination (259/304 [85.2%] in the Men-AC group versus 271/308 [88.0%] in the Lanzhou-AC group; median titer in both groups: 256). Similarly, for serogroup C also, the percentages of participants with titers ≥1:128 were essentially similar between vaccine groups both before (3/304 [1.0%] versus 5/308 [1.6%] in the Men-AC and Lanzhou-AC groups, respectively; median titer in both groups: 4.0) and 30 d after vaccination (244/304 [80.3%] versus 254/308 [82.5%] in the Men-AC and Lanzhou-AC groups, respectively; median titer in both groups: 256). These data were for the per-protocol analysis set, with results for the full analysis set being comparable.
Geometric mean titers (GMTs) against serogroups A and C increased from baseline for both Men-Ac and Lanzhou-AC at 30 d postvaccination (). Postvaccination GMTs and postvaccination:prevaccination geometric mean titer ratios (GMTRs) were higher for serogroup A than for serogroup C and were similar between vaccines.
Safety
Solicited injection site reactions were reported by 101 (30.5%) and 95 (28.7%) of participants who received Men-AC and Lanzhou-AC, respectively (). The most frequently reported solicited injection site reaction in both vaccine groups was pain at the injection site. Most reactions were grade 1 in intensity and resolved within 3 d. Solicited systemic reactions were reported by 87 (26.3%) of participants receiving Men-AC and 85 (25.7%) participants receiving Lanzhou-AC (). Fever was the most commonly reported systemic reaction in participants who received Men-AC and myalgia was the most common systemic reaction in participants who received Lanzhou-AC. Most solicited systemic reactions occurred within the first 3 d after vaccination, were grade 1 in intensity, and resolved within 3 d.
One participant (Men-AC group) experienced an immediate non-serious systemic AE of worsening allergy, with symptoms of erythema on the left groin and left anterolateral thigh (). The event was grade 2 in intensity, lasted for 1 d, and required healthcare provider contact but no prescription of new medication. The event was considered to be related to vaccination per the investigator.
Unsolicited AEs occurred in 110 (33.0%) and 106 (31.9%) of participants receiving Men-AC and Lanzhou-AC, respectively (). The most commonly reported unsolicited AEs in the Men-AC group were nasopharyngitis (18.0%), cough (5.1%), and diarrhea (3.3%). All of these events were grade 1 or grade 2 in intensity except for a grade 3 event of pyrexia, which was considered by the investigator to be unrelated to vaccination. The most commonly reported unsolicited AEs in the Lanzhou-AC group were nasopharyngitis (15.4%) and cough (8.7%). All of these events were grade 1 or grade 2 in intensity. Unsolicited ARs were reported by 3 (0.9%) participants receiving Men-AC and 2 (0.6%) participants receiving Lanzhou-AC ().
Three SAEs were reported during the study (). None of the SAEs was considered to be related to vaccination per the investigator. One participant who received Men-AC experienced bronchopneumonia that required hospitalization. The event started 15 d after vaccination and lasted 12 d. One participant who received Men-AC had abdominal pain that required hospitalization, which started 6 d after vaccination and lasted 15 d. One participant who received Lanzhou-AC experienced bronchitis that required hospitalization, which started 24 d after vaccination and lasted 5 d. No deaths were reported during the study.
Discussion
This Phase IV, single-center, randomized, controlled, blind-observer, non-inferiority study undertaken as required by the China SFDA demonstrated that Men-AC and Lanzhou-AC had similar immunogenicity and safety profiles; both vaccines induced robust immune responses and were well tolerated. A single dose of Men-AC was immunologically non-inferior to Lanzhou-AC, both with regard to the seroconversion rates for serogroups A and C and with regard to seroprotection rates against the two serogroups. Over 94.7% of participants demonstrated seroconversion, while over 96.8% had postvaccination titers ≥1:8 against the two serogroups with the two vaccines. Percentages of participants with SBA-BR titers ≥1:128, shown to correlate well with protection,Citation21 did not differ appreciably between vaccine groups for both serogroups. As for safety, solicited injection site reactions (the most common being pain at the injection site) and solicited systemic reactions (the most common being fever) were of grade 1 intensity and most resolved within 3 d. A single participant in the Men-AC group experienced a grade 2 non-serious systemic AE (worsening allergy with symptoms of erythema on the left groin and left anterolateral thigh) that lasted for a single day and required medical attention but no new medications and was considered to be vaccine-related. Unsolicited AEs occurred in similar proportions of participants in both groups and were mostly of grade 1 or 2 in intensity. Unsolicited ARs occurred in similar proportions of participants in both groups. There were no SAEs deemed to be related to vaccination, and no deaths occurred during the study.
Several participants in both vaccine groups had lower post- than pre-vaccination titers for serogroups A and C: six and four participants, respectively, in the Men-AC group, and three and five participants, respectively, in the Lanzhou-AC group. These data were verified and confirmed by the NIFDC laboratory. Given that the low frequencies of these abnormal postvaccination titers were similar in the two groups and that the lack of antibody responses in individual participants was not seen with both serogroups, the abnormal titers were deemed not to reflect vaccine, vaccine batch, vaccine storage, or vaccine administration issues. Although the clinical relevance of this finding is not known, it may be due to the well-recognized phenomenon of hyporesponsiveness seen in individuals receiving subsequent doses of meningococcal A polysaccharide vaccines.Citation22 However, hyporesponsiveness would not explain the observed lower responses against serogroup C polysaccharides since none of the participants had received a vaccine containing meningococcal serogroup C polysaccharides prior to receiving the study vaccines that contained polysaccharides from both serogroups A and C. Capsular polysaccharides of most meningococcal serogroup C strains contain O-acetyl groups within the sialic acid residues (OAc+),Citation23 while 12–15% of isolates have been shown to lack these O-acetyl groups (OAc−).Citation24,Citation25 The degree of O-acetylation of meningococcal serogroup C capsular polysaccharides is known to affect their immunogenicity, with de-O-acetylated capsular polysaccharides (OAc−) being significantly more immunogenic, as evidenced by higher levels of IgG antibodies elicited and higher serum bactericidal activity.Citation26–28 Information on the strains used in the manufacture of the Lanzhou-AC vaccine is not available and information on the degree of O-acetylation of the capsular polysaccharide of the C2241 Gotschlich strain of serogroup C used in the manufacture of the Men-AC vaccine is not available either. However, given that the lower post-vaccination titers against serogroup C were observed in both vaccine groups in the study, this phenomenon is not likely to be related to either vaccine product.
This study had one potential limitation. There was uncertainty regarding the prior meningococcal immunization history of a subset of participants in this study, who were between 2 and 6 y of age and whose vaccinations within the first 18 months of life were scheduled to include 2 doses of a meningococcal serogroup A vaccine. Parents or legal representatives of participants were required, per the study protocol, to bring with them documents establishing the prior meningococcal immunization history. However, the immunization history could not be accurately established in a small subset of the participants because the parents or legal representatives could not provide the required documents, leaving open the possibility that some participants may have received only a single dose of meningococcal serogroup A vaccine in the first year of life. However, this study was randomized and participants with uncertain prior meningococcal immunization history were equally distributed among both study arms. The impact of the uncertain meningococcal immunization history on the findings of this study was therefore likely to have been minimal.
In conclusion, Men-AC was demonstrated to be similar to Lanzhou-AC with regard to both immunogenicity and safety. This study satisfies the China SFDA requirement for the conduct of an active-controlled study comparing Men-AC to a vaccine already available in the market. Taking into account the burden of meningococcal disease attributable to serogroups A and C in China, Men-AC vaccines can help protect children, who are especially vulnerable to the devastating effects of IMD caused by these serogroups. However, the emergence of serogroups B and W may in the future prompt recommendation of the use of quadrivalent meningococcal conjugate (Men ACWY) and serogroup B meningococcal (Men B) vaccines. Disease surveillance and implementation of standardized laboratory techniques across provinces will be important to ensure optimal epidemiological monitoring.Citation9
Disclosure of potential conflicts of interest
Drs. Zhu, Hu, and Li have no conflicts of interest to disclose. Drs. Shu and Oster are employees of Sanofi Pasteur and have no conflicts to disclose outside the submitted work.
Acknowledgments
We thank Bret Wing, PhD and Robert A. Lersch, PhD of Sanofi Pasteur Inc., Swiftwater, Pennsylvania, USA for managing the development of the manuscript. Prasad Kulkarni, PhD, CMPP of Asclepius Medical Communications LLC, Ridgewood, New Jersey, USA and Julia R. Gage, PhD of Gage Medical Writing LLC, Moorpark, California, USA assisted in the development of the manuscript with funding provided by Sanofi Pasteur SA, Lyon, France.
Additional information
Funding
References
- Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(Suppl 2):B3–9. doi:10.1016/j.vaccine.2011.12.062.
- World Health Organization (WHO). Meningococcal meningitis - Fact sheet. Geneva (Switzerland): World Health Organization (WHO); 2018.
- Harrison LH, Pelton SI, Wilder-Smith A, Holst J, Safadi MA, Vazquez JA, Taha M-K, LaForce FM, Von Gottberg A, Borrow R, et al. The global meningococcal initiative: recommendations for reducing the global burden of meningococcal disease. Vaccine. 2011;29(18):3363–71. doi:10.1016/j.vaccine.2011.02.058.
- Xie O, Pollard AJ, Mueller JE, Norheim G. Emergence of serogroup X meningococcal disease in Africa: need for a vaccine. Vaccine. 2013;31:2852–61. doi:10.1016/j.vaccine.2013.04.036.
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20.
- Wang JF, Caugant DA, Li X, Hu X, Poolman JT, Crowe BA, Achtman M. Clonal and antigenic analysis of serogroup A Neisseria meningitidis with particular reference to epidemiological features of epidemic meningitis in the People’s Republic of China. Infect Immun. 1992;60:5267–82. doi:10.1128/IAI.60.12.5267-5282.1992.
- Li J, Li Y, Yin Z, Yang J, Shao Z, Ning X. [Epidemiological analysis of the meningococcal disease in China during 1997–2006]. Chin J Vacc Immunization. 2007;13:453–56.
- Chen M, Guo Q, Wang Y, Zou Y, Wang G, Zhang X, Xu X, Zhao M, Hu F, Qu D, et al. Shifts in the antibiotic susceptibility, serogroups, and clonal complexes of Neisseria meningitidis in Shanghai, China: a time trend analysis of the pre-quinolone and quinolone eras. PLoS Med. 2015;12(6):e1001838. doi:10.1371/journal.pmed.1001838.
- Li J, Shao Z, Liu G, Bai X, Borrow R, Chen M, Guo Q, Han Y, Li Y, Taha M-K, et al. Meningococcal disease and control in China: findings and updates from the Global Meningococcal Initiative (GMI). J Infect. 2018;76(5):429–37. doi:10.1016/j.jinf.2018.01.007.
- Li J, Li Y, Shao Z, Li L, Yin Z, Ning G, Xu L, Luo H. Prevalence of meningococcal meningitis in China from 2005 to 2010. Vaccine. 2015;33(8):1092–97. doi:10.1016/j.vaccine.2014.10.072.
- Zhang Y, Wei D, Guo X, Han M, Yuan L, Kyaw MH. Burden of Neisseria meningitidis infections in China: a systematic review and meta-analysis. J Glob Health. 2016;6:020409. doi:10.7189/jogh.06.020409.
- Shao Z, Li W, Ren J, Liang X, Xu L, Diao B, Li M, Lu M, Ren H, Cui Z, et al. Identification of a new Neisseria meningitidis serogroup C clone from Anhui province, China. Lancet. 2006;367(9508):419–23. doi:10.1016/S0140-6736(06)68141-5.
- Zhang X, Shao Z, Zhu Y, Xu L, Xu X, Mayer LW, Xu J, Jin Q. Genetic characteristics of serogroup A meningococci circulating in China, 1956-2005. Clin Microbiol Infect. 2008;14:555–61. doi:10.1111/j.1469-0691.2008.01977.x.
- Yang L, Shao Z, Zhang X, Xu L, Peng J, Xu X, Liang X, Qi Y, Jin Q. Genotypic characterization of Neisseria meningitidis serogroup B strains circulating in China. J Infect. 2008;56(3):211–18. doi:10.1016/j.jinf.2007.12.005.
- Zhang TG, Chen C, He JG, Wu J, Chen LJ, Pang XH, Yang J, Shao ZJ, Huang YC. Molecular characterizations of serogroup B Neisseria meningitidis strains circulating in Beijing. Chin Med J (Engl). 2009;122:584–87. PMID 19323912.
- Xu XH, Ye Y, Hu LF, Jin YH, Jiang QQ, Li JB. Emergence of serogroup C meningococcal disease associated with a high mortality rate in Hefei, China. BMC Infect Dis. 2012;12:205. doi:10.1186/1471-2334-12-205.
- Zhou H, Liu W, Xu L, Deng L, Deng Q, Zhuo J, Shao Z. Spread of Neisseria meningitidis serogroup W clone, China. Emerg Infect Dis. 2013;19:1496–99. doi:10.3201/eid1909.130160. PMID 23965378.
- World Health Organization (WHO). Requirements for meningococcal polysaccharide vaccine. World Health Organ Tech Rep Ser; Geneva, Switzerland; 1976.
- Wong KH, Barrera O, Sutton A, May J, Hochstein DH, Robbins JD, Robbins JB, Parkman PD, Seligmann EB Jr. Standardization and control of meningococcal vaccines, group A and group C polysaccharides. J Biol Stand. 1977;5:197–215. doi:10.1016/S0092-1157(77)80005-X. PMID 408354.
- Jodar L, Cartwright K, Feavers IM. Standardisation and validation of serological assays for the evaluation of immune responses to Neisseria meningitidis serogroup A and C vaccines. Biologicals. 2000;28:193–97. doi:10.1006/biol.2000.0253.
- Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun. 2001;69:1568–73. doi:10.1128/IAI.69.3.1568-1573.2001.
- Granoff DM, Pollard AJ. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr Infect Dis J. 2007;26:716–22. doi:10.1097/INF.0b013e3180cc2c25.
- Arakere G, Frasch CE. Specificity of antibodies to O-acetyl-positive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect Immun. 1991;59:4349–56. doi:10.1128/IAI.59.12.4349-4356.1991.
- Apicella MA, Feldman HA. Meningococcal group C subgroup determinant detected by immunofluorescence. Proc Soc Exp Biol Med. 1976;152:289–91. doi:10.3181/00379727-152-39381.
- Borrow R, Longworth E, Gray SJ, Kaczmarski EB. Prevalence of de-O-acetylated serogroup C meningococci before the introduction of meningococcal serogroup C conjugate vaccines in the United Kingdom. FEMS Immunol Med Microbiol. 2000;28:189–91. doi:10.1111/j.1574-695X.2000.tb01475.x.
- Michon F, Huang CH, Farley EK, Hronowski L, Di J, Fusco PC. Structure activity studies on group C meningococcal polysaccharide-protein conjugate vaccines: effect of O-acetylation on the nature of the protective epitope. Dev Biol (Basel). 2000;103:151–60.
- Richmond P, Borrow R, Findlow J, Martin S, Thornton C, Cartwright K, Miller E. Evaluation of De-O-acetylated meningococcal C polysaccharide-tetanus toxoid conjugate vaccine in infancy: reactogenicity, immunogenicity, immunologic priming, and bactericidal activity against O-acetylated and De-O-acetylated serogroup C strains. Infect Immun. 2001;69:2378–82. doi:10.1128/IAI.69.4.2378-2382.2001.
- Fusco PC, Farley EK, Huang CH, Moore S, Michon F. Protective meningococcal capsular polysaccharide epitopes and the role of O acetylation. Clin Vaccine Immunol. 2007;14:577–84. doi:10.1128/CVI.00009-07.