ABSTRACT
The sudden emergence of a highly transmissible and pathogenic coronavirus SARS-CoV-2 in December 2019 from China and its rapid global spread has posed an international health emergency. The rapid development of an effective vaccine is imperative to control the spread of SARS-CoV-2. A number of concurrent efforts to find an effective therapeutic agent or vaccine for COVID-19 (coronavirus disease 2019) are being undertaken globally. Oral and nasal mucosal surfaces serve as the primary portal of entry for pathogens like coronaviruses in the human body. As evidenced by studies on similar coronaviruses (SARS-CoV and MERS-CoV), mucosal vaccination can provide a safe and effective means for the induction of long-lasting systemic and mucosal immunity to confer protection against SARS-CoV-2. This article summarizes the approaches to an effective mucosal vaccine formulation which can be a rewarding approach to combat the unprecedented threat posed by this emerging global pandemic.
Introduction
In the 21st century, we have seen a worldwide spread of three previously unknown coronaviruses. The first outbreak of Severe Acute Respiratory Syndrome (SARS) occurred in November 2002 in the Guangdong province, China. The causative agent of the 2002 SARS outbreak was identified as SARS coronavirus (SARS-CoV).Citation1 Another coronavirus, Middle East respiratory syndrome coronavirus (MERS-CoV) was first identified in Saudi Arabia in 2012.Citation2 On December 31, 2019, several cases of pneumonia were reported in Wuhan, China.Citation3 The etiological agent of the 2019 outbreak was later identified as SARS coronavirus 2 (SARS-CoV-2) because the genomic sequence was closely related to that of the SARS-CoV from 2003.Citation4,Citation5 On February 12, 2020, the world health organization (WHO) named the disease caused by the novel coronavirus (SARS-CoV-2) as Coronavirus Disease 2019 (COVID-19).
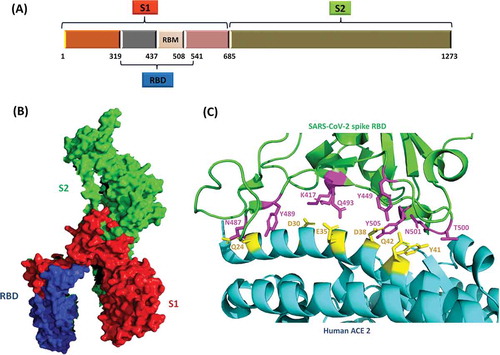
Coronaviruses belong to the Coronaviridae family. The family members are enveloped, positive-stranded RNA viruses which appear as crown-like entities under the electron microscope due to spikes of glycoproteins protruding from their viral envelopes, thus exhibiting a corona-like appearance.Citation6 The Coronaviridae family is divided into two subfamilies: Letovirinae and Orthocoronavirinae. The latter consists of the genera Alphacoronvirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. The pathogenic coronaviruses: SARS-CoV, MERS-CoV, and SARS-CoV-2 are all betacoronaviruses.Citation6 Among known RNA viruses, coronaviruses have the largest genomes size in the range of 26 to 32 kb in length.Citation7 Two-third of the viral genome at 5ˊ end encodes up to 16 nonstructural replicase proteins which are translated as two polyproteins: pp1a and pp1ab. The genes encoding structural proteins, including spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins are present at 3ˊ end of genomic RNA.Citation7,Citation8 The S protein of coronaviruses is one of the most important targets for the development of SARS vaccines and therapeutics because it is involved in receptor recognition, as well as virus attachment and entry. The S protein is made up of S1 and S2 subunits. S1 subunit has the receptor-binding domain (RBD) which binds with host receptor and then the S2 subunit mediates the fusion of viral and host membranes.Citation9 Host receptor for SARS-CoV is angiotensin-converting enzyme 2 (ACE2), whereas MERS-CoV recognizes dipeptidyl peptidase 4 (DPP4) as its receptor.Citation10,Citation11
The common symptoms of coronavirus infection are fever, cough, and sore throat. Clinically, patients with SARS suffer from atypical pneumonia.Citation12,Citation13 Clinical presentation of SARS-CoV-2 patients is similar to patients infected with SARS-CoV. COVID-19 manifests itself with symptoms including fever, dry cough, fatigue, and acute respiratory distress syndrome.Citation3,Citation14 These clinical features are a direct consequence of massive alveolar epithelial cell and vascular endothelial cell damage which is also accompanied by an exuberant release of proinflammatory cytokines and chemokines.Citation15 The disease severity and lung damage in the case of SARS-CoV-2 infection can be directly correlated with the dysregulated immune response at 7–10 days after symptom onset and is characterized by exuberant production of cytokines including IL-2, IL-7, IL-10, MIP-1A, IP-10, and TNF-α.Citation3,Citation15 This ‘cytokine storm’ was also reported in animal studies with SARS-CoV infection and was responsible for the dampening of adaptive immunity of patients in the later phase of infection.Citation16 As of now, the spread of SARS-CoV-2 is being quelled by strict policy measures such as travel restrictions, social distancing, patient isolation, and nationwide lockdown in several parts of the world. There are no approved vaccines available against any of the coronaviruses. This emerging global pandemic has instigated an unprecedented search for an effective prophylactic or therapeutic intervention against COVID-19.Citation17–20 In this review, we have discussed the potential and challenges for the development of a successful mucosal vaccine against SARS-CoV-2.
Design strategies for mucosal vaccine against SARS-CoV-2
Vaccines have been one of the major contributors in the eradication of most of the infectious diseases in the last century. Vaccination of a population interrupts the transmission chain of a communicable pathogen by not only protecting the immunized subjects but also by halting the transmission of the virus by ‘breaking the chain’ within a population by induction of herd immunity in the vaccine recipients. Vaccines can be administered by either intramuscular or subcutaneous injection to introduce them into the systemic circulation. Vaccination through systemic routes elicits strong systemic immune response but is not effective in generating efficient mucosal immunity.Citation21 Mucosal vaccines are advantageous not just in evoking strong immune response at both mucosal sites and systemic circulation but also offer greater practicality in terms of cost and administration.Citation22 Mucosal vaccines can be produced at considerably low cost for mass immunization, the administration is needle-free and convenient compared to systemic vaccines.Citation23 Majority of mucosal vaccines are administered through oral or intranasal routes while rectal, vaginal, ocular, and sublingual routes can also be used. The selection of administration route depends on the nature of the antigen and the desired site for induction of immune response. Upon oral immunization, immune responses are induced strongly in gastrointestinal (GI) tract, mammary glands, and salivary glands while intranasal vaccination induces marked antigen-specific immune response in respiratory, GI, and genital tracts.
The primary mode of transmission of SARS-CoV is through mucosal membranes of the eyes, nose, or mouth.Citation24 Studies with S protein of SARS-CoV-2 have revealed that it also recognizes human ACE2 (hACE2) receptor on the host cells similar to SARS-CoV.Citation25–27 ACE2 is abundantly expressed in nasal and oral mucosa rendering them the primary targets for the viral entry and dissemination of SARS-CoV-2.Citation28 Concomitant gastrointestinal symptoms are reported in confirmed COVID-19 patients along with pulmonary pathology, characteristic of SARS-CoV-2 infection. The occurrence of acute hemorrhagic colitis in a few cases and the presence of SARS-CoV-2 RNA in fecal samples of COVID-19 patients indicate enteric involvement in COVID-19 reasserting the similarity in tissue tropism with SARS-CoV.Citation29–31 Considering the prominent role of nasal and gastric mucosa in the transmission and the clinical progression of SARS-CoV-2, mucosal immunization using oral or intranasal vaccine could be an effective strategy for immunoprophylaxis against SARS-CoV-2. Vaccination at the entry site such as intranasal or oral immunization induces a strong local immune response in the case of SARS-CoV.Citation32,Citation33 Consequently, vaccination at the mucosal sites reduces the risk of antibody-dependent disease enhancement (ADE) by blocking the virus at the entry site. Administration of mucosal vaccine has also been shown to elicit strong systemic humoral immunity thereby neutralizing any virus particle that evades the primary immune response at the mucosal site. Currently, several strategies are being examined for the development of a mucosal vaccine against SARS-CoV-2.Citation34
Mucosal immune system
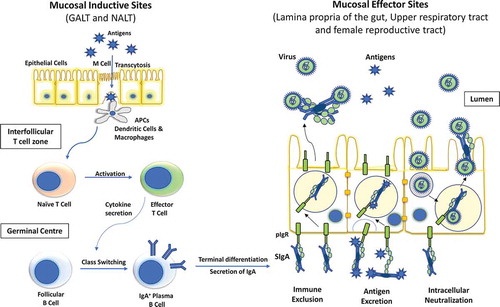
A unique mucosal system exists independent of the systemic immune system to protect exposed mucosal surfaces against environmental antigens and downregulation of systemic immune responses. It acts as the first line of defense against most of the antigens and prevents them from evoking systemic immune system.Citation35 The mucosal system serves as the most common portal for pathogen entry in the human body. The mucosal immune system consists of a complex network of tissues, non-lymphoid/lymphoid cells, and effector molecules including cytokines, chemokines, and antibodies.Citation36 The mucosal immune system is compartmentalized into immune inductive sites- where antigen sampling from mucosal surface occurs and then priming of B cells and T cells takes place, and immune effector site- where the activated immune effector cells move after extravasation and secrete cytokines to promote IgA class-switch recombination.Citation37,Citation38 Inductive site for the mucosal immune system is constituted by mucosa-associated lymphoid tissue (MALT). MALT comprises of highly organized structures such as appendix and Peyer’s patches in the intestine and tonsils in the upper airway. The effector sites of the mucosal immune system include lamina propria of various mucosae, surface epithelia, and exocrine glands. Gut-associated lymphoid tissue (GALT) and nasopharyngeal-associated lymphoid tissue (NALT) represent the major mucosal inductive sites. Waldeyer’s ring and oropharyngeal lymphoid tissues (paired palatine tonsils) in humans are considered anatomical equivalent of murine NALT.Citation39 Larynx-associated lymphoid tissue (LALT), salivary duct-associated lymphoid tissue (SDALT), lacrimal duct-associated lymphoid tissue (LDALT), and conjunctiva-associated lymphoid tissue (CALT) are also considered part of human MALT.Citation40 These mucosal inductive sites are covered with a layer of enterocytes and microfold cells (M cells). M cells are specialized thin epithelial cells that move soluble antigen from the gut lumen to the underlying lymphoid tissues via transcytosis. Exogenous antigens can activate T cells directly or are handed off to dendritic cells (DCs) that can act as antigen-presenting cells (APCs).Citation41,Citation42 These primed T cells move to the germinal centers and secrete cytokines to promote B-cell isotype switching to IgA production.Citation43
Secretory IgA (sIgA) antibodies are the most essential effector molecule in the mucosa. It is recognized as the first line of protection against foreign toxins, pathogens, and overgrowth of commensal microbes. It is secreted as a dimer joined together by a joining chain and is actively transported exclusively across the mucosal surface via a polymeric IgA receptor.Citation44 The activated T and B cells retain immunological memory and contribute to long-lasting protective immunity against the pathogen in systemic and mucosal systems. sIgA neutralizes toxins or pathogens in the mucosal environment using three mechanisms: immune exclusion, antigen excretion, and intracellular neutralization ().Citation45 In addition to sIgA, transudated IgA and IgG, which are generated in response to both systemic and mucosal vaccination, also contribute to local surface defense in the genitourinary mucosa and in the lower respiratory tract which are more permeable to serum-derived antibodies than intestine. These antibodies employ a diverse range of effector functions to protect against pathogens. They neutralize toxins; can mediate opsonization and facilitate internalization of invading pathogens by phagocytes. Transudated IgG exert its immunopathological effect when sIgA-dependent elimination of pathogen is unsuccessful.Citation46 Serum IgG antibodies protect against viremia and are crucial for virus clearance from systemic circulation.
Figure 1. Diagrammatic representation of inductive and effector sites for mucosal immunity. Two most important mucosal inductive sites are gut-associated lymphoid tissue (GALT) and nasopharynx-associated lymphoid tissue (NALT). These inductive sites are lined with follicle-associated epithelium which consists of microfold (M) cells responsible for the transport of antigens to the antigen presenting cells (APCs). These APC’s then trigger the cellular immunity by activating effector T cells which in turn elicit the IgA class switching in follicular plasma B cells. These IgA producing B cells then reach the effector sites through systemic circulation and release secretory IgA (sIgA). The polymeric immunoglobulin receptor (pIgR) located at the basal surface of effector sites, such as lamina propria etc. transfers sIgA to the luminal surface, where it inhibits the pathogen by three different mechanisms, namely, immune exclusion, antigen excretion, and intracellular neutralization
Mucosal vaccine platforms
A mucosal vaccine can be developed based on one of the several vaccine platforms including viral vectors, virus-like particle (VLP)-based, DNAs, subunit, inactivated whole virus, or live-attenuated vaccine.Citation6,Citation47,Citation48 Each of these platforms has its own advantages and disadvantages. VLP-based vaccines are composed of viral structural proteins capable of self-assembly. DNA vaccine comprises viral immunogens encoded by a recombinant plasmid that is delivered to the site of administration where the plasmid expresses itself and elicits the desired immune response.Citation49 Both these safe vaccine platforms preserve the inherent antigenic structure of viral immunogens and are noninfectious but often suffer from weak immunogenicity. Viral vectors encode the antigenic protein and are delivered to the host cell where the antigen is expressed and is presented on the surface of APCs to induce a cellular and humoral immune response.Citation50 These vaccines are highly efficient but preexisting immunity against the viral vector might cause a harmful immune response in some recipients.
Live-attenuated vaccines or inactivated vaccines are based on pathogens that have been attenuated or inactivated by heat or chemical treatment.Citation46 As of now, all the mucosal vaccines licensed for human use are based on live-attenuated approach including oral polio vaccine (OPV) and intranasal influenza vaccine (FluMist®). Safety concerns are often associated with attenuated vaccines due to the possibility of incomplete inactivation of harmful pathogens. Subunit vaccines consist of specific antigenic fragments of virus capable of eliciting strong antibody-mediated and cellular immune response.Citation51 Inactivated whole virus vaccines and subunit vaccines are relatively inexpensive, inert, and nontoxic but the possibility of alteration of immunogenicity of antigens needs to be confirmed consistently. Few SARS-CoV and MERS-CoV vaccines based on the different platforms are summarized in .
Table 1. SARS-CoV and MERS-CoV mucosal vaccines based on different vaccine platforms*
Antigen selection
S protein is the main antigenic component of SARS-CoV, SARS-CoV-2, and MERS-CoV among four structural proteins (S, E, M, and N proteins). Vaccines using S protein elicit a potent immune response and inhibit viral infection.Citation9,Citation53,Citation64 But the use of full-length S protein as an antigen for vaccine design has raised few safety issues due to ADE of viral infection in vaccinated subjects. A study on the Chinese macaque models for SARS-CoV showed greater lung damage in vaccinated animals (vaccinated with full-length S proteins) relative to unvaccinated subjects upon virus challenge.Citation65 Anti-S protein IgG antibodies have been implicated in this acute alveolar damage on exposure to the virus. Similar symptoms have been observed in critical patients with SARS-CoV infection.Citation66 It is speculated that ADE of viral infection is mediated through the binding of virus-neutralizing antibody complex to the Fc receptors on the monocytes/macrophages leading to increased production of pro-inflammatory cytokines. Additionally, this virus-antibody complex might activate the classical pathway of complement system or antibody-mediated cytotoxicity leading to cellular damage.Citation67 Taking a cue from studies with SARS-CoV and MERS-CoV, caution must be exercised in selecting an antigen target that minimize ADE while inducing a potent immune response against future exposure to SARS-CoV-2.
The use of RBD of S protein as an antigen in the case of SARS-CoV or MERS-CoV has been extensively explored.Citation9,Citation68 RBD of S protein is highly immunogenic and confers neutralizing capability against multiple strains of SARS-CoV and MERS-CoV. RBD-based vaccine minimizes the risk of ADE upon exposure to virus in vaccinated individuals as it lacks the non-neutralizing immunodominant region of S protein.Citation69 Other fragments of S protein of SARS-CoV and MERS-CoV that have been used for vaccine design include S1 and S2 fragments ().Citation71,Citation72 A mucosal vaccine based on N protein of SARS-CoV has shown the induction of both cellular and humoral immunity in mice.Citation61 Several immunogenic domains of N protein are highly conserved in coronaviruses.Citation73 N protein of SARS-CoV-2 shares high sequence identity (~91%) with N protein of SARS-CoV and hence can be considered for the development of a broad spectrum coronavirus vaccine.Citation74 A challenging alternative that can warrant protection against future outbreaks of similar coronaviruses is to identify a universal epitope in the whole virus family or genera. This strategy is being undertaken for influenza viruses and can be extended to coronaviruses as well.Citation75 Immunoinformatics can also be a powerful tool in prediction of epitopes on the viral surface proteins.Citation76
Figure 2. (a) Schematic of SARS-CoV-2 S protein and its subunits. S protein is the major determinant of receptor binding and pathogenesis in SARS-CoV-2. (b) SARS-CoV-2 S protein monomer (PDB ID: 6VXX). The S1 domain which contains the receptor binding domain (RBD) and receptor binding motif (RBM), is critical to the viral infectivity as it initiates the attachment of viral particle to the host cell. The S2 domain is responsible for the fusion of viral and host membranes leading to internalization of the virus. (c) Receptor binding domain (RBD) of SARS-CoV-2 bound to its host cell receptor, human angiotensin-converting enzyme 2 (hACE2) via specific amino-acid interactions (PDB ID: 6M0J). Interacting residues are shown as sticks at RBD-hACE2 interface.Citation70
Mucosal adjuvants
Traditionally, mucosal immune induction needs a higher dose of antigen in comparison to parenteral immunization as antigenic preparation may get diluted in mucus in the nasal cavity or get expelled by mucus and ciliary movement in the respiratory tract.Citation77 Effective intranasal vaccination requires the antigen to reach mucosal sites, cross the mucus layer, and induce local IgA production. A vaccine administered through the oral route has to endure the low pH environment in the upper GI tract and a variety of nucleases and proteases present in the digestive tract before it can reach the immunological sites. Mucosal antigens when administered alone lead to a weak induction of immune response. To overcome these physical and biochemical obstacles in priming mucosal immune cells, vaccines delivered through oral or nasal routes are often administered complemented with an adjuvant.Citation78 Adjuvants are the supplementary materials (natural or synthetic) in a vaccine formulation that potentiates the immune-induction capacity of a vaccine.Citation79 Adjuvant in a vaccine formulation plays a critical role in maintaining the structural integrity of the antigen, augmenting antigen bioavailability, and enhancing antigenic stimulation. Adjuvants are broadly divided into two classes: adjuvants that can serve as carrier systems to facilitate antigen delivery to immune induction sites and adjuvants that can act as immunostimulators through enhanced internalization, presentation, and processing of the antigen in the APCs.Citation80
A number of polymers including chitosan and poly lactic-co-glycolic acid (PLGA) have been used as carriers in various vaccine formulations for immunostimulation due to their high affinity toward mucosal surfaces.Citation81,Citation82 Emulsions and liposomes are the other carriers routinely tested for mucosal vaccine.Citation48 DC and M cells are a major determinant for induction of mucosal immune response at immune inductive sites and thus represent an ideal site for antigen presentation. Surface markers at the surface of epithelial cells and DCs can be strategically targeted for antigen delivery with specialized molecules such as lectins or nanoparticles for increased antigen uptake by APCs.Citation83,Citation84 Pathogen recognition receptor agonists such as synthetic poly-(I:C), or toll like receptor agonists such as CpG oligodeoxynucleotides, engage these receptors present on the surface of APCs to potentiate the immunogenicity of the vaccine.Citation85,Citation86 Other commonly used adjuvants including cholera enterotoxin (CT) and heat-labile enterotoxin (LT) from E. coli exert their adjuvanticity by interacting with GM1 gangliosides on the surface of follicular dendritic cells and in turn increase the induction of B-cell clones.Citation87 The use of immune-stimulating complexes (ISCOMs) has also proven to be highly effective for administration with mucosal vaccines.Citation88
Immunotolerance
One of the major factors influencing the rational design of a mucosal vaccine is immunotolerance at the mucosal sites. Immunotolerance is the ingenious modulation of the mucosal microenvironment to avoid unnecessary induction of host immune cells against foreign antigens or commensal microbes. Mucosal surfaces are exposed continuously to environmental, food, or self-antigens which lead to the development of a tolerogenic microenvironment, especially in gastric mucosa.Citation89 Some vaccination strategies fail to develop effective immunity and can induce immunotolerance. This suppression of immune response is affected mainly by vaccine formulation, antigenic dose, and frequency of administration. Administration of antigen at low doses for a long time leads to low-dose immunotolerance.Citation48 Contrastingly, high dose of antigen administered at a low rate induces high-dose tolerance instead of immunostimulation. This hypo-responsiveness at the mucosal induction sites can be overcome by strategically determining the antigen dose, vaccine formulation, and timing of vaccine delivery. The design of a novel mucosal vaccine also aims at mimicking the kinetics of pathogen infection for increasing the clinical efficacy of the vaccine. Optimal release timing of antigen at the inductive site helps in dodging the mucosal immunotolerance. To this end, the antigen is conjugated with adjuvants like TLR ligands or CD-40 specific antibodies.Citation90,Citation91
Immunosenescence
Several issues are needed to be addressed for the design and development of an efficacious mucosal vaccine against SARS-CoV-2. It has been observed in human challenge studies that immunity acquired with coronavirus infection is often short-lived and in some cases, re-infection with the same virus was possible after an extended period.Citation92 It was reported in some cases that immunity acquired with SARS-CoV or MERS-CoV also declines considerably 2–3 years after viral infection.Citation93,Citation94 Another concern for designing a vaccine against SARS-CoV-2 is that the viral infection is associated with severe pathology specifically in patients of higher age group (typically >50 years).Citation14 People in this age group do not respond very well to vaccination in terms of neutralizing antibody titers and require a higher amount of antigen to produce sufficient immunogenicity. Specialized dose-regime in terms of the amount of antigen, use of specific adjuvants, or the immunization effects of follow-up doses after a single prime dose can be investigated in vulnerable age groups and immunocompromised people before extending vaccination to them.
Correlates of protection and testing in animal models
For most parenterally administered vaccines and mucosal vaccines, correlates and precise mechanism of their efficacy remain poorly defined. Most of the licensed vaccines rely on the measurement of a single parameter that is statistically correlated with protection afforded by the vaccine. There is no absolute method of sampling, and the choice of sampling method varies according to the parameter to be evaluated. Conventionally, quantification and qualification of secreted antibodies is performed using sero-immunoassays in body secretions such as saliva, tears, nasal, blood samples or genital secretions, and gut or organ lavages.Citation95 Cellular correlates of immunity are analyzed using assays such as enzyme-linked immunospot assays (ELISPOTs), reverse-transcriptase PCR (RT-PCR) or cell-sorting techniques.Citation96 A modern system biology approach can also be used that utilizes functional genomics to identify molecular signatures that correlate well with traditional biological markers for evaluating vaccine efficacy.Citation97 Correlates of protection differ greatly based on whether the objective of vaccination is to prevent a mucosal or a systemic infection.Citation96
Several animal models including mice, ferrets, macaques, hamsters, and non-human primates have been used for evaluating safety and efficacy of SARS-CoV and MERS-CoV vaccines.Citation53,Citation98-102 Ferrets are a suitable animal model for SARS-CoV vaccine evaluation as they support viral replication in the respiratory tract, develop similar disease symptoms, and display severe lung pathology.Citation103,Citation104 Smaller animal models like rabbits and mice are a preferred choice for vaccine evaluation in animals because of inexpensive maintenance, ease of genetic manipulation, and standardized methods of testing. Wild type mice are nonpermissive to SARS-CoV-2 viral replication. A transgenic mice model expressing hACE2 has recently been developed by genetic manipulation to make the mice susceptible to SARS-CoV-2 infection.Citation105 This mice model mirrors the pathological features of SARS-CoV-2 infection in human patients albeit at a moderate level as compared to SARS-CoV. Moreover, a young animal/mice model can effectively exhibit excellent neutralizing efficiency induced by a vaccine candidate but it cannot effectively mimic the clinical manifestations of COVID-19 in an elderly population. Hence, robust lethal-challenged mice models using senescent mice that can recapitulate the clinical disease in the aged human population are needed to be developed for assessing the efficacy of a COVID-19 vaccine candidate. Following animal model-based preclinical studies, good manufacturing practices are employed for the scale-up of the vaccine production.
Parenteral vaccines against mucosal pathogens
Currently, mucosal vaccines form a small proportion of licensed vaccines for humans. Limited choice of adjuvants for human mucosal vaccines, immunotolerance, and differential degree of vaccine-induced immune response in different populations are some of the impediments associated with the development of mucosal vaccines. Efficacies of different mucosal vaccines depend on myriad of factors such as age, environment, host genetics, the microbiome of the recipient, and the regimen of immunization. It ranges from 70% to 90% for rotavirus vaccines to 85%– to 90% for oral vaccines against influenza and polio viruses.Citation22 Cholera, polio, and rotavirus oral vaccines are found to be less efficacious in developing countries. This weak induction of immune response can be attributed to nutritional deficiencies such as vitamin A or zinc, concomitant bacterial, helminth, or viral infections, and the presence of high levels of maternal antibodies in the breast milk.Citation106,Citation107 Intranasal immunization using live attenuated or live vectors is also associated with the risk of the harmful antigens and adjuvants (such as CT and LT) accessing the central nervous system through the cribiform plate.Citation108
A number of infectious diseases have been successfully controlled with the use of parenterally administered vaccine that may or may not lead to the induction of mucosal immune response. Administration of the vaccine through systemic circulation might not necessarily recapitulate the immune response generated by mucosal administration but is adequate for evoking immune response against mucosal pathogens such as human papilloma virus (HPV), influenza virus, or polio virus. All three licensed VLP-based HPV vaccines are administered intramuscularly and induce a high level of durable IgG antibody response against the virus.Citation109,Citation110 These antibodies are also detectable at mucosal sites of infection such as oral cavity and cervix after vaccination and serve as the primary effectors of protection against HPV.Citation111,Citation112
Currently, there are two types of licensed influenza vaccines: parenterally administered inactivated influenza vaccine and live attenuated influenza virus vaccine (LAIV) which is delivered intranasally. Inactivated influenza vaccine elicits some degree of local IgA antibodies and high levels of systemic IgG antibodies to mediate protection by diffusing into the mucosal sites. LAIV, in turn, mimics natural infection and induce highly cross-reactive serum IgG, mucosal IgA, and cellular immunity in the recipients.Citation113,Citation114 Yearly influenza vaccines are adjusted according to the prevalent strains in the coming flu season. In clinical settings, inactivated vaccine is found to be more effective than LAIV in preventing symptomatic disease in healthy adults while LAIV offers greater protection against influenza disease in children.Citation115–117 Similarly, two types of vaccines are available for polio virus: an injectable polio vaccine (IPV) and OPV. Sabin OPV, with its enormous impact in disease control worldwide, is considered to be the prototype oral vaccine. Superiority of OPV over IPV is attributed to the induction of high titers of mucosal IgA antibodies.Citation118,Citation119 Despite the generation of higher serum antibody titers, IPV provides limited intestinal immunity.Citation118 Due to the risk of rare cases of vaccine associated paralytic polio, most developed countries have switched to either IPV or a sequential combination of IPV and OPV.Citation120,Citation121
Based on the substantial experience in control of mucosal pathogens using parenterally administered vaccines, several parenteral vaccines are being developed or evaluated against SARS-CoV-2. Notably, DNA-based vaccine by Inovio Pharmaceuticals (INO-4800), mRNA-based vaccine by Moderna (mRNA-1273), adenovirus-vectored vaccine (ChAdOx1), and an inactivated virus vaccine (PiCoVacc) are currently under investigation in human clinical trials.Citation122–125
Rapid licensure and production of a mucosal vaccine
Time is the most valuable asset in the rapidly emerging COVID-19 pandemic, especially in high-mortality areas. Rapid development and testing of a viable vaccine can be achieved by adopting a number of novel strategies. Instead of developing a novel vaccine development platform, utilizing an existing vaccine platform will accelerate the design and production of vaccines against SARS-CoV-2 and will enable a quicker future response against newly emerging viruses. Since the outbreaks of SARS-CoV and MERS-CoV, several vaccine candidates were developed using different production platforms. These established platforms are being used for the development of vaccine candidate against SARS-CoV-2.Citation122,Citation124,Citation126
To expedite the process for licensure and use of the vaccine against SARS-CoV-2 in the wake of the current pandemic, Eyal et al. have suggested an alternative model for accelerated rollout of an effective SARS-CoV-2 vaccine by skipping phase 3 clinical trials.Citation127 This study proposes a controlled human challenge model wherein nonsusceptible adult volunteers from a healthy group will be challenged with an increasing dose of live virus to determine a dose that induces clinical symptoms similar to the natural infection in the individuals of similar age (Preparatory phase). After this dose for controlled human challenge model has been optimized, a randomized cohort of volunteers will receive the candidate vaccine or placebo before being challenged with the controlled dose of the live virus (Phase 1). The successful vaccine candidates can then be administered to a large cohort of individuals for evaluation of short-term safety prior to vaccine rollout (Phase 2). This accelerated licensure of the vaccine can be followed up with post-approval surveillance to monitor the occurrence of rare side effects and long-term efficacy for future regulatory approvals.
The overwhelming demand for a SARS-CoV-2 vaccine in the current pandemic by far exceeds the existing production capacity. To deliver required quantities of the successful vaccine, production process will have to be ramped up to an enormous scale. The necessary infrastructure can be developed simultaneously with the continued production on existing capacity. Alternatively, rapid manufacturing of a successful vaccine can be facilitated by adopting a new pandemic paradigm, with a fast start and many steps of vaccine development and production executed in parallel before confirming a successful outcome of another step.Citation128 In recent years, rapid production of live attenuated vaccines is being facilitated by utilizing reverse genetics process that involves the precise deletion of specific genes of viral genome.Citation129 Deletion of pathogenic components of the pathogen is advantageous over traditional methods of viral attenuation in terms of safety and efficacy of a vaccine.
Concluding remarks
The current COVID-19 pandemic has virtually brought most world economies to a halt and severely impacted the lives of a large proportion of the world population. Development of a viable SARS-CoV vaccine is imperative to reduce mortality and morbidity associated with this novel virus outbreak. Mucosal vaccines offer better patient compliance in terms of the physical and psychological comfort due to absence of needlestick injury and thus are highly compatible for mass immunization in a pandemic scenario. Vaccine administration using injection involves the cost of injection device, it’s safe disposal, and the employment of trained medical staff which adds a considerable cost for mass vaccination especially in developing countries. Mucosally administered vaccines also abrogate the risk of transmission of infections by injection devices. Despite our detailed understanding of mucosal system in mice, the complex cellular and molecular interplay of different component of innate immune response to mucosal vaccination in humans is still poorly deciphered. Additionally, the emergence and rapid global spread of SARS-CoV-2 has provided a very small window for basic and translational studies that propel the development and evaluation of vaccine against a pathogen. While the knowledge gained from previous studies on SARS-CoV and MERS-CoV can be used for SARS-CoV-2 vaccine development, it is yet uncertain as to what extent it will work for SARS-CoV-2 or whether correlates of protection used will faithfully predict protective efficacy. Potential ADE and waning of vaccine-induced immune response represent other obstacles in the development of a mucosal vaccine against SARS-CoV-2.
The currently licensed mucosal vaccines against viral pathogens are exclusively live-attenuated. The development of a safe and immunogenic live-attenuated vaccine is an attractive possibility for mucosal vaccine against SARS-CoV-2. Live attenuated vaccines, by definition establish a mild infection at the immunization site and exhibit some level of in-built adjuvanticity thereby ensuring the delivery of a higher antigen dose and better targeting of mucosal inductive sites for immunostimulation. This candidate vaccine can be easily administered orally as encapsulated antigens or can be delivered through the intranasal route via droplets and aerosol spray. COVID-19 pandemic demands for adopting nonconventional approaches for a viable mucosal vaccine development, hastening the vaccine licensure process, utilizing existing vaccine development and manufacturing platforms, and ensuring global distribution of the licensed vaccine in time to minimize the impact of COVID-19 across the globe. It will also need an unprecedented scale-up of the manufacturing process and concerted efforts of the supply chains to make the vaccine available before this pandemic is over. Learning from the COVID-19 pandemic, sustained investments in vaccine development and production infrastructure should be made to save human lives and curtail the economic impact associated with a looming viral pandemic. Moreover, continued global surveillance and vigilance in the post-pandemic scenario should be practiced to help the world counter a second wave of COVID-19 or other possible future coronavirus outbreaks.
Disclosure of potential conflict of interest
The authors declare no potential conflict of interest.
Additional information
Funding
References
- Zhong NS, Zheng BJ, Li YM, Poon N, Xie ZH, Chan KH, Li PH, Tan SY, Chang Q, Xie JP, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362:1353–58.
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506.
- Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiol. 2020;5:536–44.
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–73.
- Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–89.
- Cui J, Li F, Shi Z-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–92.
- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis [Internet]. In: Maier HJ, Bickerton E, Britton P, editors. Coronaviruses: methods and Protocols. New York (NY): Springer; 2015, p. 1–23 [accessed 2020 Apr 23]. doi:https://doi.org/10.1007/978-1-4939-2438-7_1
- Du L, He Y, Zhou Y, Liu S, Zheng B-J, Jiang S. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–36.
- Raj VS, Mou H, Smits SL, Dekkers DHW, Müller MA, Dijkman R, Muth D, Demmers JAA, Zaki A, Fouchier RAM, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–54.
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–54.
- Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, Lam WK, Seto WH, Yam LY, Cheung TM, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–85.
- Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005.
- Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, et al. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020. [accessed 2020 Apr 3] http://www.jci.org/articles/view/137244
- Liu Y, Yan L-M, Wan L, Xiang T-X, Le A, Liu J-M, Peiris M, Poon LLM, Zhang W Viral dynamics in mild and severe cases of COVID-19. The Lancet Infectious Diseases; 2020 [accessed 2020 Apr 23]. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30232-2/abstract
- Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–93.
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;1–7.
- Rani R, Singh A, Pareek A, Tomar S In silico guided drug repurposing to combat SARS-CoV-2 by Targeting Mpro, the key virus specific protease; 2020 [accessed 2020 Apr 28]. https://chemrxiv.org/articles/In_Silico_Guided_Drug_Repurposing_to_Combat_SARS-CoV-2_by_Targeting_Mpro_the_Key_Virus_Specific_Protease/12030345
- Choudhary S, Malik YS, Tomar S Identification of SARS-CoV-2 cell entry inhibitors by drug repurposing using in silico structure-based virtual screening approach; 2020 [accessed 2020 Apr 28]. https://chemrxiv.org/articles/Identification_of_SARS-CoV-2_Cell_Entry_Inhibitors_by_Drug_Repurposing_Using_in_Silico_Structure-Based_Virtual_Screening_Approach/12005988/2
- Singh Tomar PP, Arkin IT. SARS-CoV-2 E protein is a potential ion channel that can Be inhibited by Gliclazide and Memantine. Biochem Biophys Res Commun. 2020 . [accessed 2020 Jun 26] http://www.sciencedirect.com/science/article/pii/S0006291X20311530
- Park S, Sestak K, Hodgins DC, Shoup DI, Ward LA, Jackwood DJ, Saif LJ. Immune response of sows vaccinated with attenuated transmissible gastroenteritis virus (TGEV) and recombinant TGEV spike protein vaccines and protection of their suckling pigs against virulent TGEV challenge exposure. Am J Vet Res. 1998;59:1002–08.
- Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605.
- Li M, Wang Y, Sun Y, Cui H, Zhu SJ, Qiu H-J. Mucosal vaccines: strategies and challenges. Immunol Lett. 2020;217:116–25.
- Peiris JSM, Yuen KY, Osterhaus ADME, Stöhr K. The Severe Acute Respiratory Syndrome. N Eng J Med. 2003;349:2431–41.
- Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–69.
- Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020 . [accessed 2020 Apr 9] http://www.nature.com/articles/s41586-020-2179-y
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8.
- Hamming I, Timens W, Bulthuis M, Lely A, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–37.
- Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, Yin H, Xiao Q, Tang Y, Qu X, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–35.
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 . [accessed 2020 Apr 23] https://jamanetwork.com/journals/jama/fullarticle/2762997
- Leung WK, To K-F, Chan PKS, Chan HLY, Wu AKL, Lee N, Yuen KY, Sung JJY. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–17.
- Qu D, Zheng B, Yao X, Guan Y, Yuan Z-H, Zhong N-S, Lu L-W, Xie J-P, Wen Y-M. Intranasal immunization with inactivated SARS-CoV (SARS-associated coronavirus) induced local and serum antibodies in mice. Vaccine. 2005;23:924–31.
- Ma C, Li Y, Wang L, Zhao G, Tao X, Tseng C-TK, Zhou Y, Du L, Jiang S. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: implication for designing novel mucosal MERS vaccines. Vaccine. 2014;32:2100–08.
- novel-coronavirus-landscape-ncov.pdf [Internet]. [accessed 2020 Apr 23]. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf
- McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88.
- Vajdy M. Immunity against mucosal pathogens. Dordrecht, Netherlands: Springer; 2008.
- Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418.
- Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711.
- Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Path. 2006;34:599–608.
- Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immuno. 2008;1:31–37.
- Jensen VB, Harty JT, Jones BD. Interactions of the Invasive PathogensSalmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M Cells and Murine Peyer’s Patches. Infect Immun. 1998;66:3758–66.
- Jang MH, Kweon M-N, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, Rennert PD. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci. 2004;101:6110–15.
- Bemark M, Boysen P, Lycke NY. Induction of gut IgA production through T cell-dependent and T cell-independent pathways. Annals New York Acad Sci. 2012;1247:97–116.
- Pabst O. New concepts in the generation and functions of IgA. Nature Rev Immunol. 2012;12:821–32.
- Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8:656–67.
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–S53.
- Yong CY, Ong HK, Yeap SK, Ho KL, Tan WS. Recent advances in the vaccine development against middle east respiratory syndrome-coronavirus. Front Microbiol. 2019;10. [accessed 2020 Apr 24] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6688523/
- Woodrow KA, Bennett KM, Lo DD. Mucosal Vaccine Design and Delivery. Annual Rev Biomed Eng. 2012;14:17–46.
- Gurunathan S, Wu CY, Freidag BL, Seder RA. DNA vaccines: a key for inducing long-term cellular immunity. Curr Opin Immunol. 2000;12:442–47.
- Rocha CD, Caetano BC, Machado AV, Bruna-Romero O. Recombinant viruses as tools to induce protective cellular immunity against infectious diseases. Int Microbiol. 2004;7:83–94.
- Moyle PM, Toth I. Modern subunit vaccines: development, components, and research opportunities. ChemMedChem. 2013;8:360–76.
- See RH, Zakhartchouk AN, Petric M, Lawrence DJ, Mok CPY, Hogan RJ, Rowe T, Zitzow LA, Karunakaran KP, Hitt MM, et al. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J Gen Virol. 2006;87:641–50.
- Bukreyev A, Lamirande EW, Buchholz UJ, Vogel LN, Elkins WR, St Claire M, Murphy BR, Subbarao K, Collins PL. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363:2122–27.
- Du L, Zhao G, Lin Y, Sui H, Chan C, Ma S, He Y, Jiang S, Wu C, Yuen K-Y, et al. Intranasal vaccination of recombinant adeno-associated virus encoding receptor-binding domain of severe acute respiratory syndrome coronavirus (SARS-CoV) spike protein induces strong mucosal immune responses and provides long-term protection against SARS-CoV infection. J Immunol. 2008;180:948–56.
- Li K, Li Z, Wohlford-Lenane C, Meyerholz DK, Channappanavar R, An D, Perlman S, McCray PB, He B. Single-dose, intranasal immunization with recombinant parainfluenza virus 5 expressing middle east respiratory syndrome coronavirus (MERS-CoV) Spike protein protects mice from fatal MERS-CoV infection. mBio. 2020;11. [accessed 2020 Apr 25] https://mbio.asm.org/content/11/2/e00554-20
- Jia W, Channappanavar R, Zhang C, Li M, Zhou H, Zhang S, Zhou P, Xu J, Shan S, Shi X, et al. Single intranasal immunization with chimpanzee adenovirus-based vaccine induces sustained and protective immunity against MERS-CoV infection. Emerg Microb Infec. 2019;8:760–72.
- Kim MH, Kim HJ, Chang J. Superior immune responses induced by intranasal immunization with recombinant adenovirus-based vaccine expressing full-length Spike protein of Middle East respiratory syndrome coronavirus. Plos One. 2019;14:e0220196.
- Liu R, Wang J, Shao Y, Wang X, Zhang H, Shuai L, Ge J, Wen Z, Bu Z. A recombinant VSV-vectored MERS-CoV vaccine induces neutralizing antibody and T cell responses in rhesus monkeys after single dose immunization. Antiviral Res. 2018;150:30–38.
- Pei H, Liu J, Cheng Y, Sun C, Wang C, Lu Y, Ding J, Zhou J, Xiang H. Expression of SARS-coronavirus nucleocapsid protein in Escherichia coli and Lactococcus lactis for serodiagnosis and mucosal vaccination. Appl Microbiol Biotechnol. 2005;68:220–27.
- Shim B-S, Park S-M, Quan J-S, Jere D, Chu H, Song MK, Kim DW, Jang Y-S, Yang M-S, Han SH, et al. Intranasal immunization with plasmid DNA encoding spike protein of SARS-coronavirus/polyethylenimine nanoparticles elicits antigen-specific humoral and cellular immune responses. BMC Immunol. 2010;11:65.
- Raghuwanshi D, Mishra V, Das D, Kaur K, Suresh MR. Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Mol Pharm. 2012;9:946–56.
- Lu B, Huang Y, Huang L, Li B, Zheng Z, Chen Z, Chen J, Hu Q, Wang H. Effect of mucosal and systemic immunization with virus-like particles of severe acute respiratory syndrome coronavirus in mice. Immunology. 2010;130:254–61.
- Menachery VD, Gralinski LE, Mitchell HD, Dinnon KH, Leist SR, Yount BL, McAnarney ET, Graham RL, Waters KM, Baric RS. Combination Attenuation Offers Strategy for Live Attenuated Coronavirus Vaccines. J Virol. 2018;92. [accessed 2020 Apr 25] https://jvi.asm.org/content/92/17/e00710-18
- Pallesen J, Wang N, Corbett KS, Wrapp D, Kirchdoerfer RN, Turner HL, Cottrell CA, Becker MM, Wang L, Shi W, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci. 2017;114:E7348–57.
- Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, Tang H, Nishiura K, Peng J, Tan Z, et al. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4. [accessed 2020 Apr 3] https://insight.jci.org/articles/view/123158
- Peiris JSM, Chu CM, Cheng VCC, Chan KS, Hung IFN, Poon LLM, Law KI, Tang BSF, Hon TYW, Chan CS, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–72.
- Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020. [accessed 2020 Apr 24]. doi:https://doi.org/10.1007/s12250-020-00207-4.
- He Y, Zhou Y, Liu S, Kou Z, Li W, Farzan M, Jiang S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun. 2004;324:773–81.
- Jiang S, Lu L, Liu Q, Xu W, Du L. Receptor-binding domains of spike proteins of emerging or re-emerging viruses as targets for development of antiviral vaccines. Emerg Microb Infec. 2012;1:1–8.
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020; 581: 215-220..
- Li J, Ulitzky L, Silberstein E, Taylor DR, Viscidi R. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral Immunol. 2013;26:126–32.
- Guo Y, Sun S, Wang K, Zhang S, Zhu W, Chen Z. Elicitation of immunity in mice after immunization with the S2 subunit of the severe acute respiratory syndrome coronavirus. DNA Cell Biol. 2005;24:510–15.
- McBride R, van Zyl M, Fielding BC. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6:2991–3018.
- Srinivasan S, Cui H, Gao Z, Liu M, Lu S, Mkandawire W, Narykov O, Sun M, Korkin D. Structural Genomics of SARS-CoV-2 indicates evolutionary conserved functional regions of viral proteins. Viruses. 2020;12:360.
- Erbelding EJ, Post DJ, Stemmy EJ, Roberts PC, Augustine AD, Ferguson S, Paules CI, Graham BS, Fauci AS. A Universal Influenza Vaccine: the Strategic Plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis. 2018;218:347–54.
- Zheng M, Song L. Novel antibody epitopes dominate the antigenicity of spike glycoprotein in SARS-CoV-2 compared to SARS-CoV. Cell Mol Immunol. 2020 . [accessed 2020 Apr 6] http://www.nature.com/articles/s41423-020-0385-z
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–58.
- Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–96.
- Rappuoli R, Aderem AA. 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473:463–69.
- Kim S-H, Jang Y-S. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clin Exp Vaccine Res. 2017;6:15–21.
- Manocha M, Pal PC, Chitralekha KT, Thomas BE, Tripathi V, Gupta SD, Paranjape R, Kulkarni S, Rao DN. Enhanced mucosal and systemic immune response with intranasal immunization of mice with HIV peptides entrapped in PLG microparticles in combination with Ulex Europaeus-I lectin as M cell target. Vaccine. 2005;23:5599–617.
- Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Delivery Rev. 2005;57:1556–68.
- Clark MA, Jepson MA, Simmons NL, Booth TA, Hirst BH. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J Histochem Cytochem. 1993;41:1679–87.
- Makidon PE, Bielinska AU, Nigavekar SS, Janczak KW, Knowlton J, Scott AJ, Mank N, Cao Z, Rathinavelu S, Beer MR. Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PloS One. 2008;3: e2954.
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503.
- Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499–511.
- Liang S, Hajishengallis G. Heat-labile enterotoxins as adjuvants or anti-inflammatory agents. Immunol Invest. 2010;39:449–67.
- Mowat AM. Dendritic cells and immune responses to orally administered antigens. Vaccine. 2005;23:1797–99.
- Weiner HL, Friedman A, Miller A, Khoury SJ, Al-Sabbagh A, Santos L, Sayegh M, Nussenblatt RB, Trentham DE, Hafler DA. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annual Rev Immunol. 1994;12:809–37.
- Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S-I, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–24.
- Van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27:49–55.
- Callow KA, Parry HF, Sergeant M, Tyrrell DA. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105:435–46.
- Liu W, Fontanet A, Zhang P-H, Zhan L, Xin Z-T, Baril L, Tang F, Lv H, Cao W-C. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis. 2006;193:792–95.
- Wu L-P, Wang N-C, Chang Y-H, Tian X-Y, Na D-Y, Zhang L-Y, Zheng L, Lan T, Wang L-F, Liang G-D. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infec Dis J- CDC.2007;13:10 [accessed 2020 Apr 24] https://wwwnc.cdc.gov/eid/article/13/10/07-0576_article
- Guy B. Evaluation of events occurring at mucosal surfaces: techniques used to collect and analyze mucosal secretions and cells. Clin Diagn Lab Immunol. 2002;9:753–62.
- Plotkin SA. Correlates of protection induced by vaccination. Clinical Vaccine Immunol. 2010;17:1055–65.
- Pulendran B. Systems biological approaches for mucosal vaccine development. In: Mucosal vaccines. Academic Press: Elsevier; 2020. p. 753–72.
- de Wit E, Rasmussen AL, Falzarano D, Bushmaker T, Feldmann F, Brining DL, Fischer ER, Martellaro C, Okumura A, Chang J, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci USA. 2013;110:16598–603.
- Munster VJ, de Wit E, Feldmann H. Pneumonia from human coronavirus in a macaque model. N Engl J Med. 2013;368:1560–62.
- Martina BE, Haagmans BL, Kuiken T, Fouchier RA, Rimmelzwaan GF, Van Amerongen G, Peiris JM, Lim W, Osterhaus AD. SARS virus infection of cats and ferrets. Nature. 2003;425:915–915.
- Subbarao K, McAuliffe J, Vogel L, Fahle G, Fischer S, Tatti K, Packard M, Shieh W-J, Zaki S, Murphy B. Prior Infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol. 2004;78:3572–77.
- Roberts A, Vogel L, Guarner J, Hayes N, Murphy B, Zaki S, Subbarao K. Severe Acute Respiratory Syndrome Coronavirus Infection of Golden Syrian Hamsters. J Virol. 2005;79:503–11.
- See RH, Petric M, Lawrence DJ, Mok CPY, Rowe T, Zitzow LA, Karunakaran KP, Voss TG, Brunham RC, Gauldie J, et al. Severe acute respiratory syndrome vaccine efficacy in ferrets: whole killed virus and adenovirus-vectored vaccines. J Gen Virol. 2008;89:2136–46.
- Kobinger GP, Figueredo JM, Rowe T, Zhi Y, Gao G, Sanmiguel JC, Bell P, Wivel NA, Zitzow LA, Flieder DB. Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine. 2007;25:5220–31.
- Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Wei Q, Yu P, Xu Y, Qi F, et al. The Pathogenicity of SARS-CoV-2 in hACE2 Transgenic Mice [Internet]. Microbiology. 2020. [accessed 2020 Apr 24] http://biorxiv.org/lookup/doi/10.1101/2020.02.07.939389
- Ahmed T, Svennerholm A-M, Al Tarique A, Sultana GN, Qadri F. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine. 2009;27:1433–39.
- Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries. Rev Infect Dis. 1991;13:926–39.
- Otczyk DC, Cripps AW. Mucosal immunization: a realistic alternative. Hum Vaccin. 2010;6:978–1006.
- Pinto LA, Castle PE, Roden RB, Harro CD, Lowy DR, Schiller JT, Wallace D, Williams M, Kopp W, Frazer IH. HPV-16 L1 VLP vaccine elicits a broad-spectrum of cytokine responses in whole blood. Vaccine. 2005;23:3555–64.
- Nygaard M, Saah A, Munk C, Tryggvadottir L, Enerly E, Hortlund M, Sigurdardottir LG, Vuocolo S, Kjaer SK, Dillner J. Evaluation of the long-term anti-human papillomavirus 6 (HPV6), 11, 16, and 18 immune responses generated by the quadrivalent HPV vaccine. Clinical Vaccine Immunol. 2015;22:943–48.
- Pinto LA, Kemp TJ, Torres BN, Isaacs-Soriano K, Ingles D, Abrahamsen M, Pan Y, Lazcano-Ponce E, Salmeron J, Giuliano AR. Quadrivalent human papillomavirus (HPV) vaccine induces HPV-specific antibodies in the oral cavity: results from the mid-adult male vaccine trial. J Infect Dis. 2016;214:1276–83.
- Petäjä T, Pedersen C, Poder A, Strauss G, Catteau G, Thomas F, Lehtinen M, Descamps D. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int J Cancer. 2011;129:2147–57.
- Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, Abate G, Sakala IG, Edwards KM, Creech CB, et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse t-cell responses in young children. J Infect Dis. 2011;204:845–53.
- Clements ML, Murphy BR. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986;23:66–72.
- Monto AS, Ohmit SE, Petrie JG, Johnson E, Truscon R, Teich E, Rotthoff J, Boulton M, Victor JC. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Eng J Med. 2009;361:1260–67.
- Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM. Live attenuated versus inactivated influenza vaccine in infants and young children. N Eng J Med. 2007;356:685–96.
- Ambrose CS, Luke C, Coelingh K. Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. Influenza Other Respi Viruses. 2008;2:193–202.
- Onorato IM, Modlin JF, McBean AM, Thoms ML, Losonsky GA, Bernier RH. Mucosal Immunity Induced by Enhanced-Potency Inactivated and Oral Polio Vaccines. J Infect Dis. 1991;163:1–6.
- Ogra PL, Karzon DT, Righthand F, MacGillivray M. Immunoglobulin response in serum and secretions after immunization with live and inactivated poliovaccine and natural infection. N Eng J Med. 1968;279:893–900.
- Ni L, Seward JF, Santibanez TA, Pallansch MA, Kew OM, Prevots DR, Strebel PM, Cono J, Wharton M, Orenstein WA. Vaccine policy changes and epidemiology of poliomyelitis in the United States. JAMA. 2004;292:1696–701.
- Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. FutureMicrobiol. 2015;10:791–808.
- Smith TRF, Patel A, Ramos S, Elwood D, Zhu X, Yan J, Gary EN, Walker SN, Schultheis K, Purwar M, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nature Comm. 2020;11:2601.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020 Jul 14. https://www.nejm.org/doi/full/10.1056/NEJMoa2022483
- van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, Avanzato V, Bushmaker T, Flaxman A, Ulaszewska M, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. 2020. [accessed 2020 Jun 24] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7241103/
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020. [accessed 2020 Jun 24] https://science.sciencemag.org/content/early/2020/05/06/science.abc1932
- Kim E, Erdos G, Huang S, Kenniston TW, Balmert SC, Carey CD, Raj VS, Epperly MW, Klimstra WB, Haagmans BL, et al. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 2020;55:102743.
- Eyal N, Lipsitch M, Smith PG. Human challenge studies to accelerate coronavirus vaccine licensure. J Infect Dis. 2020;221:1752-56.
- Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. N Eng J Med. 2020;382:1969-73.
- Neumann G, Fujii K, Kino Y, Kawaoka Y. An improved reverse genetics system for influenza A virus generation and its implications for vaccine production. Proc Natl Acad Sci. 2005;102:16825–29.
