ABSTRACT
The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still spreading globally. The scientific community is attempting to procure an effective treatment and prevention strategy for COVID-19. A rising number of vaccines for COVID-19 are being developed at an unprecedented speed. Development platforms include traditional inactivated or live attenuated virus vaccines, DNA or RNA vaccines, recombinant viral vector vaccines, and protein or peptide subunit vaccines. There are 23 vaccines in the clinical evaluation stage and at least 140 candidate vaccines in preclinical evaluation. In this review, we describe research regarding basic knowledge on the virus, updates on the animal models, current landscape of vaccines in clinical evaluation and updated research results on vaccine development. Safe and effective COVID-19 vaccines require further investigation.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19, was first reported in December 2019,Citation1 and confirmed cases are approaching 20 million globally as of August 2020. Coronaviruses are positive-strand RNA viruses with average genomes ranging from 27 to 31 kb in size.Citation2 Coronaviruses belong to the Coronaviridae family, which are divided into four genera, the alphacoronavirus (alpha-CoV), betacoronavirus (beta-CoV), gammacoronavirus (gamma-CoV), and deltacoronavirus (delta-CoV).Citation2 Only the alphacoronavirus and betacoronavirus were reported to infect human beings. Seven coronaviruses have been reported to infect humans so far, including human coronavirus 229E (HCoV-229E), coronavirus (SARS-CoV), human coronavirus NL63 (HCoV-NL63), human coronavirus HKU1 (HCoV-HKU1), middle east respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV-2.Citation3 HCoV-229E and HCoV-OC43 were isolated in the mid-1960s, whereas SARS-CoV, HCoV-NL63, HCoV-HKU1, and MERS-CoV were reported in 2002, 2004, 2005, and 2012, respectively.Citation4 SARS-CoV-2, with close relations to SARS-CoV by evolutionary analysis, was first reported in Wuhan, China in December, 2019 and instigated severe pneumonia of unknown causes.Citation5 Bats and rodents were natural sources of the virus, which was determined by comparing genome sequences.Citation6 The intermediate hosts that connected the origin of the virus and humans were different. Four of the coronaviruses, the HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1, can lead to mild infections like the common cold or asymptomatic infections.Citation2 SARS-CoV, MERS-CoV, and SARS-CoV-2 can cause mild, severe, or fatal infections.
SARS-CoV, that causes severe acute respiratory syndrome, was the first reported coronavirus that led to a worldwide pandemic, which infected more than 8,098 people in 29 counties from November 2002 to August 2003 with a 10% mortality rate.Citation7 MERS-CoV, responsible for a devastating pandemic first reported in Saudi Arabia in 2012, caused 2,494 laboratory confirmed cases of MERS, resulting in 858 associated deaths in 27 countries (34.4% mortality rate) by December 2019.Citation4 SARS-CoV-2 is the third coronavirus that can cause severe acute respiratory syndrome (COVID-19). The COVID-19 pneumonia has expanded to almost all the countries and regions all over the world in less than half a year. According to the WHO, SARS-CoV-2 has infected almost 20 million people and killed well over a half-million people as of August 2020.Citation8 COVID-19 is an immense threat to global public health. Vaccines are the most economic and effective method to control and prevent infectious diseases. Hence, successful vaccines are in urgent need to combat COVID-19. In this article, research on the genome and protein of SARS-CoV-2, the host receptor and structural analysis of the spike protein, the animal models for COVID-19, the platforms, and progress of the vaccines are reviewed.
Genome and phylogenetic evolution of SARS-CoV-2
After unexplained pneumonia cases were reported in Wuhan, researchers attempted to determine the exact causative pathogen. With the help of next-generation meta-transcriptomic sequencing, the complete viral genome of the causative pathogen was obtained on January 5, 2020 and released on the open access virological website (http://virological.org/) in early January 11, 2020.Citation6 Multiple-sequence alignments of the genome sequences indicated approximately 79% similarity to SARS-CoV. Phylogenetic analyses were performed to determine the evolution of the new virus and it was clear that SARS-CoV-2 was a member of the genus, Betacoronavirus, which falls into a subgenus that includes SARS-CoV.Citation9 Later, it was reported that BatCoV RaTG13, which was previously detected in Rhinolophus affinis from Yunnan Province, showed high sequence identity to SARS-CoV-2 (2019-nCoV) at the nucleic acid level.Citation10 However, SARS-CoV-2 and RaTG13 differed significantly in a number of key genomic features, but the sequence identity may indicate the possibility that SARS-CoV-2 originated from bats. Intermediate hosts could possibly link the ecologically separated bats to humans, as the intermediate hosts for SARS was civetsCitation11 and camels for MERS.Citation12 The most likely intermediate host for SARS-CoV-2 was Malayan pangolins, illegally imported into southern China since there is high similarity in amino acid sequence and similar key mutations in the critical RBD domain.Citation6 One study then showed the isolation of a coronavirus from a Malayan pangolin with high amino acid identity with SARS-CoV-2 and confirmed the intermediate host of the virus, indicating the importance of controlling wildlife tradesCitation13.
Structural proteins and host receptor of the SARS-CoV-2
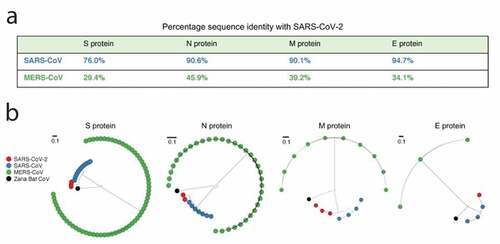
The SARS-CoV-2 genome (~30 kb) was composed of a 5ʹuntranslated region (UTR), replicase complex (orf1ab), S gene, E gene, M gene, N gene, 3ʹUTR, and several unidentified nonstructural open reading frames.Citation14 The S gene, E gene, M gene, and N gene encodes four structural proteins of SARS-CoV-2 similar to other coronaviruses, which consists of the surface glycoprotein (S protein), envelope protein (E protein), membrane protein (M protein) and nucleocapsid protein (N protein).Citation15 The genome also encodes 16 non-structural proteins (nsp1-nsp16) and several accessory proteins. The identity of the four structural proteins was compared with the corresponding proteins of SARS and MERS and phylogenetic analysis was conducted using the sequences of four structural proteins ().Citation16 The identity between SARS-CoV-2 and SARS proteins was high, which was 76% for S protein, 90.6% for N protein, 90.1% for M protein, and 94.7% for E protein, respectively, whereas the identity between SARS-CoV-2 and MERS proteins was very low. The close relationship between SARS-CoV and SARS-CoV-2 could also be observed by the phylogenetic trees.
Figure 1. Comparison of the similarity of structural proteins of SARS-CoV-2 with the corresponding proteins of SARS-CoV and MERS-CoV.Citation16 (a) Percentage genetic similarity of the individual structural proteins of SARS‐CoV‐2 with those of SARS‐CoV and MERSCoV. (b) Circular phylogram of the phylogenetic trees of the four structural proteins. All trees were constructed based on the available unique sequences using PASTA and rooted with the out group Zaria Bat CoV strain (accession ID: HQ166910.1). Reprint from reference.16
S protein, as a transmembrane glycoprotein, mediates the entry of the coronavirus into the host cells through interaction with the cell receptor. The cell receptor was angiotensin-converting enzyme II (ACE2) for SARS, and dipeptidyl peptidase 4 (DPP4) for MERS.Citation17 The cellular entry receptor of SARS-CoV-2 was preliminarily tested using the HeLa cells expressing or not expressing ACE2 proteins, and it was indicated that ACE2 was the likely receptor of SARS-CoV-2, not DPP4 or aminopeptidase.Citation5 Following, a detailed analysis by flow cytometry and competitive inhibition experiment using soluble hACE2 confirmed that ACE2 was the cell receptor of SARS-CoV-2.Citation18 A comparable affinity of SARS-CoV and SARS-CoV-2 to the human ACE2 was observed.Citation19 The determination of the cell receptor of SARS-CoV-2 would benefit studies on the treatment and prevention of COVID-19.
Structure of the S protein, RBD and ACE2
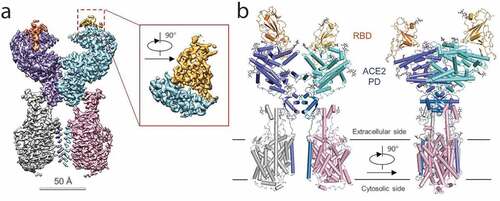
S protein is composed of two functional subunits responsible for binding to the host cell receptor (S1 subunit) through the receptor-binding domain (RBD), and fusion of the viral and cellular membranes (S2 subunit) ().Citation20 The Cryo-EM structure of the SARS-CoV-2 S protein in the prefusion conformation was determined ().Citation20 The trimer S protein rotated to let one of the three RBD domains up to make the receptor accessible. An amino acid mutation was reported in the 614 position of S protein converting the Aspartate to a Glycine residue (D614 G),Citation21,Citation22 which is located on the SD2 region of the RBD-containing S1 subunit. Zhang et al. reported that the mutated S protein could be more stable and increase the infectivity of the virus with no effect on the binding capacity of the ACE2 receptor.Citation23 The structure of the full length human ACE2 protein with or without the RBD domain of the S protein of SARS-CoV-2 made the molecular basis for coronavirus recognition and infection clear ().Citation24 The overall structure of the RBD-ACE2-B0AT1 complex showed that only the closed state of ACE2 was observed in this complex, whereas the open and closed conformation was found in the ACE2-B0AT1 complex. Hence, S protein is always a main target for the vaccine development for its essential role in viral infection. The RBD of the S1 protein is a promising target for vaccine development and it was proven that the RBD protein of the S1 subunit was firmly bound to ACE2 receptors in humans and bats.Citation25 Compared to SARS-CoV RBD, SARS-CoV-2 RBD has a much higher binding affinity to the ACE2 receptor.Citation18,Citation25 The S2 subunit shares 99% identity with that of the bat SARS-CoV and a vaccine based on this subunit has the potential of a broad-spectrum antiviral effect. The E and M proteins have important functions in coronavirus assembly and the N protein is critical for viral RNA synthesis. Other than structural proteins, non-structural proteins also have the potential to be an antigen for SARS-CoV-2 vaccine development, as was reviewed by Zhang et al.Citation26
Figure 2. Structure of 2019-nCoV S in the prefusion conformation.Citation20 (a) Schematic of 2019-nCoV S primary structure colored by domain. Domains that were excluded from the ectodomain expression construct or could not be visualized in the final map are colored white. SS, signal sequence; S2′, S2′ protease cleavage site; FP, fusion peptide; HR1, heptad repeat 1; CH, central helix; CD, connector domain; HR2, heptad repeat 2; TM, transmembrane domain; CT, cytoplasmic tail. Arrows denote protease cleavage sites. (b) Side and top views of the prefusion structure of the 2019-nCoV S protein with a single RBD in the up conformation. The two RBD down protomers are shown as cryo-EM density in either white or gray and the RBD up protomer is shown in ribbons colored corresponding to the schematic in (a). Reprint from reference.20
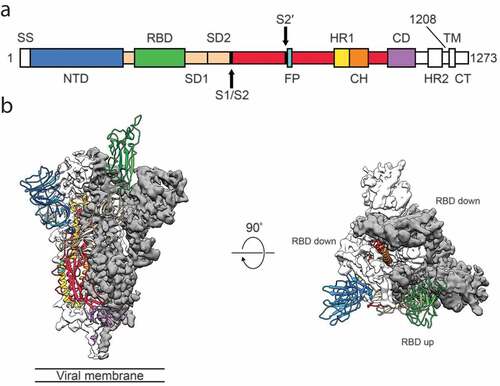
Figure 3. Overall structure of the RBD-ACE2-B0AT1 complex.Citation21 (a) Cryo-EM map of the RBD-ACE2-B0AT1 complex. The overall reconstruction of the ternary complex at 2.9 Å is shown on the left. The inset shows the focused refined map of RBD. Protomer A of ACE2 (cyan), protomer B of ACE2 (blue), protomer A of B0AT1 (pink) and protomer B of B0AT1 (gray) are shown. The red and gold color represent RBD protomers. (b) Overall structure of the RBD-ACE2-B0AT1 complex. The complex is colored by subunits, with the PD and CLD in one ACE2 protomer colored cyan and blue, respectively. The glycosylation moieties are shown as sticks. Reprint from reference.21
Animal models for SARS-CoV-2
An effective animal model is critical for the vaccine development and many vaccine developments are slowed down by lack of proper animal models, as was for SARS and MERS. Yuan et al. summarized the progress in developing animal models, focusing mainly on SARS and MERS.Citation27 We will summarize the recent progress on SARS-CoV-2 animal models. Transgenic mice were constructed expressing human ACE2 where the pathogenicity of SARS-CoV-2 was studied and observed.Citation28 This hACE2 transgenic model may be used to conduct initial animal evaluations of candidate vaccines. Three non-human primates were also utilized to evaluate the infections caused by SARS-CoV-2.Citation29 Effective NHP models were established, the most susceptible was Macaca mulatta followed by Macaca fascicularis and Callithrix jacchus.Citation29 Cynomolgus macaques were used to compare the pathogenesis of SARS, MERS, and SARS-CoV-2, and COVID-19-like symptoms were observed after SARS-CoV-2 inoculation, which suggested that an effective animal model was achieved.Citation30 The susceptibility of ferrets and domesticated animals to SARS-CoV-2 was studied. Ferrets and cats were found to be susceptible to the virus, whereas dogs, pigs, chickens, and ducks were not.Citation31 A ferret model of SARS-CoV-2 was also reported to have infectious symptoms without fatality.Citation32 Rhesus macaques infection model was used to mimic the age-related infections of the virus and respiratory disease in these models was analyzed.Citation33 A golden Syrian hamster model was reported to resemble mild infections in humans.Citation34 Chandrashekar et al. developed a SARS-CoV-2 infection rhesus macaques model to test the protective immunity to viral re-infection.Citation35 Prevention against the relapse of SARS-CoV-2 was observed, which indicated the possible utility of this animal model to test the effect of the vaccines. The establishment of effective animal models will benefit the development of effective vaccines and therapies against SARS-CoV-2, but severe disease models with respiratory failure or mortality is still needed to better mimic the infection in humans.
Platforms and progress on SARS-CoV-2 vaccine development
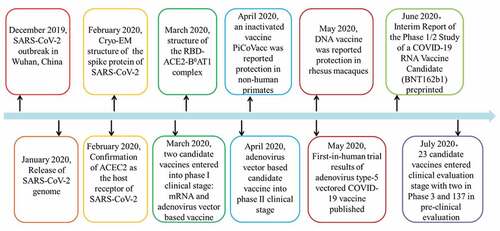
Currently, there are no specific medicines for COVID-19, yet the infection is still spreading worldwide. Therefore, vaccine development is urgently required for disease prevention and control. Experts have called for efforts made to develop effective vaccines for SARS-CoV-2.Citation36–39 Several types of SARS-CoV-2 vaccines are under development at an unprecedented speed, including live attenuated vaccine, inactivated vaccine, subunit vaccine, recombinant vector vaccine, DNA vaccine, and peptide vaccine.Citation40 The progress of COVID-19 vaccine development has noticeably accelerated. In the short period of time since the outbreak of the disease, studies on the viral genomes, protein structures, candidate vaccines, and clinical and pre-clinical trials have been reported and conducted (). Overall, at least 23 vaccines are in clinical evaluation and more than 140 candidates in pre-clinical stage (July 14, 2020). More importantly, at least four vaccine candidates have entered phase 3 evaluation.Citation41
Inactivated and attenuated vaccines
Inactivated vaccine is a whole virus vaccine, which is prepared by in vitro culture of a large number of live viruses and then treated with an inactivated agent.Citation42 Inactivated vaccines can be prepared in a relative short development cycle, but have high safety requirements due to the safety problems of leakage and incomplete inactivation. Live attenuated vaccines can be obtained by serial passage or targeted genetic engineering, but due to the high reverse-mutation rate of RNA, they may also produce more virulent strains or viruses that are susceptible to other hosts, which can be potentially dangerous.Citation40 Despite these safety concerns, live-attenuated or inactive whole virus vaccines represent a classic strategy for viral vaccinations and several successful vaccines have been widely used in humans, such as Polio vaccine and hepatitis vaccine. Phase I clinical results of the inactivated SARS-CoV vaccine showed that the candidate vaccine was safe and immunogenic,Citation43 whereas this form of the MERS vaccine was still in the pre-clinical development stage.
At least 13 inactivated SARS-CoV-2 virus vaccines have been under development with 5 under clinical evaluation and 8 in the pre-clinical stages.Citation41 The five inactivated virus vaccines in were developed in clinical trials, including a vaccine (ChiCTR2000031809) sponsored by Wuhan Institute of Biological Products/Sinopharm, a vaccine (ChiCTR2000032459) sponsored by Beijing Institute of Biological Products/Sinopharm, a vaccine by Sinovac (NCT04383574, NCT04352608, NCT04456595), a vaccine by Institute of Medical Biology/Chinese Academy of Medical Sciences (NCT04412538), and a whole-virion inactivated vaccine by Bharat Biotech (CTRI/2020/07/026300). The phase I/II clinical trial of vaccine (ChiCTR2000031809), an inactivated Novel Coronavirus Pneumonia (COVID-19) vaccine (Vero cells), was registered on April 11, 2020 to evaluate its safety and immunogenicity in healthy population aged 6 years and above.Citation44 The clinical trial of vaccine (NCT04352608) started on April 16, 2020 was a randomized, double-blinded, placebo-controlled Phase I/II Clinical Trial designed to evaluate the safety and immunogenicity of the vaccine in healthy adults aged 18 ~ 59 years.Citation45 This vaccine, manufactured by Sinovac, has entered a phase 3 clinical trial (NCT04456595) in order to evaluate the efficacy and safety of the vaccine where two age groups consisting of adults (18–59 years) and elderly participants (60 years and above) are compared.Citation46 The phase I/II clinical trial evaluation of the safety and immunogenicity of the vaccine (Vero cells, ChiCTR2000032459, registered on April 29) was designed to be tested in healthy populations aged 3 years old and above.Citation47 The phase Ia/IIa trial of the vaccine (NCT04412538) was designed to evaluate the safety and immunogenicity of the vaccine in healthy people aged 18 ~ 59 years.Citation48 The whole-virion inactivated SARS-CoV-2 Vaccine (BBV152) was registered to conduct a phase 1 and phase 2 randomized, double-blind, multicenter study to evaluate its safety and immunogenicity.Citation49
A pilot-scale production of an inactivated SARS-CoV-2 virus vaccine candidate (PiCoVacc, Sinovac) was first reported to have complete protection against SARS-CoV-2 virus in non-human primates.Citation50 Vero cells were used to culture the virus and the genetic stability of the selected strain was tested. The strain with special genetic stability was selected. The proteins S, N and M could be detected in purified PiCoVacc. PiCoVacc induced SARS-CoV-2-specific neutralizing antibodies in mice, rats, and non-human primates, which neutralized 10 representative SARS-CoV-2 strains. Complete protection could be achieved by immunization with a 6 μg dose and the safety of the vaccine was also confirmed by systematic analysis of clinical signs in macaques. Animal experiments supported further clinical development of PiCoVacc for human application. Currently, clinical research has begun for the PiCoVacc vaccine.
The inactivated SARS-CoV-2 vaccine candidate (BBIBP-CorV) in pilot-scale production was also reported. High levels of neutralizing antibodies were induced by the vaccine BBIBP-CorV in several animal models such as mice, rats, guinea pigs, rabbits, and nonhuman primates (cynomolgus monkeys and rhesus macaques). Two-dose immunizations using 2 mg/dose of BBIBP-CorV could induce efficient protection against SARS-CoV-2 intratracheal challenge in rhesus macaques and no antibody-dependent enhancement immune response was detected. The safety, immunogenicity, and stability of the BBIBP-CorV vaccine in animal models supported further clinical evaluation of this vaccine candidate.Citation51
Three live attenuated virus vaccines have been reported in the pre-clinical evaluation stage. Two were designed by the genetic codon deoptimized to attenuate the virus, sponsored by Codagenix/Serum Institute of India and Indian Immunologicals Ltd/Griffith University. One was designed using the measles virus with the target S and N by the German Center for Infection Research. Engineered E protein deleted live-attenuated vaccines for MERS were evaluated to be safe in vitro,Citation52 which may indicate a promising future for this type vaccine.
Recombinant vector vaccine
Recombinant vector vaccine is a designed to produce coronavirus proteins in the body by using genetically engineered viruses that cannot cause diseases, which includes the replicating viral vector vaccine and non-replicating viral vector vaccine.Citation40 At least 41 recombinant vector vaccines for COVID-19 have been in urgent development including 23 non-replicating and 18 replicating viral vector vaccines. Three of the 23 non-replicating viral vector vaccines were in the clinical evaluation stage.
Adenovirus, a non-enveloped DNA virus, is one of the most promising vaccine delivery vectors for its lower production cost and induction of long-term protective immunity.Citation53 A COVID-19 vaccine candidate based on the adenoviral type 5 vectors entered the phase I clinical trial (ChiCTR2000030906 or NCT04313127) on March 16, 2020 in Wuhan to evaluate the dose-escalation safety and tolerance in healthy adults aged between 18 and 60 years.Citation54 All volunteers completed the first vaccination. The vaccine proceeded to a phase II clinical trial (ChiCTR2000031781)Citation55 on April 12 in order to conduct a randomized, double-blinded, placebo-controlled clinical evaluation with an estimated 125 participants in the low-dose group, 250 participants in the medium-dose group, and 125 participants in placebo group (healthy adults above 18 years). This vaccine was the first COVID-19 vaccine candidate that entered a phase II clinical trial. The preliminary results of the phase I experiment have already been published.Citation56 One hundred and eight participants were successfully enrolled (51% male, 49% female; mean age 36.3 years) and were divided into low dose, medium dose, and high dose groups to receive the Ad5 vectored COVID-19 vaccine shots. The vaccines were tolerated in participants with transient and self-limiting severe adverse reactions and common adverse reactions consisted of fever, fatigue, headache, and muscle pain. Successful humoral and T-cell immune response indicated the immunogenicity of the vaccine, but the preexisting anti-Ad5 immunity would reduce the antibody and T-cell immune response of the vaccination. The current results at 28 days post-vaccination demonstrated the safety, tolerability, and immunogenicity of the vaccine, but further research was needed to confirm whether the Ad5 vectored COVID-19 vaccine was effective in preventing SARS-CoV-2 infection. The phase 1 clinical trial of the Adeno-based vaccine “Gam-COVID-Vac” by Gamaleya Research Institute was also registered to assess its safety, tolerability, and immunogenicity in healthy volunteers.Citation57,Citation58
Another recombinant vector vaccine in clinical evaluation is the one from University of Oxford / Astra-Zeneca expressing the surface spike protein of SARS-CoV-2 virus using the chimpanzee adenovirus vector ChAdOx1. A phase I/II single-blinded, randomized, multi-center study was registered March 27, 2020 to determine efficacy, safety, and immunogenicity of the candidate vaccine ChAdOx1 nCoV-19 (COV001, NCT04324606) in UK and conducted in healthy adult volunteers aged 18–55 years.Citation59 The vaccine will be administered intramuscularly (IM). There will be four study groups and it is anticipated that a total of 1112 volunteers will be enrolled. A Phase 3 trial is underway. The animal experiments results of ChAdOx1 nCoV-19 were preprinted on the bioRxiv.Citation60 ChAdOx1-40 vectored vaccine was designed to encode a codon optimized full-length spike protein of SARS-CoV-2 (YP_009724390.1) with a human tPA leader sequence. The vaccination could induce humoral and cell-mediated response in mice and rhesus macaques without immune-enhanced disease in vaccinated animals. The infection experiments indicated that a single vaccination with ChAdOx1 nCoV-19 could reduced the viral loads in BAL fluid and lung tissue of vaccinated animals and prevent the vaccinated rhesus macaques from interstitial pneumonia. But the vaccination did not block the infection of the virus, and further investigation may be required to evaluate the efficacy of the vaccine. The phase III study (ISRCTN899514) of ChAdOx1 nCoV-19 vaccine is ongoing in Brazil to assess whether the vaccine can protect healthy people aged 18 to 55 from COVID-19, meanwhile the safety and immunogenicity of the vaccine will be analyzed further.Citation61
Subunit vaccine
Subunit vaccines are recombinant expressed proteins or synthetic peptides based vaccines.Citation4 The potential safety problem of whole virus vaccines are avoided by using the pure antigen and the immunogenicity of subunit vaccine makes it a promising candidate vaccine. Wang et al.Citation4 reviewed the advances in subunit vaccines against human coronaviruses with a special focus on SARS-CoV and MERS-CoV in early 2020. S protein was the main target for the development of the subunit vaccine against SARS-CoV, MERS-CoV, and SARS-CoV-2 for its critical role in the entry of the virus into host cells.Citation62,Citation63
At least 52 subunit vaccines against COVID-19 were under development and 5 protein subunit vaccines have entered clinical evaluation. Most of these vaccines used either the full length or part of the S protein as the target. The recombinant protein was expressed using different expression systems mainly in eukaryotic cells and designed to be delivered with different adjuvants and procedures. The SARS-CoV-2 rS was a recombinant glycoprotein nanoparticle vaccine sponsored by Novavax. A phase I clinical trial was registered to evaluate the safety and immunogenicity of this nanoparticle vaccine with or without matrix-M adjuvant (NCT04368988).Citation64 The study would recruit 131 participants between 18 and 59 years old to receive two IM injections. The phase 1 clinical trial of SCB 2019, a recombinant SARS-CoV-2 Trimeric S Protein Subunit Vaccine sponsored by Clover Biopharmaceuticals Inc./GSK/Dynavax, was registered (NCT04405908).Citation65 An adjuvanted recombinant protein vaccine against SARS-CoV2 based on the RBD-Dimer by Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences was registered to conduct the phase 1 stage evaluation in 50 subjects (NCT04445194).Citation66 Covax-19 is a protein subunit vaccine made of recombinant spike protein with Advax™ adjuvant sponsored by Vaxine Pty Ltd/Medytox, and the phase 1 clinical trial was registered to evaluate the safety and efficacy of it in adults between 18 and 65 years old (NCT04453852).Citation67 A protein subunit vaccine candidate was designed by University of Queensland/CSL/Seqirus using molecular clamp stabilized Spike protein with MF59 adjuvant, and registered to conduct a phase 1 clinical evaluation to assess its safety and immune response (ACTRN12620000674932p).Citation68
Immunogenicity of recombinant SARS-CoV-2 S1-Fc fusion protein expressed in mammalian CHO-K1 cells was reported to be effective in mice, rabbits, and monkeys, which can be evaluated for its potential as a candidate COVID-19 subunit vaccine.Citation69 The immunogenicity of recombinant SARS-CoV-2 S1-Fc fusion protein was evaluated by the antibody levels and neutralizing titers. The purified recombinant S1-Fc fusion protein was formulated with the adjuvant and then used to immunize animals, in which the first immunization used CFA (Freund’s complete adjuvant) and the boost immunization used AD11.10 (saponin-based microemulsion). High levels of the anti-S1 antibodies and neutralizing activities against live SARS-CoV-2 were measured.
Nucleic acid vaccine
Nucleic acid vaccines include DNA and RNA vaccines, which are safe and easy to design based on the genetic sequence of the virus.Citation70,Citation71 These vaccines can induce both humoral and cellular immunity, and has shown protective effects in animal models. There are currently no licensed vaccines using this platform.
At least 11 DNA vaccines against SARS-CoV-2 were in pre-clinical stages and four have entered clinical evaluation. A DNA plasmid vaccine (INO-4800) for COVID-19, delivered by electroporation from Inovio Pharmaceuticals, was in a Phase I clinical trial (NCT04336410).Citation72 The phase I clinical trial of INO-4800 vaccine started on April 3, 2020, with 40 participants aged from 18 years to 50 years. The trial was designed to employ intradermal administration with one or two injections per administration on Day 0 and Week 4 to evaluate the safety, tolerability, and immunogenicity of INO-4800. The phase I/IIa trial (NCT04447781) of INO-4800 was registered on June 25, 2020 to evaluate the safety, tolerability, and immunological profile of this DNA vaccine in 160 participants aged 19 to 64 years in Republic of Korea.Citation73 A Phase 1/2 clinical trial of COVID-19 DNA Vaccine (AG0301-COVID19, NCT04463472) sponsored by Osaka University/AnGes/Takara Bio was registered to assess the safety and immunogenicity of the vaccine in 30 healthy volunteers aged 20–65 divided into the low and high dose groups.Citation74 The DNA plasmid vaccine by Cadila Healthcare Limited was registered for the Phase 1/2 (CTRI/2020/07/026352) clinical trial to test the performance of the vaccine by intradermal route in healthy subjects between 18 and 55 years old.Citation75 GX-19 was another DNA vaccine candidate for COVID-19 registered for the phase 1/2a clinical trial (NCT04445389) to evaluate its safety, tolerability, and immunogenicity. GX-19 developed by Genexine Consortium would be intramusculary administered with 40 subjects in phase 1 and 150 in phase 2a.Citation76 One candidate DNA vaccine for SARS was proven to be well tolerated and immunogenic in healthy adults through a phase I clinical trial.Citation77 MERS DNA vaccine GLS-5300 was evaluated by the phase I clinical study and the results released in 2019 showed that GLS-5300 was safe and immunogenic in healthy adult humans.Citation78 Antibody and cellular immune responses were induced by this vaccine without serious adverse events. The safety showed in the clinical evaluation of the DNA vaccine for SARS and MERS shows the potential for similar vaccine candidates for COVID-19. The protective efficacy and safety of DNA vaccines expressing full length or different parts of SARS-CoV-2 S protection were tested in the rhesus macaques.Citation79 Reduced viral loads could be measured in the vaccinated monkeys compared to the controls, which indicated the protective effects of the vaccines against the virus. Meanwhile, antibody-dependent enhanced respiratory diseases were not observed. Positive results in nonhuman primates using DNA vaccines validate further evaluation for this vaccine.
mRNA vaccine containing the selected sequence of the viral gene can translate directly in the host cell cytoplasm to produce the target antigen.Citation70 Five mRNA vaccines were in clinical evaluation stages and more than 17 in pre-clinical studies. The first mRNA vaccine for COVID-19 was mRNA-1273 (NCT04283461) and manufactured by Moderna, which initiated the first injections on March 16, 2020.Citation80 The mRNA vaccine mRNA-1273 is a novel lipid nanoparticle (LNP)-encapsulated mRNA-based vaccine that encodes for a full-length, prefusion stabilized spike (S) protein of SARS-CoV-2, which will be administered twice by intramuscular (IM) injection on days 1 and 29.Citation81 The study aimed to enroll 105 participants from 18 years to 99 years. The Phase 2a clinical study of the candidate vaccine mRNA-1273 (NCT04405076) started on May 29, 2020, which was designed to evaluate the safety, reactogenicity, and immunogenicity of the vaccine in two dose levels in 600 adults aged over 18 years.Citation82 A Phase 3 trial is underway for this vaccine. The second mRNA vaccine in clinical evaluation for COVID-19 was BNT162 (Phase 1/2, 2020–001038-36, NCT04368728) sponsored by BioNTech/Fosun Pharma/Pfizer.Citation83,Citation84 A multi-site Phase I/II, 2-Part, dose-escalation trial is being conducted with an estimated 7600 subjects to investigate the safety and immunogenicity of four RNA Vaccines (BNT162a1, b1, b2, c2) against SARS-CoV-2 virus in healthy adults aged from 18 to 85 years by intramuscular use. The Phase 1 clinical trial (ISRCTN17072692) of a self-amplifying ribonucleic acid (saRNA) vaccine encoding the S glycoprotein of SARS-CoV-2, sponsored by the Imperial College London, was registered to assess its safety and immunogenicity.Citation85 Two groups were designed where one group was evaluated for dose escalation in adults aged 18–45 years old and the other group was evaluated for the safety of the vaccine in subjects aged 18–75 years old. CVnCoV Vaccine was also a SARS-CoV-2 mRNA Vaccine which was developed by Curevac and a phase 1 clinical trial (NCT04449276) was registered to assess the safety, reactogenicity, and immunogenicity of the vaccine in adults aged 18–60.Citation86 Another SARS-CoV-2 mRNA vaccine sponsored by the People’s Liberation Army (PLA) Academy of Military Sciences/Walvax Biotech was registered for a phase I clinical trial (ChiCTR2000034112) to evaluate its safety, tolerance, and preliminary immunogenicity in healthy participants aged 18–59 years and 60 years old and above.Citation87 The rationale of mRNA vaccine development for COVID-19 was reviewedCitation88 where four safety and efficacy advantages were summarized and potential risks were also mentioned. As a new technology, special attention must be given regarding safety issues for human use, yet the vaccine is still worth developing. Positive results in the Phase I clinical evaluation of mRNA-1273 yielded new expectations for this vaccine. Recently, the interim report of the ongoing phase 1/2 study of a COVID-19 RNA Vaccine candidate (BNT162b1) was preprinted by medRxiv. After being injected with the vaccine, dose-dependent local reactions and systemic events were observed, which varied from mild to moderate and could be relieved within a few days. Robust immunogenicity and neutralization titers were measured at Day 21 for all dose levels and Day 28 (7 days after Dose 2). The preliminary results of this vaccine indicated a well-tolerated and immunogenic dose level (10 μg and 30 μg) and the safety and immunogenicity of the vaccine. Large-scale clinical trials with more participants of different ages are being conducted to further assess the vaccine BNT162b1.Citation89
Conclusions and prospects
Many years have passed since the outbreak of SARS in 2002 and MERS in 2012. However, no licensed vaccines or therapies are available. SARS-CoV-2 is more infectious with high human-to-human transmission ability and people of all ages are susceptible where they may experience mild or severe pneumonia. Studies and government actions to control the spread of the disease had been accelerated. The entry receptor of the virus into the host cell was determined, the structure of the S protein and the RBD-AEC2 was revealed, and more than 163 candidate vaccines have been designed. Several types of vaccine candidates have entered clinical evaluation. Even though the world is waiting for a successful COVID-19 vaccine, sufficient effort must be made to establish the safety and effectiveness of the vaccine. Thus, adequate time is required to develop an effective vaccine. However, currently published results of experimental animal evaluations and phase I/II clinical trials show promising prospects for a successful COVID-19 vaccine.
Author contribution
LFL composed the manuscript. HQS, WCZ, ZDY and LFL discussed and confirmed the contents and framework of the manuscript. XMZ and PBG retrieved the latest literature. All authors contributed to the drafting and critical review of the manuscript.
Disclosure of potential conflicts of interest
The authors declare no conflict of interests.
Additional information
Funding
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727–33. doi:10.1056/NEJMoa2001017. PubMed PMID: 31978945; PubMed Central PMCID: PMCPMC7092803.
- Wevers BA, van der Hoek L Recently discovered human coronaviruses. Clin Lab Med. 2009 Dec;29(4):715–24. PubMed PMID: 19892230; PubMed Central PMCID: PMCPMC7131583. doi:10.1016/j.cll.2009.07.007.
- Weston S, Frieman MB. COVID-19: knowns, unknowns, and questions. mSphere. 2020 Mar 18;5(2). doi:10.1128/mSphere.00203-20. PubMed PMID: 32188753; PubMed Central PMCID: PMCPMC7082143.
- Wang N, Shang J, Jiang S, Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol 2020;11:298. PubMed PMID: 32265848; PubMed Central PMCID: PMCPMC7105881. doi:10.3389/fmicb.2020.00298.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270–73. doi:10.1038/s41586-020-2012-7. PubMed PMID: 32015507; PubMed Central PMCID: PMCPMC7095418.
- Zhang YZ, Holmes EC. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020 Mar 26. doi:10.1016/j.cell.2020.03.035. PubMed PMID: 32220310.
- Du L, He Y, Zhou Y, Liu S, Zheng B-J, Jiang S. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009 Mar;7(3):226–36. PubMed PMID: 19198616; PubMed Central PMCID: PMCPMC2750777. doi:10.1038/nrmicro2090.
- WHO Coronavirus Disease (COVID-19) dashboard 2020 World Health Organization; [ updated 2020 May 14]. [accessed 2020 Jul 15]. https://covid19.who.int/.
- Wu Y, Ho W, Huang Y, Jin D-Y, Li S, Liu S-L, Liu X, Qiu J, Sang Y, Wang Q, et al. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020;395(10228):949–50. doi:10.1016/s0140-6736(20)30557-2.
- Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Current Bio 2020 Apr 6;30(7):1346–1351.e2. doi:10.1016/j.cub.2020.03.022. PubMed PMID: 32197085; PubMed Central PMCID: PMCPMC7156161. eng.
- Tu C, Crameri G, Kong X, Chen J, Sun Y, Yu M, Xiang H, Xia X, Liu S, Ren T, et al. Antibodies to SARS coronavirus in civets. Emerg Infect Dis. 2004 Dec;10(12):2244–48. doi:10.3201/eid1012.040520. PubMed PMID: 15663874; PubMed Central PMCID: PMCPMC3323399. eng.
- Ji JS. Origins of MERS-CoV, and lessons for 2019-nCoV. Lancet Planet Health. 2020;4(3):e93. doi:10.1016/s2542-5196(20)30032-2.
- Xiao K, Zhai J, Feng Y, Zhou N, Zhang X, Zou -J-J, Li N, Guo Y, Li X, Shen X, et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020 May 7;583:286–89. doi:10.1038/s41586-020-2313-x. PubMed PMID: 32380510.
- Srinivasan S, Cui H, Gao Z, Liu M, Lu S, Mkandawire W, Narykov O, Sun M, Korkin D. Structural genomics of SARS-CoV-2 indicates evolutionary conserved functional regions of viral proteins. Viruses 2020 Mar 25;12(4):360. doi:10.3390/v12040360. PubMed PMID: 32218151.
- Zhang W, Zhang P, Wang G, Cheng W, Chen J, Zhang X. Recent advances of therapeutic targets and potential drugs of COVID-19. Die Pharmazie. 2020 May 1;75(5):160–62. doi:10.1691/ph.2020.0431. PubMed PMID: 32393419; eng.
- Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses 2020 Feb 25;12(3):254. doi:10.3390/v12030254. PubMed PMID: 32106567.
- Li K, Wohlford-Lenane CL, Channappanavar R, Park J-E, Earnest JT, Bair TB, Bates AM, Brogden KA, Flaherty HA, Gallagher T, et al. Mouse-adapted MERS coronavirus causes lethal lung disease in human DPP4 knockin mice. Proc Natl Acad Sci U S A. 2017 Apr 11;114(15):E3119–e3128. doi:10.1073/pnas.1619109114. PubMed PMID: 28348219; PubMed Central PMCID: PMCPMC5393213. eng.
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020 Mar 27;11(1):1620. doi:10.1038/s41467-020-15562-9. PubMed PMID: 32221306; PubMed Central PMCID: PMCPMC7100515.
- Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020 Apr 16;181(2):281–292 e6. doi:10.1016/j.cell.2020.02.058. PubMed PMID: 32155444; PubMed Central PMCID: PMCPMC7102599.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020 Mar 13;367(6483):1260–63. doi:10.1126/science.abb2507. PubMed PMID: 32075877; PubMed Central PMCID: PMCPMC7164637. eng.
- Zehender G, Lai A, Bergna A, Meroni L, Riva A, Balotta C, Tarkowski M, Gabrieli A, Bernacchia D, Rusconi S, et al. Genomic characterization and phylogenetic analysis of SARS-COV-2 in Italy. J Med Virol. 2020 Mar 29. doi:10.1002/jmv.25794. PubMed PMID: 32222993; PubMed Central PMCID: PMCPMC7228393. eng.
- Eaaswarkhanth M, Madhoun AA, Al-Mulla F. Could the D614G substitution in the SARS-CoV-2 spike (S) protein be associated with higher COVID-19 mortality. Int J Infect Dis. 2020;96:459–60. doi:10.1016/j.ijid.2020.05.071.
- Zhang L, Jackson CB, Mou H, Ojha A, Rangarajan ES, Izard T, Farzan M, Choe H. The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. bioRxiv. 2020. doi:10.1101/2020.06.12.148726.
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020 Mar 27;367(6485):1444–48. doi:10.1126/science.abb2762. PubMed PMID: 32132184; PubMed Central PMCID: PMCPMC7164635. eng.
- Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020 Mar 19;17:613–20. doi:10.1038/s41423-020-0400-4. PubMed PMID: 32203189.
- Zhang J, Zeng H, Gu J, Li H, Zheng L, Zou Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines (Basel). 2020 Mar 29;8(2). doi: 10.3390/vaccines8020153. PubMed PMID: 32235387.
- Yuan L, Tang Q, Cheng T, Xia N. Animal models for emerging coronavirus: progress and new insights. Emerg Microbes Infect. 2020 Dec;9(1):949–61. PubMed PMID: 32378471. doi:10.1080/22221751.2020.1764871.
- Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Wei Q, Yu P, Xu Y, Qi F, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020 May 7;583:830–33. doi:10.1038/s41586-020-2312-y. PubMed PMID: 32380511; eng.
- Lu S, Zhao Y, Yu W, Yang Y, Gao J, Wang J, Kuang D, Yang M, Yang J, Ma C, Xu J. Comparison of SARS-CoV-2 infections among 3 species of non-human primates. Biorxiv. 2020. doi:10.1101/2020.04.08.031807.
- Rockx* B TKDdM, van Amerongen G, Haagmans* BL, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, van den Brand J, van Run LL P, Sikkema R, Ernst Verschoor3 Langermans4,5, Christian Drosten6 Koopmans1, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 2020. doi: 10.1126/science.abb7314.
- Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020 Apr 8;368:1016–20. doi:10.1126/science.abb7015. PubMed PMID: 32269068; PubMed Central PMCID: PMCPMC7164390. eng.
- Kim YI, Kim SG, Kim SM, Kim E-H, Park S-J, Yu K-M, Chang J-H, Kim EJ, Lee S, Casel MAB, et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020 May 13;27(5):704–709.e2. doi:10.1016/j.chom.2020.03.023. PubMed PMID: 32259477; PubMed Central PMCID: PMCPMC7144857. eng.
- Munster VJ, Feldmann F, Williamson BN, van Doremalen N, Pérez-Pérez L, Schulz J, Meade-White K, Okumura A, Callison J, Brumbaugh B, et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020 May 12. doi:10.1038/s41586-020-2324-7. PubMed PMID: 32396922; eng.
- Sia SF, Yan LM, Chin AWH, Fung K, Choy K-T, Wong AYL, Kaewpreedee P, Perera RAPM, Poon LLM, Nicholls JM, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020 May 14;583:834–38. doi:10.1038/s41586-020-2342-5. PubMed PMID: 32408338; eng.
- Chandrashekar A, Liu J, Martinot AJ, McMahan K, Mercado NB, Peter L, Tostanoski LH, Yu J, Maliga Z, Nekorchuk M, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020 May 20:eabc4776. doi:10.1126/science.abc4776. PubMed PMID: 32434946; eng.
- Shanmugaraj B, Malla A, Phoolcharoen W. Emergence of novel coronavirus 2019-nCoV: need for rapid vaccine and biologics development. Pathogens 2020 Feb 22;9(2):148. doi:10.3390/pathogens9020148. PubMed PMID: 32098302.
- Shang W, Yang Y, Rao Y, Rao X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines 2020;5:18. PubMed PMID: 32194995; PubMed Central PMCID: PMCPMC7060195. doi:10.1038/s41541-020-0170-0.
- Lu S. Timely development of vaccines against SARS-CoV-2. Emerg Microbes Infect 2020;9(1):542–44. doi:10.1080/22221751.2020.1737580. PubMed PMID: 32148172; PubMed Central PMCID: PMCPMC7144304.
- Caddy S. Developing a vaccine for covid-19. BMJ. 2020 May 4;369:m1790. doi:10.1136/bmj.m1790. PubMed PMID: 32366511.
- Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020 Apr;580(7805):576–77. PubMed PMID: 32346146; eng. doi:10.1038/d41586-020-01221-y.
- Draft landscape of COVID 19 candidate vaccines 2020 [ updated 2020 May 11]. [accessed 2020 Jul 14]. https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines.
- Yong CY, Ong HK, Yeap SK, Ho KL, Tan WS. Recent advances in the vaccine development against middle east respiratory syndrome-coronavirus. Front Microbiol 2019;10:1781. doi:10.3389/fmicb.2019.01781. PubMed PMID: 31428074; PubMed Central PMCID: PMCPMC6688523.
- Lin J, Zhang J, Liu J, Gao H, Gao Q, Ning Y, Su N, Xu G , Jia Y, Liu Y , Wang H , Li M , Wang N , Chen J, Sun R. Safety and immunogenicity from a Phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther. 2007;12(7):1107–13.
- A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated novel coronavirus pneumonia vaccine (Vero cells) Chinese clinical trail registry [ accessed 2020Apr11]. http://www.chictr.org.cn/showprojen.aspx?proj=52227.
- Safety and immunogenicity study of inactivated vaccine for prophylaxis of SARS CoV-2 infection (COVID-19). NIH-Clinical Trial.gov; [ accessed 2020 Apr 20]. https://clinicaltrials.gov/ct2/show/NCT04352608?term=Sinovac&cntry=CN&draw=2.
- Double-blind, randomized, placebo-controlled phase III Clinical trial to evaluate efficacy and safety in healthcare professionals of the adsorbed COVID-19 (Inactivated) vaccine manufactured by Sinovac 2020 [accessed 2020 Jul 2]. https://clinicaltrials.gov/ct2/show/NCT04456595?term=vaccine&cond=covid-19&draw=2&rank=1.
- Evaluation of the safety and immunogenicity of inactivated novel coronavirus (2019-CoV) vaccine (Vero cells) in healthy population aged 3 years and above: a randomized, double-blind, placebo parallel-controlled phase I/II clinical trial. Chinese Clinical Trail Registry; [ accessed 2020 Apr 29]. http://www.chictr.org.cn/showproj.aspx?proj=53003.
- Safety and immunogenicity study of an inactivated SARS-CoV-2 vaccine for preventing against COVID-19: clinicalTrials.gov; 2020 [accessed 2020 June 2]. https://clinicaltrials.gov/ct2/show/NCT04412538?term=vaccine&cond=covid-19&draw=2.
- A seamless phase 1, followed by Phase 2 randomized, double-blind, multicenter study to evaluate the safety, reactogenicity, tolerability and immunogenicity of the whole-virion inactivated SARS-CoV-2 Vaccine (BBV152) in healthy volunteers. 2020 [accessed 2020 Jul 7]. http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=45184&EncHid=&userName=bbv152.
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi:10.1126/science.abc1932.
- Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, Xu W, Zhao Y, Li N, Zhang J, Liang H. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182:1–9. doi:10.1016/j.cell.2020.06.008.
- Almazán F, DeDiego ML, Sola I, Zuñiga S, Nieto-Torres JL, Marquez-Jurado S, Andrés G, Enjuanes L. Engineering a replication-competent, propagation-defective middle east respiratory syndrome coronavirus as a vaccine candidate. mbio. 2013;4(5):e00650–13. doi:10.1128/mBio.00650-13.
- Xiang K, Ying G, Yan Z, Shanshan Y, Lei Z, Hongjun L, Maosheng S. Progress on adenovirus-vectored universal influenza vaccines. Human Vaccines % Immunother. 2015;11(5):1209–22. doi:10.1080/21645515.2015.1016674.
- A phase I clinical trial for recombinant novel coronavirus (2019-COV) vaccine (adenoviral vector): Chinese Clinical Trail Registry; [ accessed 2020 Mar 17]. http://www.chictr.org.cn/showprojen.aspx?proj=51154.
- A randomized, double-blinded, placebo-controlled phase II clinical trial for recombinant novel coronavirus (2019-nCOV) vaccine (Adenovirus Vector). Chinese Clinical Trail Registry: Chinese Clinical Trail Registry; 2020 [accessed 2020 Apr 10]. http://www.chictr.org.cn/showprojen.aspx?proj=52006.
- Zhu F, Li Y, Guan X, Wang W, Li J, Wu S, Wang B, Wang Z, Wang L, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet (London, England). 2020 May 22. doi:10.1016/s0140-6736(20)31208-3. PubMed PMID: 32450106; eng.
- An open study of the safety, tolerability and immunogenicity of the drug “Gam-COVID-Vac” vaccine against COVID-19 2020 [ accessed 2020 Jun 18]. https://clinicaltrials.gov/ct2/show/NCT04436471?term=vaccine&cond=covid-19&draw=4.
- An open study of the safety, tolerability and immunogenicity of “Gam-COVID-Vac Lyo” vaccine against COVID-19 2020 [ accessed 2020 Jun 18]. https://clinicaltrials.gov/ct2/show/NCT04437875?term=vaccine&cond=covid-19&draw=4.
- A study of a candidate COVID-19 vaccine (COV001). NIH-Clinical Trial.gov; [ accessed 2020 Mar 27]. https://clinicaltrials.gov/ct2/show/NCT04324606?term=vaccine&cond=covid-19&draw=2.
- van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, Avanzato V, Bushmaker T, Flaxman A, Ulaszewska M, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. 2020. doi:10.1101/2020.05.13.093195.
- A phase III study to investigate a vaccine against COVID-19 2020 [ updated 2020 12 Jun; 2020 26 May]. https://doi.org/10.1186/ISRCTN89951424
- Zhang N, Jiang S, Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev Vaccines. 2014;13(6):761–74. doi:10.1586/14760584.2014.912134.
- Padron-Regalado E. Vaccines for SARS-CoV-2: lessons from other coronavirus strains. Infect Dis Ther. 2020;9:255–74. doi:10.1007/s40121-020-00300-x.
- Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle vaccine with/without matrix-M adjuvant. 2020.
- A phase 1, randomized, double-blind, placebo-controlled, first-in-human study to evaluate the safety and immunogenicity of SCB 2019, a recombinant SARS-CoV-2 trimeric S protein subunit vaccine for COVID-19 in healthy volunteers: ClinicalTrials.gov; 2020 [updated 2020 Jun 23]. https://clinicaltrials.gov/ct2/show/NCT04405908?term=clover&cond=covid-19&draw=2&rank=1.
- A multi-center, double-blind, randomized, placebo parallel controlled, safety and tolerability phase I clinical trial of recombinant novel coronavirus vaccine (CHO Cells) in healthy people between 18 and 59 years of age clinicaltrials.gov2020 [ updated July 2, 2020]. https://clinicaltrials.gov/ct2/show/NCT04445194?term=longcom&draw=2
- A randomised, controlled, phase 1 study to evaluate the safety and immunogenicity of a candidate adjuvanted recombinant protein SARS-COV-2 vaccine in healthy adult subjects 2020 [ accessed 2020 July 1]. https://clinicaltrials.gov/ct2/show/NCT04453852?term=vaccine&cond=covid-19&draw=5
- A phase 1 randomised, double-blind, placebo-controlled, dosage-escalation, single centre study to evaluate the safety and immunogenicity of an adjuvanted SARS-CoV-2 sclamp protein subunit vaccine (COVID-19 vaccine) in healthy adults aged 18 to 55 years old 2020 [ accessed 2020 12 Jun]. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379861&isReview=true
- Ren W, Sun H, Gao GF, Chen J, Sun S, Zhao R, Gao G, Hu Y, Zhao G, Chen Y, Jin X, et al. Recombinant SARS-CoV-2 spike S1-Fc fusion protein induced high levels of neutralizing responses in nonhuman primates. bioRxiv. 2020. doi:10.1101/2020.04.21.052209.
- Rauch S, Jasny E, Schmidt KE, Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.01963.
- Okuda K, Wada Y, Shimada M. Recent developments in preclinical DNA vaccination. Vaccines (Basel) 2014 Jan 13;2(1):89–106. doi:10.3390/vaccines2010089. PubMed PMID: 26344468; PubMed Central PMCID: PMCPMC4494203.
- Safety, tolerability and immunogenicity of INO-4800 for COVID-19 in healthy volunteers. NIH-Clinical Trial.gov. [ accessed 2020 Apr 7]. https://clinicaltrials.gov/ct2/show/NCT04336410?term=inovio&cond=covid-19&draw=2&rank=1.
- A phase I/IIa, dose-ranging trial to evaluate safety, tolerability and immunogenicity of INO-4800, a prophylactic vaccine against SARS-CoV-2, administered intradermally followed by electroporation in healthy volunteers 2020 [ updated 2020 June 25]. https://clinicaltrials.gov/ct2/show/NCT04447781
- A non-randomized, open-label, non-controlled phase I/II study to assess safety and immunogenicity of two doses of intramuscular AG0301-COVID19 (1mg/2mg) in healthy adults clinicaltrials.gov2020 [ accessed 2020 July 9]. https://clinicaltrials.gov/ct2/show/NCT04463472?term=NCT04463472&draw=2&rank=1
- A prospective, randomized, adaptive, phase I/II clinical study to evaluate the safety and immunogenicity of novel corona virus −2019-nCov vaccine candidate of M/s Cadila Healthcare Limited by intradermal route in healthy subjects 2020 [ accessed 2020 04 Jul]. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45306&EncHid=&userName=vaccine
- A phase 1/2a, multi-center, randomized, double-blind, placebo-controlled study to investigate the safety, tolerability, and immunogenicity of GX-19, a COVID-19 preventive DNA vaccine in healthy subjects 2020 [ updated June 26, 2020]. https://clinicaltrials.gov/ct2/show/NCT04445389?term=vaccine&cond=covid-19&draw=3
- Martina JE, Loudera MK, Holmana LA, Gordon IJ, Enama ME, Larkin BD, Andrews CA, Vogel L, Koup RA, Roederer M, et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune_responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26:6338–43. doi:10.1016/j.vaccine.2008.09.026.
- Modjarrad K, Roberts CC, Mills KT, Castellano AR, Paolino K, Muthumani K, Reuschel EL, Robb ML, Racine T, Oh M-D, et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19:1013–22. doi:10.1016/S1473-3099(19)30266-X.
- Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, Mahrokhian SH, Nkolola JP, Liu J, Li Z, Chandrashekar A, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020 May 20:eabc6284. doi:10.1126/science.abc6284. PubMed PMID: 32434945; eng.
- Cohen J. Vaccine designers take first shots at COVID-19. Nature. 2020;368:14–16.
- Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19) NIH-clinical trial.gov. [ accessed 2020 February 25]. https://clinicaltrials.gov/ct2/show/NCT04283461?term=vaccine&cond=covid-19&draw=2.
- A phase 2a, randomized, observer-blind, placebo controlled, dose-confirmation study to evaluate the safety, reactogenicity, and immunogenicity of mRNA-1273 SARS-COV-2 vaccine in adults aged 18 years and older 2020 [ updated 2020 July 10]. https://clinicaltrials.gov/ct2/show/NCT04405076?term=moderna&cond=covid-19&draw=2.
- A multi-site phase I/II, 2-part, dose-escalation trial investigating the safety and immunogenicity of four prophylactic SARS-CoV-2 RNA vaccines against COVID-2019 using different dosing regimens in healthy adults. EU Clinical Trails Register. [ accessed 2020 Apr 14]. https://www.clinicaltrialsregister.eu/ctr-search/search?query=BNT162-01.
- A phase 1/2, placebo-controlled, randomized, observer blind, dose-discovery study to describe the safety, tolerability, immunogenicity, and potential efficacy of SARS-COV-2 RNA vaccine candidates against COVID-19 in healthy adults 2020 [ updated 2020 June 29; accessed 2020 April 30]. https://clinicaltrials.gov/ct2/show/NCT04368728?term=vaccine&cond=covid-19&draw=3.
- A first-in-human clinical trial to assess the safety and immunogenicity of a self-amplifying ribonucleic acid (saRNA) vaccine encoding the S glycoprotein of SARS-CoV-2, the causative agent of COVID-19 2020 [ accessed 2020 Apr 06]. http://www.isrctn.com/ISRCTN17072692.
- A phase 1, partially blind, placebo-controlled, dose-escalation, first-in-human, clinical trial to evaluate the safety, reactogenicity and immunogenicity after 1 and 2 doses of the investigational SARS-CoV-2 mRNA vaccine CVnCoV administered intramuscularly in healthy adults 2020 [ accessed 2020 June 26]. https://clinicaltrials.gov/ct2/show/NCT04449276?term=vaccine&cond=covid-19&draw=6.
- A Phase I clinical trial to evaluate the safety, tolerance and preliminary immunogenicity of different doses of a SARS-CoV-2 mRNA vaccine in population aged 18–59 years and 60 years and above. 2020.
- Wang F, Kream RM, Stefano GB. An evidence based perspective on mRNA-SARS-CoV-2 vaccine development. Med Sci Monit. 2020 May 5;26:e924700. doi:10.12659/MSM.924700. PubMed PMID: 32366816.
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson AK, et al. Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 years of age: interim report. medRxiv. 2020. doi:10.1101/2020.06.30.20142570.